Journal of Traditional Chinese Medicine ›› 2025, Vol. 45 ›› Issue (2): 272-280.DOI: 10.19852/j.cnki.jtcm.2025.02.018
• Original articles • Previous Articles Next Articles
Fuzheng Xuanfei Huashi prescription (扶正宣肺化湿方) suppresses inflammation in lipopolysaccharide-induced lung injury in mice via toll-like recptor 4/nuclear transcription factor κB and cyclooxygenase-2/prostaglandin E2 pathway
HUANG Haiyang1, ZHU Shumin2, ZHONG Shaowen3, LIU Ying3, HOU Shaozhen3,4, GAO Jie3, OU Jianzhao1, DONG Mingguo1( ), NING Weimin1(
), NING Weimin1( )
)
- 1 Institute of Traditional Chinese Medicine, Dongguan Hospital of Traditional Chinese Medicine, Dongguan 523000, China
2 Department of Traditional Chinese Medicine, Guangdong Food and Drug Vocational College, Guangzhou 510520, China
3 School of Pharmaceutical Sciences, Guangzhou University of Chinese Medicine, Guangzhou 510006, China
4 Dongguan Institute of Guangzhou University of Chinese Medicine, Dongguan 523808, China
-
Received:2023-12-08Accepted:2024-05-27Online:2025-04-15Published:2025-03-10 -
Contact:NING Weimin, Institute of Traditional Chinese Medicine, Dongguan Hospital of Traditional Chinese Medicine, Dongguan 523000, China. ningweiming11@126.com; DONG Mingguo, Institute of Traditional Chinese Medicine, Dongguan Hospital of Traditional Chinese Medicine, Dongguan 523000, China. dgdongmingguo@163.com, Telephone: +86-769-26385763 -
Supported by:Emergency Corona Virus Disease 2019 (COVID-19) Response Project of Dongguan: Clinical Efficacy Observation and Mechanism Study of Fuzheng Xuanfei Huashi Formula in the Treatment of COVID-19 Based on the Lingnan Theory of Epidemic Diseases(202071715002124);National Natural Science Foundation of China: Study on the Mechanism of Lung Inflammatory Injury Induced by Gut-derived Lipopolysaccharide and Skatole in Spleen Deficiency Animals based on Pulmonary Alveolus Macrophage Heterogeneity(82274381);Guangdong Basic and Applied Basic Research Foundation: Development and Industrialization of Traditional Chinese Medicine Classic and Famous Prescription Compound Formulations(2021ZD006)
Cite this article
HUANG Haiyang, ZHU Shumin, ZHONG Shaowen, LIU Ying, HOU Shaozhen, GAO Jie, OU Jianzhao, DONG Mingguo, NING Weimin. Fuzheng Xuanfei Huashi prescription (扶正宣肺化湿方) suppresses inflammation in lipopolysaccharide-induced lung injury in mice via toll-like recptor 4/nuclear transcription factor κB and cyclooxygenase-2/prostaglandin E2 pathway[J]. Journal of Traditional Chinese Medicine, 2025, 45(2): 272-280.
share this article
| Group | n | Liver index (%) | AST (U/L) | ALT (U/L) | Thymus index (%) | Spleen index (%) | WBC (109/L) |
|---|---|---|---|---|---|---|---|
| NC | 6 | 4.24±0.29 | 16.13±1.21 | 14.66±4.02 | 0.23±0.03 | 0.31±0.04 | 78.10±3.11 |
| LPS | 6 | 6.75±0.60a | 47.41±2.35a | 26.15±1.75a | 0.29±0.04 | 0.92±0.12a | 94.50±11.10a |
| DEX | 6 | 6.78±0.61 | 30.85±2.15b | 18.16±2.95c | 0.22±0.06 | 0.56±0.09b | 85.29±6.64c |
| FZXFH | 6 | 5.90±0.59c | 25.24±3.50b | 14.74±2.99b | 0.22±0.05 | 0.75±0.21 | 83.04±3.13b |
| FZXFM | 6 | 5.82±0.44c | 24.07±3.45b | 16.76±2.94b | 0.28±0.05 | 0.76±0.15 | 89.99±3.96 |
| FZXFL | 6 | 6.34±0.68 | 25.24±5.78b | 17.41±1.80c | 0.29±0.03 | 0.95±0.16 | 90.13±4.49 |
Table 1 The level of organ impairment in mice
| Group | n | Liver index (%) | AST (U/L) | ALT (U/L) | Thymus index (%) | Spleen index (%) | WBC (109/L) |
|---|---|---|---|---|---|---|---|
| NC | 6 | 4.24±0.29 | 16.13±1.21 | 14.66±4.02 | 0.23±0.03 | 0.31±0.04 | 78.10±3.11 |
| LPS | 6 | 6.75±0.60a | 47.41±2.35a | 26.15±1.75a | 0.29±0.04 | 0.92±0.12a | 94.50±11.10a |
| DEX | 6 | 6.78±0.61 | 30.85±2.15b | 18.16±2.95c | 0.22±0.06 | 0.56±0.09b | 85.29±6.64c |
| FZXFH | 6 | 5.90±0.59c | 25.24±3.50b | 14.74±2.99b | 0.22±0.05 | 0.75±0.21 | 83.04±3.13b |
| FZXFM | 6 | 5.82±0.44c | 24.07±3.45b | 16.76±2.94b | 0.28±0.05 | 0.76±0.15 | 89.99±3.96 |
| FZXFL | 6 | 6.34±0.68 | 25.24±5.78b | 17.41±1.80c | 0.29±0.03 | 0.95±0.16 | 90.13±4.49 |

Figure 1 Histological injuries in the lungs of mice and W/D A-F: HE staining of mouse lung tissue (× 200); A: NC group; B: LPS group; C: DEX group; D: FZXFH group; E: FZXFM group; F: FZXFL group; G: the lungs of mice lung wet: dry weight ratio. NC: treated only with physiological saline; LPS: treated with lipopolysaccharide and physiological saline; DEX: treated with lipopolysaccharide and DEX of 0.25 mg/kg; FZXFH: treated with lipopolysaccharide and FZXF of 4.58 g/kg; FZXFM: treated with lipopolysaccharide and FZXF of 2.29 g/kg; FZXFL: treated with lipopolysaccharide and FZXF of 1.14 g/kg. W/D: lung wet weight∶dry weight; HE: hematoxylin-eosin; LPS: lipopolysaccharide and physiological saline; DEX: dexamethasone; FZXF: Fuzheng Xuanfei Huashi prescription; FZXFH: FZXF high; FZXFM: FZXF middle; FZXFL: FZXF low; ANOVA: analysis of variance. Data are expressed as mean ± standard deviation (n = 6). The difference among multiple groups was analyzed by one-way ANOVA statistical method. Compared with the NC group, aP < 0.01; compared with the LPS group, bP < 0.01.
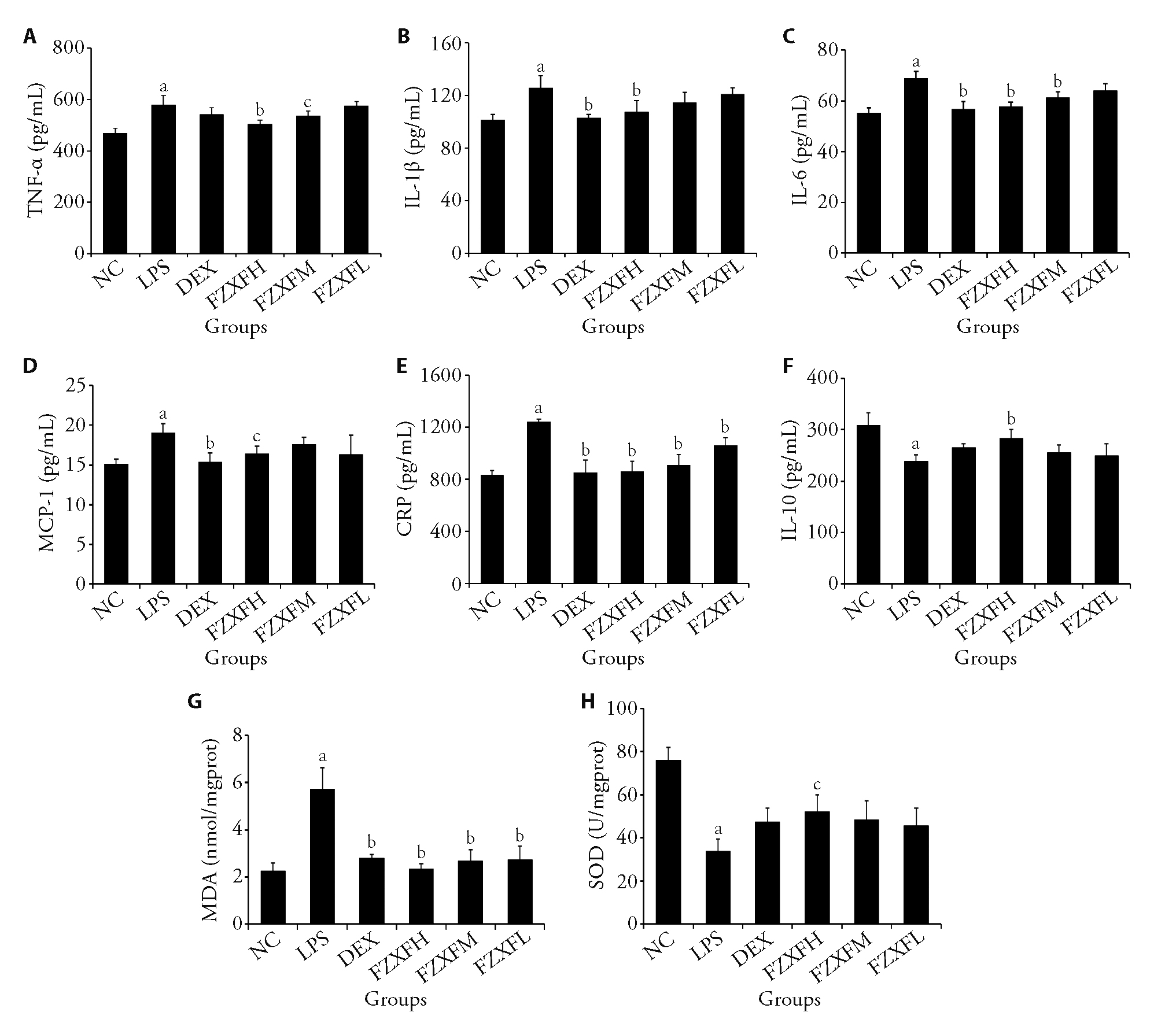
Figure 2 FZXF attenuates inflammation and oxidative stress in LPS-induced mice A: level of TNF-α; B: level of IL-1β; C: level of IL-6; D: level of MCP-1; E: level of CRP; F: level of IL-10; G: level of MDA; H: level of SOD. NC: treated only with physiological saline; LPS: treated with lipopolysaccharide and physiological saline; DEX: treated with lipopolysaccharide and DEX of 0.25 mg/kg; FZXFH: treated with lipopolysaccharide and FZXF of 4.58 g/kg; FZXFM: treated with lipopolysaccharide and FZXF of 2.29 g/kg; FZXFL: treated with lipopolysaccharide and FZXF of 1.14 g/kg. TNF-α: necrosis factor-α; IL-1β: interleukin-1β; IL-6: interleukin-6; MCP-1: monocyte chemotactic protein-1; CRP: C-reactive protein; IL-10: interleukin-10; MDA: malondialdehyde; SOD: superoxide dismutase; LPS: lipopolysaccharide and physiological saline; DEX: dexamethasone; FZXF: Fuzheng Xuanfei Huashi prescription; FZXFH: FZXF high; FZXFM: FZXF middle; FZXFL: FZXF low; ANOVA: analysis of variance. Data are expressed as mean ± standard deviation (n = 6). The difference among multiple groups was analyzed by one-way ANOVA statistical method. Compared with the NC group, aP < 0.01; compared with the LPS group, bP < 0.01, cP < 0.05.

Figure 3 Immunofluorescence of lung macrophages (scale bar = 100 μm) A-F: expression of F4/80 (red) and cell nuclei (blue) in mouse lung tissue. G: relative expression level of F4/80. A1-F1: expression of F4/80 (red) in mouse lung tissue. A2-F2: The expression of cell nuclei (blue) in mouse lung tissue. A, A1, A2: NC group; B, B1, B2: LPS group; C, C1, C2: DEX group; D, D1, D2: FZXFH group; E, E1, E2: FZXFM group; F, F1, F2: FZXFL group. NC: treated only with physiological saline; LPS: treated with lipopolysaccharide and physiological saline; DEX: treated with lipopolysaccharide and DEX of 0.25 mg/kg; FZXFH: treated with lipopolysaccharide and FZXF of 4.58 g/kg; FZXFM: treated with lipopolysaccharide and FZXF of 2.29 g/kg; FZXFL: treated with lipopolysaccharide and FZXF of 1.14 g/kg. LPS: lipopolysaccharide and physiological saline; DEX: dexamethasone; FZXF: Fuzheng Xuanfei Huashi prescription; FZXFH: FZXF high; FZXFM: FZXF middle; FZXFL: FZXF low; ANOVA: analysis of variance. Data are expressed as mean±standard deviation (n = 3). The difference among multiple groups was analyzed by one-way ANOVA statistical method. Compared with the NC group, aP < 0.01; compared with the LPS group, bP < 0.01.
| Group | n | mRNA TLR4 | mRNA NF-κB | mRNA COX-2 | mRNA PGE2 |
|---|---|---|---|---|---|
| NC | 6 | 0.96±0.20 | 1.22±0.14 | 1.34±0.14 | 1.01±0.11 |
| LPS | 6 | 3.21±0.08a | 2.57±0.14a | 4.44±0.89a | 3.68±0.44a |
| DEX | 6 | 1.50±0.19b | 1.59±0.06b | 1.96±0.32b | 1.76±0.31b |
| FZXFH | 6 | 1.71±0.15b | 1.54±0.28b | 2.66±0.23c | 1.75±0.46b |
| FZXFM | 6 | 2.19±0.48b | 2.14±0.30 | 3.02±0.72c | 3.30±0.13 |
| FZXFL | 6 | 2.54±0.32 | 2.41±0.36 | 3.99±0.96 | 3.51±0.09 |
Table 2 The mRNA expression levels of TLR4, NF-κB, COX-2 and PGE2 in the lung
| Group | n | mRNA TLR4 | mRNA NF-κB | mRNA COX-2 | mRNA PGE2 |
|---|---|---|---|---|---|
| NC | 6 | 0.96±0.20 | 1.22±0.14 | 1.34±0.14 | 1.01±0.11 |
| LPS | 6 | 3.21±0.08a | 2.57±0.14a | 4.44±0.89a | 3.68±0.44a |
| DEX | 6 | 1.50±0.19b | 1.59±0.06b | 1.96±0.32b | 1.76±0.31b |
| FZXFH | 6 | 1.71±0.15b | 1.54±0.28b | 2.66±0.23c | 1.75±0.46b |
| FZXFM | 6 | 2.19±0.48b | 2.14±0.30 | 3.02±0.72c | 3.30±0.13 |
| FZXFL | 6 | 2.54±0.32 | 2.41±0.36 | 3.99±0.96 | 3.51±0.09 |

Figure 4 Protein expression of the TLR4/NF-κB and COX-2/PGE2 signaling pathways in the lung A: Western blot bands of TLR4/ NF-κB signaling pathway; B: protein expression levels of TLR4; C: protein expression levels of p-p65/ p65; D: Western blot bands of COX-2/PGE2 signaling pathway; E: protein expression levels of COX-2; F: expression level of PGE2 in lung tissue. NC: treated only with physiological saline; LPS: treated with lipopolysaccharide and physiological saline; DEX: treated with lipopolysaccharide and DEX of 0.25 mg/kg; FZXFH: treated with lipopolysaccharide and FZXF of 4.58 g/kg; FZXFM: treated with lipopolysaccharide and FZXF of 2.29 g/kg; FZXFL: treated with lipopolysaccharide and FZXF of 1.14 g/kg. TLR4: toll-like receptor 4; p65: RelA, nuclear transcription factor κB3; p-p65: phosphorylated p65; COX-2: cyclooxygenase-2; GAPDH: glyceraldehyde-3-phosphate dehydrogenase; LPS: lipopolysaccharide and physiological saline; DEX: dexamethasone; FZXF: Fuzheng Xuanfei Huashi prescription; FZXFH: FZXF high; FZXFM: FZXF middle; FZXFL: FZXF low; ANOVA: analysis of variance. Data are expressed as mean ± standard deviation (n = 3). The difference among multiple groups was analyzed by one-way ANOVA statistical method. Compared with the NC group, aP < 0.01; compared with the LPS group, bP < 0.01, cP < 0.05.
| 1. |
Kirkcaldy RD, King BA, Brooks JT. COVID-19 and postinfection immunity: limited evidence, many remaining questions. JAMA 2020; 323: 2245-6.
DOI PMID |
| 2. | Mizgerd JP. Lung infection--a public health priority. PLoS Med 2006; 3: e76. |
| 3. | Jin Y, Yang H, Ji W, et al. Virology, epidemiology, pathogenesis, and control of COVID-19. Viruses 2020; 12. |
| 4. |
Long ME, Mallampalli RK, Horowitz JC. Pathogenesis of pneumonia and acute lung injury. Clin Sci (Lond) 2022; 136: 747-69.
DOI PMID |
| 5. | Li Y, Chu F, Li P, et al. Potential effect of Maxing Shigan decoction against coronavirus disease 2019 (COVID-19) revealed by network pharmacology and experimental verification. J Ethnopharmacol 2021; 271: 113854. |
| 6. | Kabra SK, Singhal T, Lodha R. Pneumonia. Indian J Pediatr 2001; 68: S19-23. |
| 7. |
Ruan X, Du P, Zhao K, et al. Mechanism of Dayuanyin in the treatment of coronavirus disease 2019 based on network pharmacology and molecular docking. Chin Med-uk 2020; 15: 62.
DOI PMID |
| 8. |
An X, Duan L, Zhang YH, et al. The three syndromes and six Chinese patent medicine study during the recovery phase of COVID-19. Chin Med-uk 2021; 16: 44.
DOI PMID |
| 9. | Kao ST, Yeh TJ, Hsieh CC, Yeh FT, Lin JG. Effect of San-Ao-Tang on immediate and late airway response and leukocyte infiltration in asthmatic guinea pigs. Immunopharmacol Immunotoxicol 2000; 22: 143-62. |
| 10. | Su KL, Xiong XJ. Treatment strategy and thought on classical herbal formulae for coronavirus disease 2019. Zhong Guo Zhong Yao Za Zhi 2021; 46: 494-503. |
| 11. | Ou J. Clinical observation of Fuzheng Xuanfeihuashi prescription on COVID-19. Zhong Guo Chu Fang Yao 2021; 19: 127-30. |
| 12. | Anka AU, Tahir MI, Abubakar SD, et al. Coronavirus disease 2019 (COVID-19): an overview of the immunopathology, serological diagnosis and management. Scand J Immunol 2021; 93: e12998. |
| 13. |
Zeng M, Sang W, Chen S, et al. 4-PBA inhibits LPS-induced inflammation through regulating ER stress and autophagy in acute lung injury models. Toxicol Lett 2017; 271: 26-37.
DOI PMID |
| 14. |
Tan Y, Kagan JC. A cross-disciplinary perspective on the innate immune responses to bacterial lipopolysaccharide. Mol Cell 2014; 54: 212-23.
DOI PMID |
| 15. | Zhang Y, Sun K, Liu YY, et al. Ginsenoside Rb1 ameliorates lipopolysaccharide-induced albumin leakage from rat mesenteric venules by intervening in both trans- and paracellular pathway. Am J Physiol-gastr L 2014; 306: G289-300. |
| 16. | Ruan Y, Yuan PP, Li PY, et al. Tingli Dazao Xiefei decoction ameliorates asthma in vivo and in vitro from lung to intestine by modifying NO-CO metabolic disorder mediated inflammation, immune imbalance, cellular barrier damage, oxidative stress and intestinal bacterial disorders. J Ethnopharmacol 2023; 313: 116503. |
| 17. | Liu B, Lu R, Li H, et al. Zhen-wu-tang ameliorates membranous nephropathy rats through inhibiting NF-κB pathway and NLRP 3 inflammasome. Phytomedicine 2019; 59: 152913. |
| 18. | Wu Z, Peng J, Wei Y, et al. Protective effect of Scutellariae Radix-Forsythiae fructus in an acute pneumonia mouse model induced by lipopolysaccharide. Acta Laboratorium Animalis Scientia Sinica 2022; 30: 800-9. |
| 19. | Fan Y, Wang J, Feng Z, Cao K, Xu H, Liu J. Pinitol attenuates LPS-induced pneumonia in experimental animals: possible role via inhibition of the TLR-4 and NF-kappa B/Ikappa B alpha signaling cascade pathway. J Biochem Mol Toxicol 2021; 35: e22622. |
| 20. | Shi J, Wang H, Liu J, et al. Ganoderic acid B attenuates LPS-induced lung injury. Int Immunopharmacol 2020; 88: 106990. |
| 21. |
Muravlyova L, Molotov-Luchankiy V, Bakirova R, Klyuyev D, Demidchik L, Lee V. Characteristic of the oxidative stress in blood of patients in dependence of community-acquired pneumonia severity. Open Access Maced J Med Sci 2016; 4: 122-7.
DOI PMID |
| 22. |
Arimori Y, Nakamura R, Yamada H, et al. Type I interferon limits influenza virus-induced acute lung injury by regulation of excessive inflammation in mice. Antiviral Research 2013; 99: 230-7.
DOI PMID |
| 23. |
Lee IT, Lin CC, Lin WN, Wu WL, Hsiao LD, Yang CM. Lung inflammation caused by adenosine-5'-triphosphate is mediated via Ca2+/PKCs-dependent COX-2/PGE2 induction. Int J Biochem Cell Biol 2013; 45: 1657-68.
DOI PMID |
| 24. | Su KC, Wu YC, Chen CS, et al. Bile acids increase alveolar epithelial permeability via mitogen-activated protein kinase, cytosolic phospholipase A2, cyclooxygenase-2, prostaglandin E2 and junctional proteins. Respirology 2013; 18: 848-56. |
| 25. | Luo Z, Huang J, Li E, et al. An integrated pharmacology-based strategy to investigate the potential mechanism of Xiebai San in treating pediatric pneumonia. Front Pharmacol 2022; 13: 784729. |
| 26. | Zhao J, Tian S, Lu D, et al. Systems pharmacological study illustrates the immune regulation, anti-infection, anti-inflammation, and multi-organ protection mechanism of Qing-Fei-Pai-Du decoction in the treatment of COVID-19. Phytomedicine 2021; 85: 153315. |
| 27. | Yang R, Liu H, Bai C, et al. Chemical composition and pharmacological mechanism of Qingfei Paidu decoction and Ma Xing Shi Gan decoction against Coronavirus Disease 2019 (COVID-19): in silico and experimental study. Pharmacol Res 2020; 157: 104820. |
| 28. | Desai O, Winkler J, Minasyan M, Herzog EL. The role of immune and inflammatory cells in idiopathic pulmonary fibrosis. Front Med (Lausanne) 2018; 5: 43. |
| 29. |
Laskin DL, Malaviya R, Laskin JD. Role of macrophages in acute lung injury and chronic fibrosis induced by pulmonary toxicants. Toxicol Sci 2019; 168: 287-301.
DOI PMID |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||

