Journal of Traditional Chinese Medicine ›› 2025, Vol. 45 ›› Issue (2): 281-290.DOI: 10.19852/j.cnki.jtcm.2025.02.022
• Original articles • Previous Articles Next Articles
Jinxin oral liquid (金欣口服液) reduced lung inflammation in influenza A virus infected mice through inhibiting NOD-like receptor protein 3 pathway
LI Tao1,2, WANG Xianzheng3, XIONG Yingcai2, DAI Qigang1, WANG Shouchuan1( ), JI Jianjian2(
), JI Jianjian2( )
)
- 1 Department of Pediatrics, Affiliated Hospital of Nanjing University of Chinese Medicine, Nanjing 210029, China
2 Jiangsu Key Laboratory of Children’s Health and Chinese Medicine, Nanjing University of Chinese Medicine, Nanjing 210023, China
3 Basic Medical College of Hebei University of Traditional Chinese Medicine, Shijiazhuang 050200, China
-
Received:2023-12-06Accepted:2024-05-15Online:2025-04-15Published:2025-03-10 -
Contact:Prof. JI Jianjian, Jiangsu Key Laboratory of Children’s Health and Chinese Medicine, Nanjing University of Chinese Medicine, Nanjing 210023, China. jijj@njucm.edu.cn; Prof. WANG Shouchuan, Department of Pediatrics, Affiliated Hospital of Nanjing University of Chinese Medicine, Nanjing 210029, China. wscnj@njucm.edu.cn, Telephone: +86-18260087292 -
Supported by:Young Elite Scientists Sponsorship Program by Chinese Association for Clinical Medicine(2021-QNRC2-B14);Colleges and universities in Jiangsu Province Natural Science Research: Investigating the Mechanism of Jinping Decoction Inhibiting the Differentiation of Myeloid-derived Suppressor Cells and Regulating Lipid Metabolism(22KJA360004);Jiangsu Graduate Practice Innovation Program: A Study on the Mechanism of Xiaofeng Xuanqiao Decoction in Treating Pediatric Rhinitis based on Interleukin-33/Suppression of Tumorigenicity 2(SJCX23_0803);Wuxi Health Commission Scientific Research Project: Observation on the Efficacy of Xiaofeng Xuanqiao Decoction in Treating Pediatric Rhinitis (Wind-Phlegm Obstructing Orifices Syndrome) and Its Influence on Serum IgE and Related Inflammatory Cytokine Levels(M202035);High-level Construction of Key Traditional Chinese Medicine (TCM) Disciplines, China: Research on Tic Disorders from the Perspective of the Five Viscera Syndrome Treatment(NZYEK004);High-level Construction of Key TCM Disciplines, China: Research on the Mechanism of Xiaofeng Xuanqiao Decoction in Treating Allergic Rhinitis based on the Interleukin-33/ST2 Axis(NZYEK006)
Cite this article
LI Tao, WANG Xianzheng, XIONG Yingcai, DAI Qigang, WANG Shouchuan, JI Jianjian. Jinxin oral liquid (金欣口服液) reduced lung inflammation in influenza A virus infected mice through inhibiting NOD-like receptor protein 3 pathway[J]. Journal of Traditional Chinese Medicine, 2025, 45(2): 281-290.
share this article

Figure 1 JX showed protective effects response to H1N1 infection A: representative HE-stained lung tissue (middle lobe of right lung) from mice showed histologic differences group by HE staining (× 200) (n = 4); A1: normal control group; A2: H1N1 infected group; A3: ribavirin treatment group; A4: Jxin oral liquid high-dose group; A5: Jinxin oral liquid middle-dose group; A6: Jinxin oral liquid low-dose group. B: the body temperature was measured using thermometer (n = 5); C: the viral load in the lungs of mice was measured by viral N gene expression (n = 5); D: the weight of the lungs of mice was measured (n = 5); E: the albumin level in BALF was determined (n = 3). Normal control group: treated with normal diet and physiological saline; H1N1 infected group: H1N1, 106 PFU, 2 d; ribavirin treatment group: 46 mg·kg-1·d-1, 2 d; Jxin oral liquid high-dose group: 55.2 g·kg-1·d-1, 2 d, JX-H; Jinxin oral liquid middle-dose group: 27.6 g·kg-1·d-1, 2 d, JX-M; Jinxin oral liquid low-dose group: 13.8 g·kg-1·d-1, 2 d, JX-L. NLRP3: NOD-like receptor protein 3; HE: hematoxylin and eosin; BALF: bronchoalveolar lavage fluid; PFU: plaque formation unit; Control: normal control group; H1N1: influenza a virus; JX-H: Jinxin oral liquid high-dose group; JX-M: Jinxin oral liquid middle-dose group; JX-L: Jinxin oral liquid low-dose group; ANOVA: analysis of variance. All results are expressed as the mean ± standard deviation by one-way ANOVA with Dunnett’s post hoc tests for multiple comparisons. aP < 0.01, cP < 0.05, H1N1 group vs control group; bP < 0.01, dP < 0.05, vs H1N1 group.
| No. | Compound name | Formula | Adduct | RT in STD | RTin JX | m/z | ppm |
|---|---|---|---|---|---|---|---|
| 1 | Ephedrine | C10H15NO | [M+H]+ | 2.96 | 3.01 | 166.12268 | 0.237 |
| 2 | Amygdalin | C20H27NO11 | [M+Na]+ | 4.39 | 4.41 | 480.14954 | 3.974 |
| 3 | Sinapine | C16H24NO5 | [M]+ | 4.60 | 4.60 | 310.16547 | 1.840 |
| 4 | Polydatin | C20H22O8 | [M+H]+ | 6.47 | 6.50 | 391.13983 | 2.776 |
| 5 | Baicalin | C21H18O11 | [M+H]+ | 8.26 | 8.26 | 447.09415 | 4.389 |
| 6 | Resveratrol | C14H12O3 | [M+H]+ | 8.83 | 8.82 | 229.08626 | 1.481 |
| 7 | Quercetin | C15H10O7 | [M+H]+ | 9.60 | 9.63 | 303.05054 | 2.016 |
| 8 | Emodin | C15H10O5 | [M+H]+ | 11.37 | 11.38 | 271.06082 | 2.656 |
| 9 | Praeruptorin | C24H28O7 | [M+H]+ | 20.63 | 20.60 | 429.19806 | 16.963 |
Table 1 The chemical components identified in the JX
| No. | Compound name | Formula | Adduct | RT in STD | RTin JX | m/z | ppm |
|---|---|---|---|---|---|---|---|
| 1 | Ephedrine | C10H15NO | [M+H]+ | 2.96 | 3.01 | 166.12268 | 0.237 |
| 2 | Amygdalin | C20H27NO11 | [M+Na]+ | 4.39 | 4.41 | 480.14954 | 3.974 |
| 3 | Sinapine | C16H24NO5 | [M]+ | 4.60 | 4.60 | 310.16547 | 1.840 |
| 4 | Polydatin | C20H22O8 | [M+H]+ | 6.47 | 6.50 | 391.13983 | 2.776 |
| 5 | Baicalin | C21H18O11 | [M+H]+ | 8.26 | 8.26 | 447.09415 | 4.389 |
| 6 | Resveratrol | C14H12O3 | [M+H]+ | 8.83 | 8.82 | 229.08626 | 1.481 |
| 7 | Quercetin | C15H10O7 | [M+H]+ | 9.60 | 9.63 | 303.05054 | 2.016 |
| 8 | Emodin | C15H10O5 | [M+H]+ | 11.37 | 11.38 | 271.06082 | 2.656 |
| 9 | Praeruptorin | C24H28O7 | [M+H]+ | 20.63 | 20.60 | 429.19806 | 16.963 |

Figure 2 JX reduces the expression of inflammatory cytokines and inhibits NLRP3 inflammasome activation in the lungs of H1N1-infected mice A: quantification of TNF-α in lungs from all groups of mice by qPCR (n = 6); B: quantification of IL-1β in lungs from all groups of mice by qPCR (n = 6); C: quantification of TNF-ɑ in BALF from all groups of mice by ELSIA (n = 4); D: quantification of IL-1β in BALF and serum from all groups of mice by ELISA (n = 4). Normal control group: treated with normal diet and physiological saline; H1N1 infected group: H1N1, 106 PFU, 2 d; ribavirin treatment group: 46 mg·kg-1·d-1, 2 d; Jxin oral liquid high-dose group: 55.2 g·kg-1·d-1, 2 d, JX-H; Jinxin oral liquid middle-dose group: 27.6 g·kg-1·d-1, 2 d, JX-M; Jinxin oral liquid low-dose group: 13.8 g·kg-1·d-1, 2 d, JX-L. NLRP3: nod-like receptor protein 3;TNF-α; tumor necrosis factor-α; IL-1β: interleukin-1β; qPCR: quantitative polymerase chain reaction; BALF; bronchoalveolar lavage fluid; PFU: plaque formation unit; ELISA: enzyme-linked immunosorbent assay; ANOVA: analysis of variance; Control: normal control group; H1N1: influenza a virus; JX-H: Jinxin oral liquid high-dose group; JX-M: Jinxin oral liquid middle-dose group; JX-L: Jinxin oral liquid low-dose group. All results are expressed as the mean ± standard deviation by one-way ANOVA with Dunnett’s post hoc tests for multiple comparisons. aP < 0.0001, eP < 0.001, H1N1 group vs control group; bP < 0.01, fP < 0.05, vs H1N1 group; cP < 0.001 dP < 0.0001, vs model group.

Figure 3 NLRP3 pathway is necessary for the protective effects of JX against H1N1-induced A: representative HE-stained lung tissue (middle lobe of right lung) from mice showed histologic differences (n = 4). A1: Control group; A2: H1N1 infected group; A3: H1N1 infected and treated with Jinxin oral liquid high-dose group; A4: H1N1 infected and MCC950 Inhibitor group; A5: H1N1 infected group treated with Jinxin oral liquid middle-dose group and MCC950 inhibitor. HE staining, ×200; B: the protein levels of TNF-ɑ in BALF from all groups of mice was determined by ELISA at 24 h after H1N1 infection (n = 4). C: the expression of IL-1β in lungs from all groups of mice was determined by qPCR at 24 h after H1N1 infection (n = 4). D: the protein levels of IL1β in BALF from all groups of mice was determined by ELISA at 24 h after H1N1 infection (n = 4). Normal control group: treated with normal diet and physiological saline; H1N1 infected group: H1N1, 106 PFU, 2 d; Jxin oral liquid high-dose group: 55.2 g·kg-1·d-1, 2 d, JX-H; MCC950: H1N1 infected and mcc950 Inhibitor group; JX +MCC950: H1N1 infected group treated with Jinxin oral liquid middle-dose group and mcc950 inhibitor. HE: hematoxylin and eosin; TNF-α; tumor necrosis factor-α; IL-1β: interleukin-1β; BALF: bronchoalveolar lavage fluid; PFU: plaque formation unit; ELISA: enzyme-linked immunosorbent assay; qPCR: quantitative polymerase chain reaction; ANOVA: analysis of variance. All results are expressed as the mean ± standard deviation by one-way ANOVA with Dunnett’s post hoc tests for multiple comparisons, aP < 0.001, cP < 0.05, H1N1 group vs control group; bP < 0.01, dP < 0.05, vs H1N1 group.
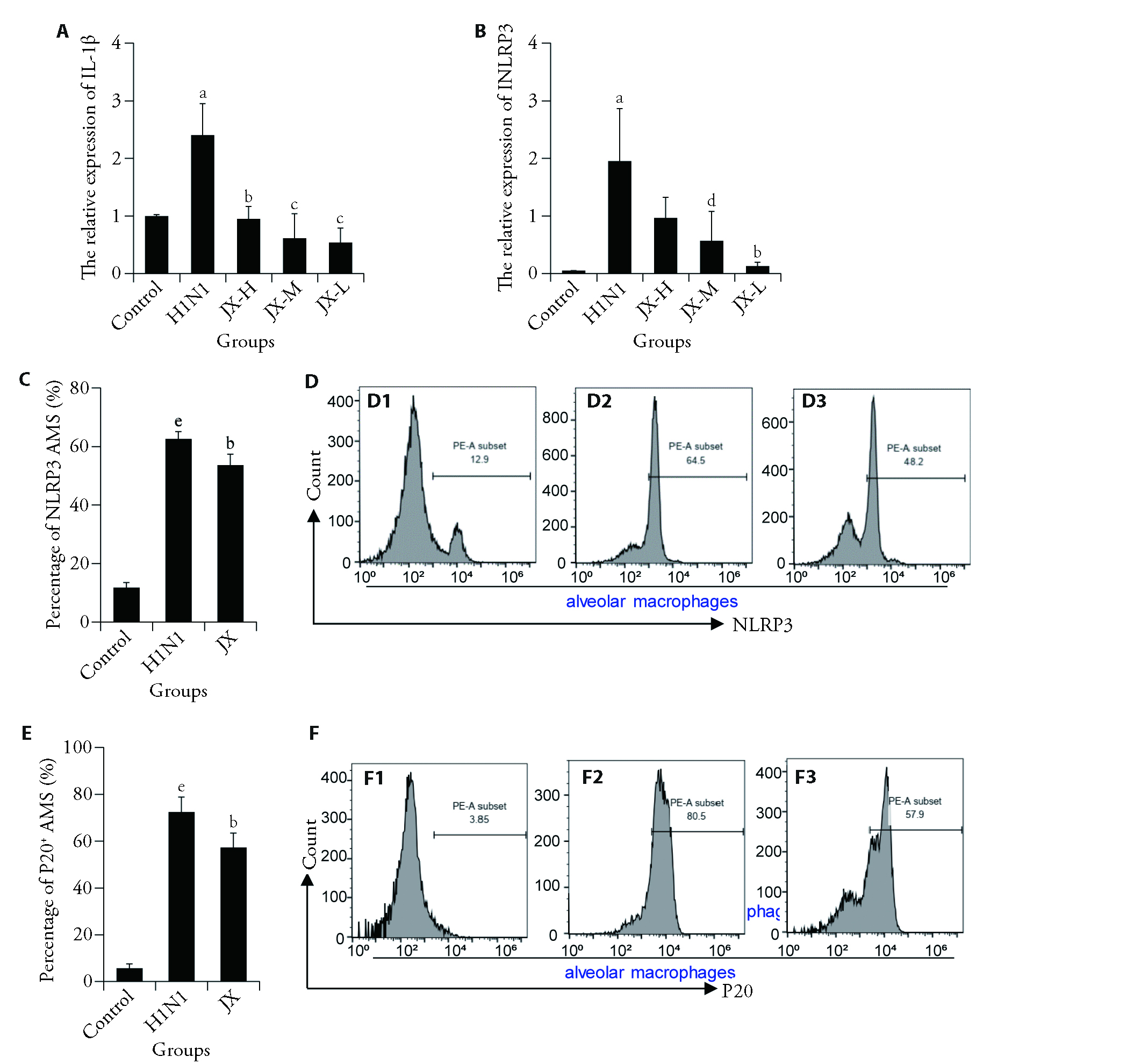
Figure 4 JX inhibits the NLRP3 inflammasome activation in the AMs A: the expression of IL-1β in isolated AMs from all groups of mice was determined by qPCR at 24 h after H1N1 infection (n = 3); B: the expression of NLRP3 in isolated AMs from all groups of mice was determined by qPCR at 24 h after H1N1 infection (n = 3); C: the expression of NLRP3 in CD11c+ Siglec-F+ AMs from all groups of mice was determined by FACS at 24 h after H1N1 infection (n = 4); D: flow cytometry histograms for NLRP3+ (D1-D3) AMS in all groups. D1: Control Group; D2: H1N1 Group; D3: JX Group. E: the expression of caspase1 p20 in CD11c+ Siglec-F+ AMs from all groups of mice was determined by FACS at 24 h after H1N1 infection (n = 4). F: flow cytometry histograms for P20+ (F1-F3) AMS in all groups, F1: Control Group; F2: H1N1 Group; F3: JX Group. Normal control group: treated with normal diet and physiological saline; H1N1 infected group: H1N1, 106 PFU, 2 d; ribavirin treatment group: 46 mg·kg-1·d-1, 2 d; Jxin oral liquid high-dose group: 55.2 g·kg-1·d-1, 2 d, JX-H; Jinxin oral liquid middle-dose group: 27.6 g·kg-1·d-1, 2 d, JX-M; Jinxin oral liquid low-dose group: 13.8 g·kg-1·d-1, 2 d, JX-L. NLRP3: nod-like receptor protein 3; TNF-α; tumor necrosis factor-α; IL-1β:interleukin-1β; qPCR: quantitative polymerase chain reaction; BALF: bronchoalveolar lavage fluid; ELISA: enzyme-linked immunosorbent assay; PFU: plaque formation unit; ANOVA: analysis of variance; Ams: Alveolar macrophages; FACS: fluorescence-activated cell sorting; Control: normal control group; H1N1: influenza a virus; JX-H: Jinxin oral liquid high-dose group; JX-M: Jinxin oral liquid middle-dose group; JX-L: Jinxin oral liquid low-dose group. All results are expressed as the mean ± standard deviation by one-way ANOVA with Dunnett’s post hoc tests for multiple comparisons. aP < 0.01, eP < 0.0001, H1N1 group vs control group; bP < 0.01, cP < 0.001, dP < 0.05, vs H1N1 group.
| 1. | Brody H. Influenza. Nature 2019; 573: S49. |
| 2. |
Liu Q, Zhou YH, Yang ZQ. The cytokine storm of severe influenza and development of immunomodulatory therapy. Cell Mol Immunol 2016; 13: 3-10.
DOI PMID |
| 3. | Guo XJ, Thomas PG. New fronts emerge in the influenza cytokine storm. Semin Immunopathol 2017; 39: 541-50. |
| 4. |
Chen S, Liu G, Chen J, et al. Ponatinib protects mice from lethal influenza infection by suppressing cytokine storm. Front Immunol 2019; 10: 1393.
DOI PMID |
| 5. | Fajgenbaum DC, June CH. Cytokine Storm. N Engl J Med 2020; 383: 2255-73. |
| 6. |
Teijaro JR, Walsh KB, Rice S, Rosen H, Oldstone MB. Mapping the innate signaling cascade essential for cytokine storm during influenza virus infection. Proc Natl Acad Sci USA 2014; 111: 3799-804.
DOI PMID |
| 7. |
Teijaro JR. The role of cytokine responses during influenza virus pathogenesis and potential therapeutic options. Curr Top Microbiol Immunol 2015; 386: 3-22.
DOI PMID |
| 8. | Hartshorn KL. Innate immunity and influenza A virus pathogenesis: lessons for COVID-19. Front Cell Infect Microbiol 2020; 10: 563850. |
| 9. |
Wang S, Le TQ, Kurihara N, et al. Influenza virus-cytokine-protease cycle in the pathogenesis of vascular hyperpermeability in severe influenza. J Infect Dis 2010; 202: 991-1001.
DOI PMID |
| 10. | Hsieh CF, Lo CW, Liu CH, et al. Mechanism by which ma-xing-shi-gan-tang inhibits the entry of influenza virus. J Ethnopharmacol 2012; 143: 57-67. |
| 11. |
Nosaka N, Martinon D, Moreira D, Crother TR, Arditi M, Shimada K. Autophagy protects against developing increased lung permeability and hypoxemia by down regulating inflammasome activity and IL-1β in LPS plus mechanical ventilation-induced acute lung injury. Front Immunol 2020; 11: 207.
DOI PMID |
| 12. |
Tuku B, Stanelle-Bertram S, Sellau J, et al. Testosterone Protects against severe influenza by reducing the pro-inflammatory cytokine response in the murine lung. Front Immunol 2020; 11: 697.
DOI PMID |
| 13. | Peiro T, Patel DF, Akthar S, et al. Neutrophils drive alveolar macrophage IL-1 beta release during respiratory viral infection. Thorax 2018; 73: 546-56. |
| 14. |
Kuriakose T, Kanneganti TD. Regulation and functions of NLRP3 inflammasome during influenza virus infection. Mol Immunol 2017; 86: 56-64.
DOI PMID |
| 15. |
Huang Y, Xu W, Zhou R. NLRP3 inflammasome activation and cell death. Cell Mol Immunol 2021; 18: 2114-27.
DOI PMID |
| 16. | Lin L, Xu L, Lv W, et al. An NLRP3 inflammasome-triggered cytokine storm contributes to streptococcal toxic shock-like syndrome (STSLS). Plos Pathog 2019; 15: e1007795. |
| 17. |
Freeman TL, Swartz TH. Targeting the NLRP3 Inflammasome in Severe COVID-19. Front Immunol 2020; 11: 1518.
DOI PMID |
| 18. |
Coates BM, Staricha KL, Ravindran N, et al. Inhibition of the NOD-like receptor protein 3 inflammasome is protective in juvenile influenza A virus infection. Front Immunol 2017; 8: 782.
DOI PMID |
| 19. | Xiong Y, Li NX, Duan N, et al. Traditional Chinese Medicine in treating influenza: from basic science to clinical applications. Front Pharmacol 2020; 11: 575803. |
| 20. | Chen ZG, Luo H, Wang SC, Xu JY, Li JX. Antiviral effects of Jinxin oral liquid against respiratory syncytial virus infection in the BALB/c mice model. J Ethnopharmacol 2015; 162: 287-95. |
| 21. |
Shen C, Zhang Z, Xie T, et al. Jinxin oral liquid inhibits human respiratory syncytial virus-induced excessive inflammation associated with blockade of the NLRP3/ASC/Caspase-1 pathway. Biomed Pharmacother 2018; 103: 1376-83.
DOI PMID |
| 22. | Jiang L, Deng L, Wu T. Chinese medicinal herbs for influenza. Cochrane Database Syst Rev 2013; 2013: D4559. |
| 23. | Zhang X, Goncalves R, Mosser DM. The isolation and characterization of murine macrophages. Curr Protoc Immunol 2008; Chapter 14: 11-4. |
| 24. | Kolli D, Gupta MR, Sbrana E, et al. Alveolar macrophages contribute to the pathogenesis of human metapneumovirus infection while protecting against respiratory syncytial virus infection. Am J Respir Cell Mol Biol 2014; 51: 502-15. |
| 25. |
Coll RC, Hill JR, Day CJ, et al. MCC950 directly targets the NLRP3 ATP-hydrolysis motif for inflammasome inhibition. Nat Chem Biol 2019; 15: 556-59.
DOI PMID |
| 26. |
Coates BM, Staricha KL, Wiese KM, Ridge KM. Influenza A virus infection, innate immunity, and childhood. JAMA Pediatr 2015; 169: 956-63.
DOI PMID |
| 27. |
Grom AA, Horne A, De Benedetti F. Macrophage activation syndrome in the era of biologic therapy. Nat Rev Rheumatol 2016; 12: 259-68.
DOI PMID |
| 28. | Allen IC, Scull MA, Moore CB, et al. The NLRP3 inflammasome mediates in vivo innate immunity to influenza A virus through recognition of viral RNA. Immunity 2009; 30: 556-65. |
| 29. |
Thomas PG, Dash P, Aldridge JJ, et al. The intracellular sensor NLRP3 mediates key innate and healing responses to influenza A virus via the regulation of caspase-1. Immunity 2009; 30: 566-75.
DOI PMID |
| 30. | McAuley JL, Tate MD, MacKenzie-Kludas CJ, et al. Activation of the NLRP3 inflammasome by IAV virulence protein PB1-F2 contributes to severe pathophysiology and disease. Plos Pathog 2013; 9: e1003392. |
| 31. | Tate MD, Ong J, Dowling JK, et al. Reassessing the role of the NLRP 3 inflammasome during pathogenic influenza A virus infection via temporal inhibition. Sci Rep 2016; 6: 27912. |
| 32. | Park HS, Liu G, Thulasi RS, Landreth SL, Liu Q, Zhou Y. NS1 protein of 2009 pandemic influenza A virus inhibits porcine NLRP3 inflammasome-mediated interleukin-1 beta production by suppressing ASC ubiquitination. J Virol 2018; 92. |
| 33. |
Liesman RM, Buchholz UJ, Luongo CL, et al. RSV-encoded NS2 promotes epithelial cell shedding and distal airway obstruction. J Clin Invest 2014; 124: 2219-33.
DOI PMID |
| 34. |
Huanbo C, Hui HU, Daihua S, Guangzhong W. Anti-inflammatory, anti-tussive effects and toxicity evaluation of Qingfei Dayuan granules. J Tradit Chin Med 2023; 43: 1110-17.
DOI |
| 35. |
Rong F, Haoyu HE, Tao T, Hanjin C. Long-term effects of Qingfei Paidu decoction in patients with coronavirus disease 2019 acute pneumonia after treatment: a protocol for systematic review and Meta-analysis. J Tradit Chin Med 2023; 43: 1068-71.
DOI |
| No related articles found! |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||

