Journal of Traditional Chinese Medicine ›› 2025, Vol. 45 ›› Issue (1): 76-88.DOI: 10.19852/j.cnki.jtcm.2025.01.007
Previous Articles Next Articles
Network pharmacology-based study on the mechanism of Tangfukang formula (糖复康方) against type 2 diabetes mellitus
YAN Kai1,2,3, WANG Wei4, WANG Yan5, GAO Huijuan6,4( ), FENG Xingzhong2,4(
), FENG Xingzhong2,4( )
)
- 1 Department of Traditional Chinese Medicine, Beijing Chao-Yang Hospital, Capital Medical University, Beijing 100043, China
2 Department of Traditional Chinese Medicine, Beijing Shijitan Hospital, Capital Medical University, Beijing 100038, China
3 Institute for Precision Medicine, Tsinghua University, Beijing 100084, China
4 Department of Endocrinology, Tsinghua University Yuquan Hospital (Tsinghua University Hospital of Integrated Traditional Chinese and Western Medicine), Beijing 100040, China
5 Department of Traditional Chinese Medicine, Civil Aviation General Hospital, Beijing 100123, China
6 Institute for Precision Medicine, Tsinghua University, Beijing 100084, China
-
Received:2023-12-22Accepted:2024-05-15Online:2025-02-15Published:2025-01-10 -
Contact:FENG Xingzhong, Department of Traditional Chinese Medicine, Beijing Shijitan Hospital, Capital Medical University, Beijing 100038, China; Department of Endocrinology, Tsinghua University Yuquan Hospital (Tsinghua University Hospital of Integrated Traditional Chinese and Western Medicine), Beijing 100040, China.fengxz9797@sina.com ; GAO Huijuan, Institute for Precision Medicine, Tsinghua University, Beijing 100084, China; Department of Endocrinology, Tsinghua University Yuquan Hospital (Tsinghua University Hospital of Integrated Traditional Chinese and Western Medicine), Beijing 100040, China.gaohuijuanghj@126.com Telephone: +86-21-88257755-6364 -
Supported by:Tsinghua Precision Medicine Foundation: Tangfukang Plays the Therapeutic Role in Type 2 Diabetes Patients with Qi and Yin Deficiency Syndrome by Regulating the Intestinal Flora Mediated Branched-chain Amino Acids-Phosphatidylinositide 3-Kinases-Protein Kinase B Signaling Pathway(grant number 10001020105);National Natural Science Foundation of China: Tangfukang Plays the Therapeutic Role in Type 2 Diabetes Mellitus by Regulating the Intestinal Flora Mediated Adiponectin-adenosine 5-Monophosphate-activated Protein Kinase-branched-chain Amino Acids Signaling Pathway(grant number 82104812)
Cite this article
YAN Kai, WANG Wei, WANG Yan, GAO Huijuan, FENG Xingzhong. Network pharmacology-based study on the mechanism of Tangfukang formula (糖复康方) against type 2 diabetes mellitus[J]. Journal of Traditional Chinese Medicine, 2025, 45(1): 76-88.
share this article
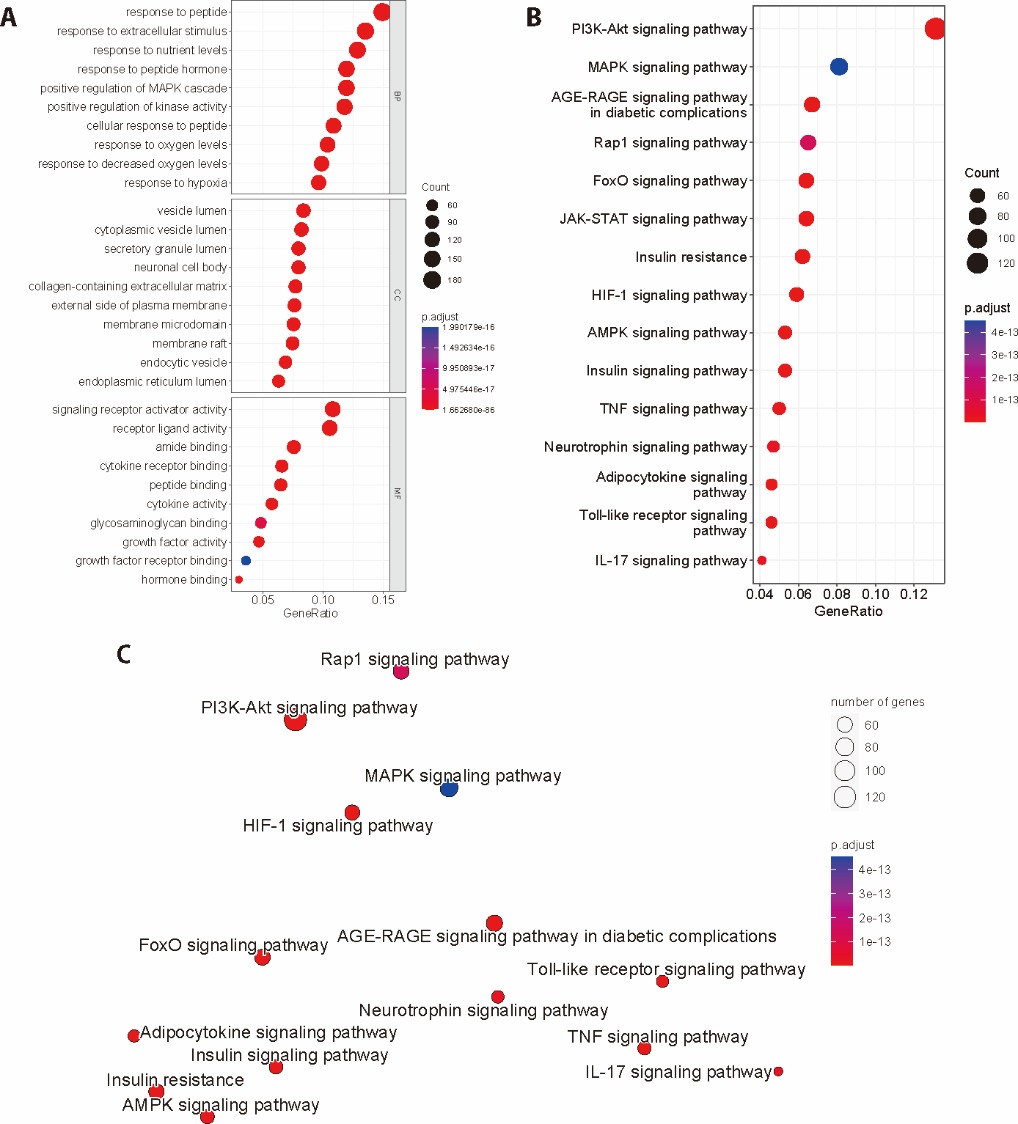
Figure 1 GO analysis and KEGG pathway enrichment analysis of TFK against T2DM A: GO analysis: barplot of BP, CC, and MF (P < 0.05); B: barplot of top 20 enriched pathways of the KEGG pathway enrichment analysis of core targets of TFK against T2DM; C: Enrichment map of top 20 enriched pathways of the KEGG pathway enrichment analysis of core targets of TFK against T2DM. GO: Gene Ontology; BP: biological process; CC: cell components; MF: molecular function; KEGG: Kyoto Encyclopedia of Genes and Genomes; TFK: Tangfukang formula; T2DM: type 2 diabetes mellitus.
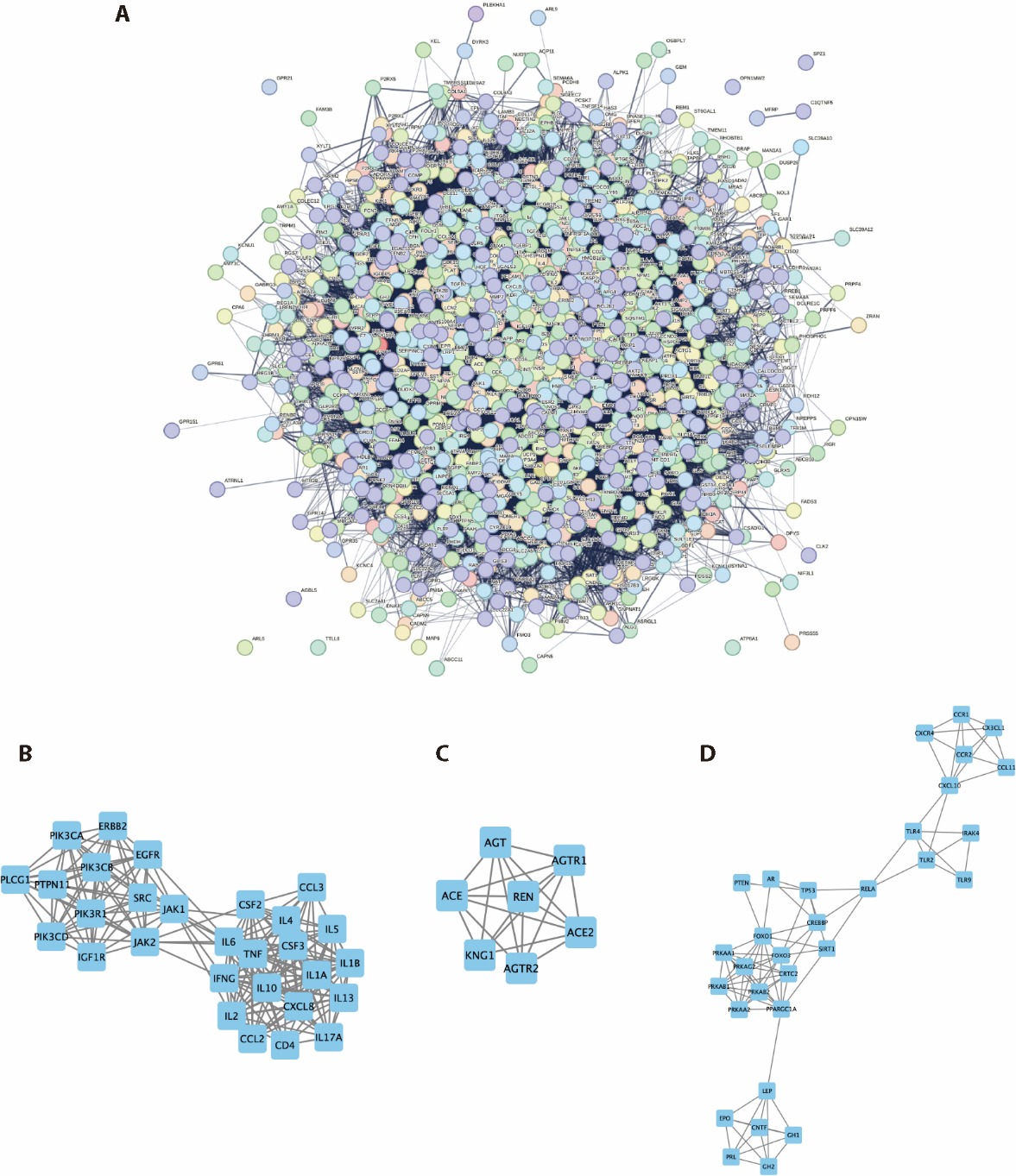
Figure 2 PPI analysis of core targets of TFK against T2DM A: PPI network of core targets; B: Cluster 1 of the core PPI network: JAK-STAT signaling pathway (LogP = -21.92); C: Cluster 2 of the core PPI network: Renin-angiotensin system (LogP = -14.91); D: Cluster 3 of the core PPI network: AMPK signaling pathway (LogP = -12.77). TFK: Tangfukang formula; T2DM: type 2 diabetes mellitus; PPI: protein-protein interaction; JAK: janus tyrosine kinase; STAT: signal transducer and activator of transcription. AMPK: adenosine 5-monophosphate-activated protein kinase.
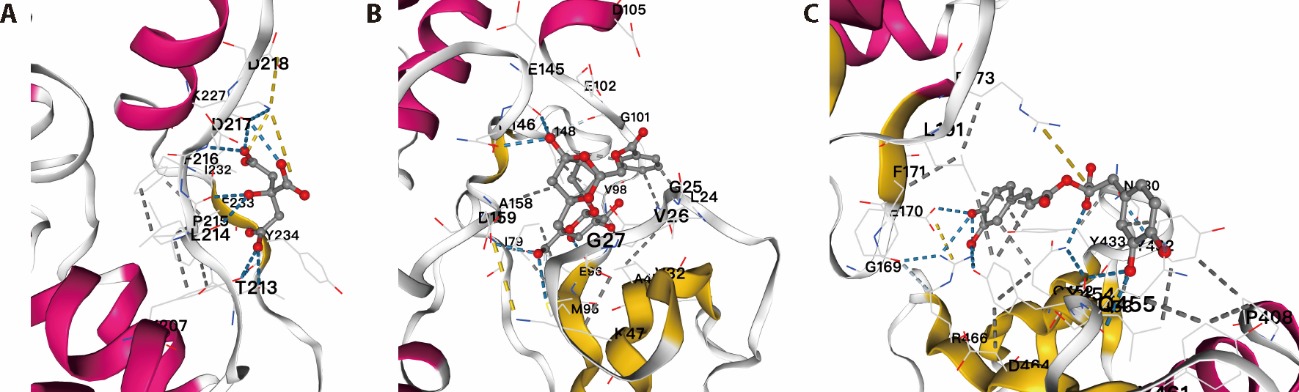
Figure 3 Molecular docking of compounds of TFK and AMPK signaling pathway A: molecular docking of citric and AMPK signaling pathway; B: molecular docking of paeoniflo and AMPK signaling pathway; C: molecular docking of rosmarinic and AMPK signaling pathway. The most likely binding conformation and the corresponding intermolecular interactions have been identified. The protein backbone is represented using a cartoon, while the ligand (carbon in red) and active site residues (carbon in yellow and magenta) are shown in stick representation. Water is represented as a white sphere, and hydrogen bonds are indicated using dashed lines. TFK: Tangfukang formula; AMPK: adenosine 5-monophosphate-activated protein kinase.
| Time (weeks) | CON group (n = 6) | KKAy group (n = 9) | TFK group (n = 9) | TFK+CC group (n = 9) | AICAR group (n = 9) |
|---|---|---|---|---|---|
| 0 | 27.8±0.6 | 36.9±0.9 | 36.3±1.9 | 36.9±1.4 | 36.9±1.7 |
| 1 | 28.4±0.7 | 38.5±1.0 | 38.4±1.9 | 37.9±1.8 | 38.0±2.0 |
| 2 | 29.2±1.1 | 39.4±1.5 | 39.1±2.1 | 39.0±1.4 | 38.3±1.7 |
| 3 | 29.1±1.2 | 40.4±1.4 | 39.1±2.3 | 39.9±1.2 | 38.1±1.6a |
| 4 | 29.1±0.9 | 40.9±1.0 | 38.9±2.4a | 40.2±1.1 | 38.8±1.8a |
Table 1 Effect of TFK on the body weight of KKAy mice (g, $\bar{x}±s$)
| Time (weeks) | CON group (n = 6) | KKAy group (n = 9) | TFK group (n = 9) | TFK+CC group (n = 9) | AICAR group (n = 9) |
|---|---|---|---|---|---|
| 0 | 27.8±0.6 | 36.9±0.9 | 36.3±1.9 | 36.9±1.4 | 36.9±1.7 |
| 1 | 28.4±0.7 | 38.5±1.0 | 38.4±1.9 | 37.9±1.8 | 38.0±2.0 |
| 2 | 29.2±1.1 | 39.4±1.5 | 39.1±2.1 | 39.0±1.4 | 38.3±1.7 |
| 3 | 29.1±1.2 | 40.4±1.4 | 39.1±2.3 | 39.9±1.2 | 38.1±1.6a |
| 4 | 29.1±0.9 | 40.9±1.0 | 38.9±2.4a | 40.2±1.1 | 38.8±1.8a |
| Time (min) | CON group (n = 6) | KKAy group (n = 9) | TFK group (n = 9) | TFK+CC group (n = 9) | AICAR group (n = 9) |
|---|---|---|---|---|---|
| 0 | 5.6±0.5 | 14.8±1.6 | 10.7±2.6a | 13.5±4.1 | 10.5±3.5a |
| 30 | 16.7±5.3 | 30.1±2.9 | 21.3±2.5b | 28.1±2.0 | 21.8±2.5b |
| 60 | 11.3±2.8 | 23.7±3.4 | 17.9±2.9c | 21.7±2.8 | 18.3±2.7d |
| 120 | 6.7±0.9 | 20.7±3.2 | 15.0±3.8c | 19.5±2.0 | 15.2±3.1d |
Table 2 Effect of TFK on the OGTT of KKAy mice (mmol/L , ? ? ± ? s)
| Time (min) | CON group (n = 6) | KKAy group (n = 9) | TFK group (n = 9) | TFK+CC group (n = 9) | AICAR group (n = 9) |
|---|---|---|---|---|---|
| 0 | 5.6±0.5 | 14.8±1.6 | 10.7±2.6a | 13.5±4.1 | 10.5±3.5a |
| 30 | 16.7±5.3 | 30.1±2.9 | 21.3±2.5b | 28.1±2.0 | 21.8±2.5b |
| 60 | 11.3±2.8 | 23.7±3.4 | 17.9±2.9c | 21.7±2.8 | 18.3±2.7d |
| 120 | 6.7±0.9 | 20.7±3.2 | 15.0±3.8c | 19.5±2.0 | 15.2±3.1d |
| Index | CON group (n = 6) | KKAy group (n = 9) | TFK group (n = 9) | TFK+CC group (n = 9) | AICAR group (n = 9) |
|---|---|---|---|---|---|
| FBG (mmol/L) | 6.0±1.2 | 14.9±2.9 | 11.0±2.9a | 14.1±3.4 | 10.8±2.3a |
| GSP (mmol/L) | 2.0±0.4 | 5.2±0.6 | 3.8±0.8b | 4.7±0.2 | 3.5±0.8c |
| FINS (μIU/ml) | 7.2±2.8 | 16.5±3.4 | 11.4±2.8a | 15.9±4.3 | 12.0±2.8a |
| HOMA-IR | 1.9±0.7 | 10.8±2.6 | 5.6±1.9b | 10.0±3.8 | 5.8±1.9d |
Table 3 Effect of TFK on the glycometabolism of KKAy mice ($\bar{x}±s$)
| Index | CON group (n = 6) | KKAy group (n = 9) | TFK group (n = 9) | TFK+CC group (n = 9) | AICAR group (n = 9) |
|---|---|---|---|---|---|
| FBG (mmol/L) | 6.0±1.2 | 14.9±2.9 | 11.0±2.9a | 14.1±3.4 | 10.8±2.3a |
| GSP (mmol/L) | 2.0±0.4 | 5.2±0.6 | 3.8±0.8b | 4.7±0.2 | 3.5±0.8c |
| FINS (μIU/ml) | 7.2±2.8 | 16.5±3.4 | 11.4±2.8a | 15.9±4.3 | 12.0±2.8a |
| HOMA-IR | 1.9±0.7 | 10.8±2.6 | 5.6±1.9b | 10.0±3.8 | 5.8±1.9d |
| Index | CON group (n = 6) | KKAy group (n = 9) | TFK group (n = 9) | TFK+CC group (n = 9) | AICAR group (n = 9) |
|---|---|---|---|---|---|
| TC | 3.29±0.47 | 5.22±0.88 | 3.71±1.24a | 5.03±1.19 | 3.79±1.15a |
| TG | 0.87±0.26 | 2.68±0.60 | 1.78±0.64a | 2.41±0.86 | 1.79±0.63a |
| HDL-C | 2.61±0.68 | 1.73±0.61 | 2.10±0.91a | 1.92±0.79 | 2.27±0.87a |
| LDL-C | 0.28±0.05 | 0.89±0.18 | 0.58±0.16b | 0.83±0.19 | 0.61±0.23a |
Table 4 Effect of TFK on the lipometabolism of KKAy mice (mmol/L, $\bar{x}±s$)
| Index | CON group (n = 6) | KKAy group (n = 9) | TFK group (n = 9) | TFK+CC group (n = 9) | AICAR group (n = 9) |
|---|---|---|---|---|---|
| TC | 3.29±0.47 | 5.22±0.88 | 3.71±1.24a | 5.03±1.19 | 3.79±1.15a |
| TG | 0.87±0.26 | 2.68±0.60 | 1.78±0.64a | 2.41±0.86 | 1.79±0.63a |
| HDL-C | 2.61±0.68 | 1.73±0.61 | 2.10±0.91a | 1.92±0.79 | 2.27±0.87a |
| LDL-C | 0.28±0.05 | 0.89±0.18 | 0.58±0.16b | 0.83±0.19 | 0.61±0.23a |
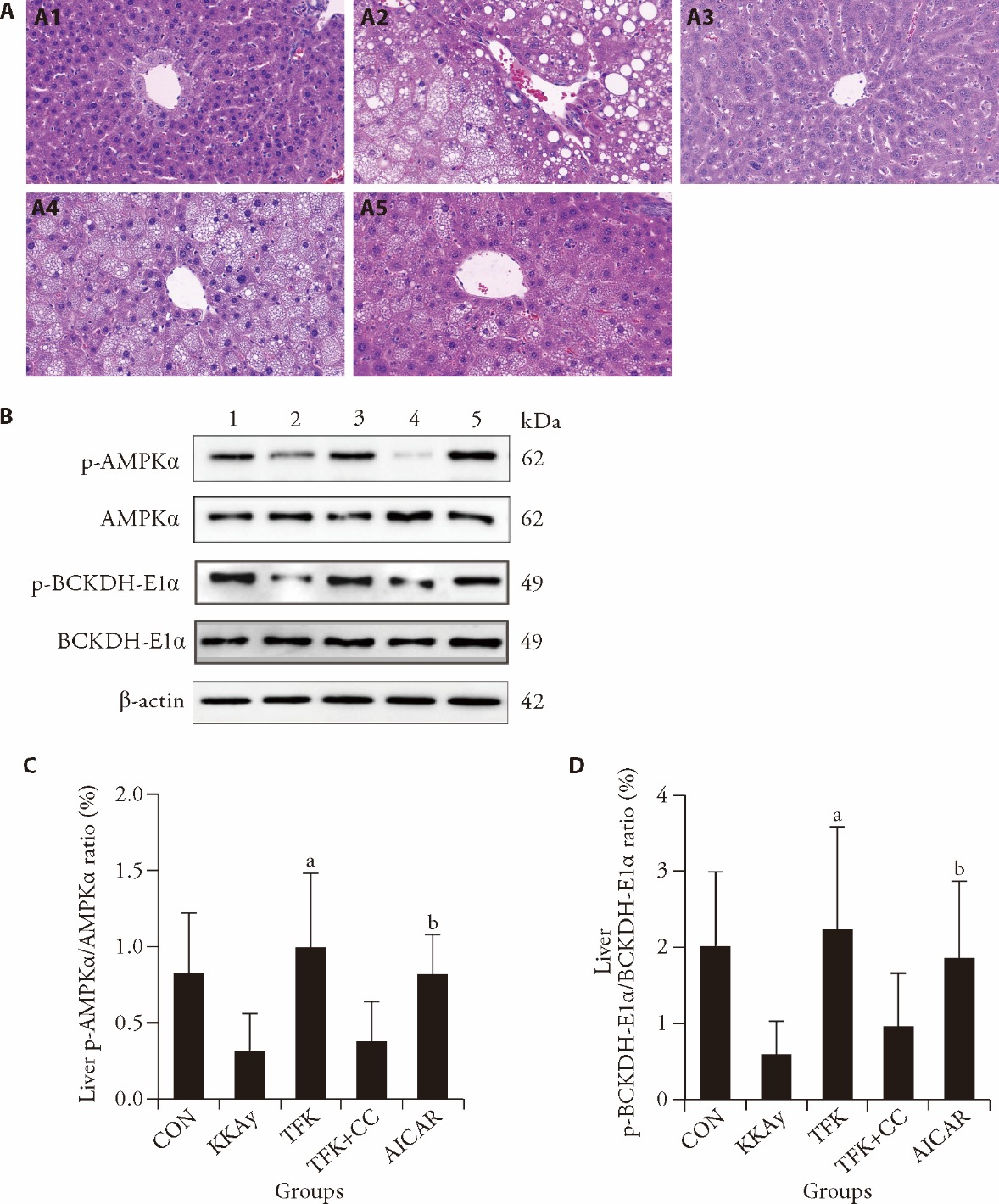
Figure 4 Effects of TFK on the AMPK signaling pathway and liver histological analysis (HE, × 400) related indicators A: liver tissue of HE staining and original magnifications was × 400 magnification: A1: liver tissue of CON group; A2: liver tissue of KKAy group; A3: liver tissue of TKF group; A4: liver tissue of TFK + CC group; A5: liver tissue of AICAR group; B: representative results of AMPK and BCKDH protein phosphorylation levels in each group: 1: CON group; 2: KKAy group; 3: TKF group; 4: TFK + CC group; 5: AICAR group; C: quantitative results of AMPK protein phosphorylation levels in each group; D: quantitative results of BCKDH protein phosphorylation levels in each group. CON group and KKAy group: treated with physiological saline by gavage and PBS via intraperitoneal injection for four weeks; TFK group: treated with TFK at a dose of 3.2 g·kg-1·d-1 by gavage and PBS via intraperitoneal injection for four weeks; TFK + CC group: treated with TFK at a dose of 3.2 g·kg-1·d-1 by gavage and CC at a dose of 5 mg·kg-1·d-1 via intraperitoneal injection for four weeks; AICAR group: treated with physiological saline by gavage and AICAR at a dose of 0.5 g·kg-1·d-1 via intraperitoneal injection for four weeks. AMPK: adenosine 5-monophosphate-activated protein kinase; BCKDH: branched-chain α-ketoacid dehydrogenase; TFK: Tangfukang formula; HE: hematoxylin-eosin; CON: control; CC: compound c; AICAR: 5-Aminoimidazole-4-carboxamide ribonucleoside; PBS: phosphate buffer saline. The normal probability plot was used to assess the distribution of the data. One-way analysis of variance was used to compare multiple conditions statistically. Values are expressed as mean ± standard deviation, n = 6 for CON group and n = 9 for each other group. aP < 0.01 vs KKAy group, bP < 0.05 vs KKAy group.
| 1. | Heald AH, Stedman M, Davies M, et al. Estimating life years lost to diabetes: outcomes from analysis of National Diabetes Audit and Office of National Statistics data. Cardiovasc Endocrinol Metab 2020; 9: 183-5. |
| 2. | Sun H, Saeedi P, Karuranga S, et al. IDF diabetes atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract 2022; 183: 109-19. |
| 3. | Pan L, Li Z, Wang Y, Zhang B, Liu G, Liu J. Network pharmacology and metabolomics study on the intervention of Traditional Chinese Medicine Huanglian decoction in rats with type 2 diabetes mellitus. J Ethnopharmacol 2020; 258: 112842. |
| 4. | Wang Y, Gao HJ, Yan K, Wang W, Feng XZ. Effect of Tangfukang formula on the intestinal flora of KK-Ay mice and its therapeutic effect. Beijing Zhong Yi Yao 2022; 41: 230-5. |
| 5. | Li S, Zhang B. Traditional Chinese Medicine network pharmacology: theory, methodology and application. Chin J Nat Med 2013; 11: 110-20. |
| 6. | Wang X, Wang ZY, Zheng JH, Li S. TCM network pharmacology: a new trend towards combining computational, experimental and clinical approaches. Chin J Nat Med 2021; 19: 1-11. |
| 7. | Li H, Zhao L, Zhang B, et al. A network pharmacology approach to determine active compounds and action mechanisms of Ge-Gen-Qin-Lian decoction for treatment of type 2 diabetes. Evid Based Complement Alternat Med 2014; 2014: 495840. |
| 8. | Chen X, Yang Z, Du L, Guan Y, Li Y, Liu C. Study on the active ingredients and mechanism of action of Jiaotai Pill in the treatment of type 2 diabetes based on network pharmacology: a review. Medicine (Baltimore) 2023; 102: e33317. |
| 9. | Long F, Zhang Z, Luo C, Lei X, Guo J, An L. Exploring the molecular mechanism of Ling-Gui-Zhu-Gan decoction for the treatment of type 2 diabetes mellitus based on network pharmacology and molecular docking: a review. Medicine (Baltimore) 2023; 102: e33210. |
| 10. | Li LZ, Zhou C, Wang P, et al. Molecular mechanism of the effect of Gegen Qinlian decoction on COVID-19 comorbid with diabetes mellitus based on network pharmacology and molecular docking: a review. Medicine (Baltimore) 2023; 102: e34683. |
| 11. | Lin C, Wu F, Zheng T, Wang X, Chen Y, Wu X. Kaempferol attenuates retinal ganglion cell death by suppressing NLRP1/NLRP3 inflammasomes and caspase-8 via JNK and NF-κB pathways in acute glaucoma. Eye (Lond) 2019; 33: 777-84. |
| 12. | Ru J, Li P, Wang J, et al. TCMSP: a database of systems pharmacology for drug discovery from herbal medicines. J Cheminform 2014; 6: 13. |
| 13. | Davis AP, Grondin CJ, Johnson RJ, et al. The comparative toxicogenomics database: update 2019. Nucleic Acids Res 2019; 47: 948-54. |
| 14. |
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985; 28: 412-9.
DOI PMID |
| 15. |
Antonioli L, Blandizzi C, Csóka B, Pacher P, Haskó G. Adenosine signalling in diabetes mellitus--pathophysiology and therapeutic considerations. Nat Rev Endocrinol 2015; 11: 228-41.
DOI PMID |
| 16. | Sanni O, Terre'Blanche G. Therapeutic potentials of agonist and antagonist of adenosine receptors in type 2 diabetes. Rev Endocr Metab Disord 2021; 22: 1073-90. |
| 17. | Khan S, Kamal MA. Wogonin alleviates hyperglycemia through increased glucose entry into cells via AKT/GLUT4 pathway. Curr Pharm Des 2019; 25: 2602-6. |
| 18. | Khan S. Wogonin and alleviation of hyperglycemia via inhibition of DAG mediated PKC expression. A brief insight. Protein Pept Lett 2021; 28: 1365-71. |
| 19. | Weng L, Zhang F, Wang R, Ma W, Song Y. A review on protective role of genistein against oxidative stress in diabetes and related complications. Chem Biol Interact 2019; 310: 108665. |
| 20. | Guo C, Huang Q, Wang Y, et al. Therapeutic application of natural products: NAD+ metabolism as potential target. Phytomedicine 2023; 114: 154768. |
| 21. |
Zhao J, Liu H, Hong Z, et al. Tanshinone I specifically suppresses NLRP3 inflammasome activation by disrupting the association of NLRP3 and ASC. Mol Med 2023; 29: 84.
DOI PMID |
| 22. | Chen X, Qian L, Wang B, et al. Synergistic hypoglycemic effects of pumpkin polysaccharides and puerarin on type II diabetes mellitus mice. Molecules 2019; 24: 955. |
| 23. | Yi H, Peng H, Wu X, et al. The therapeutic effects and mechanisms of quercetin on metabolic diseases: pharmacological data and clinical evidence. Oxid Med Cell Longev 2021; 2021: 6678662. |
| 24. | Li D, Jiang C, Mei G, et al. Quercetin alleviates ferroptosis of pancreatic β cells in type 2 diabetes. Nutrients 2020; 12: 2954. |
| 25. | Alam W, Rocca C, Khan H, et al. Current status and future perspectives on therapeutic potential of apigenin: focus on metabolic-syndrome-dependent organ dysfunction. Antioxidants (Basel) 2021; 10: 1643. |
| 26. | Subiabre M, Villalobos-Labra R, Silva L, Fuentes G, Toledo F, Sobrevia L. Role of insulin, adenosine, and adipokine receptors in the foetoplacental vascular dysfunction in gestational diabetes mellitus. Biochim Biophys Acta Mol Basis Dis 2020; 1866: 165370. |
| 27. | Liu XQ, Jiang L, Li YY, et al. Wogonin protects glomerular podocytes by targeting Bcl-2-mediated autophagy and apoptosis in diabetic kidney disease. Acta Pharmacol Sin 2022; 43: 96-110. |
| 28. | Jiang T, Dong Y, Zhu W, et al. Underlying mechanisms and molecular targets of genistein in the management of type 2 diabetes mellitus and related complications. Crit Rev Food Sci Nutr 2023: 64: 11543-55. |
| 29. | Liu H, Wang Q, Shi G, et al. Emodin ameliorates renal damage and podocyte injury in a rat model of diabetic nephropathy via regulating AMPK/mTOR-mediated autophagy signaling pathway. Diabetes Metab Syndr Obes 2021; 14: 1253-66. |
| 30. | Bai YL, Han LL, Qian JH, Wang HZ. Molecular mechanism of puerarin against diabetes and its complications. Front Pharmacol 2022; 12: 780419. |
| 31. | Dhanya R. Quercetin for managing type 2 diabetes and its complications, an insight into multitarget therapy. Biomed Pharmacother 2022; 146: 112560. |
| 32. |
Zhou Q, Cheng KW, Gong J, Li ETS, Wang M. Apigenin and its methylglyoxal-adduct inhibit advanced glycation end products-induced oxidative stress and inflammation in endothelial cells. Biochem Pharmacol 2019; 166: 231-41.
DOI PMID |
| 33. |
Huang X, Liu G, Guo J, Su Z. The PI3K/AKT pathway in obesity and type 2 diabetes. Int J Biol Sci 2018; 14: 1483-96.
DOI PMID |
| 34. | He X, Gao F, Hou J, et al. Metformin inhibits MAPK signaling and rescues pancreatic aquaporin 7 expression to induce insulin secretion in type 2 diabetes mellitus. J Biol Chem 2021; 297: 101002. |
| 35. | Marchelek-Mysliwiec M, Nalewajska M, Turoń-Skrzypińska A, et al. The role of forkhead box O in pathogenesis and therapy of diabetes mellitus. Int J Mol Sci 2022; 23: 11611. |
| 36. | Lee BW, Chae HY, Kwon SJ, Park SY, Ihm J, Ihm SH. RAGE ligands induce apoptotic cell death of pancreatic β-cells via oxidative stress. Int J Mol Med 2010; 26: 813-8. |
| 37. | Zhu Y, Shu T, Lin Y, et al. Inhibition of the receptor for advanced glycation endproducts (RAGE) protects pancreatic β-cells. Biochem Biophys Res Commun 2011; 404: 159-65. |
| 38. |
Ohshima K, Mogi M, Jing F, et al. Roles of interleukin 17 in angiotensin II type 1 receptor-mediated insulin resistance. Hypertension 2012; 59: 493-9.
DOI PMID |
| 39. | Bako HY, Ibrahim MA, Isah MS, Ibrahim S. Inhibition of JAK-STAT and NF-κB signalling systems could be a novel therapeutic target against insulin resistance and type 2 diabetes. Life Sci 2019; 239: 117045. |
| 40. | Yehualashet AS. Toll-like receptors as a potential drug target for diabetes mellitus and diabetes-associated complications. Diabetes Metab Syndr Obes 2020; 13: 4763-77. |
| 41. | Botusan IR, Sunkari VG, Savu O, et al. Stabilization of HIF-1alpha is critical to improve wound healing in diabetic mice. Proc Natl Acad Sci U S A 2008; 105: 19426-31. |
| 42. | Bento CF, Fernandes R, Ramalho J, et al. The chaperone-dependent ubiquitin ligase CHIP targets HIF-1α for degradation in the presence of methylglyoxal. PLoS One 2010; 5: e15062. |
| 43. |
Katavetin P, Miyata T, Inagi R, et al. High glucose blunts vascular endothelial growth factor response to hypoxia via the oxidative stress-regulated hypoxia-inducible factor/hypoxia-responsible element pathway. J Am Soc Nephrol 2006; 17: 1405-13.
DOI PMID |
| 44. | Juszczak F, Caron N, Mathew AV, Declèves AE. Critical role for AMPK in metabolic disease-Induced chronic kidney disease. Int J Mol Sci 2020; 21: 7994. |
| 45. |
Ahmed N, Furth AJ. Failure of common glycation assays to detect glycation by fructose. Clin Chem 1992; 38: 1301-3.
PMID |
| 46. | Selvin E, Rawlings AM, Grams M, et al. Fructosamine and glycated albumin for risk stratification and prediction of incident diabetes and microvascular complications: a prospective cohort analysis of the Atherosclerosis Risk in Communities (ARIC) study. Lancet Diabetes Endocrinol 2014; 2: 279-288. |
| 47. | Lann D, LeRoith D. Insulin resistance as the underlying cause for the metabolic syndrome. Med Clin North Am 2007; 91: 1063-77. |
| 48. |
Antuna-Puente B, Disse E, Rabasa-Lhoret R, Laville M, Capeau J, Bastard JP. How can we measure insulin sensitivity/resistance? Diabetes Metab 2011; 37: 179-88.
DOI PMID |
| 49. |
Mojiminiyi OA, Abdella NA. Effect of homeostasis model assessment computational method on the definition and associations of insulin resistance. Clin Chem Lab Med 2010; 48: 1629-34.
DOI PMID |
| 50. |
Stoppa GR, Cesquini M, Roman EA, et al. Intracerebroventricular injection of citrate inhibits hypothalamic AMPK and modulates feeding behavior and peripheral insulin signaling. J Endocrinol 2008; 198: 157-68.
DOI PMID |
| 51. |
Haikala HM, Anttila JM, Klefström J. MYC and AMPK-save energy or die! Front cell dev biol 2017; 5: 38.
DOI PMID |
| 52. | Li Q, Wu J, Huang J, et al. Paeoniflorin ameliorates skeletal muscle atrophy in chronic kidney disease via AMPK/SIRT1/PGC-1α-mediated oxidative stress and mitochondrial dysfunction. Front Pharmacol 2022; 13: 859723. |
| 53. | Liu T, Zhang N, Kong L, et al. Paeoniflorin alleviates liver injury in hypercholesterolemic rats through the ROCK/AMPK pathway. Front Pharmacol 2022; 13: 968717. |
| 54. | Han X, Han B, Zhao Y, et al. Rosmarinic acid attenuates rotenone-induced neurotoxicity in SH-SY5Y parkinson's disease cell model through abl inhibition. Nutrients 2022; 14: 3508. |
| 55. |
Shah SH, Crosslin DR, Haynes CS, et al. Branched-chain amino acid levels are associated with improvement in insulin resistance with weight loss. Diabetologia 2012; 55: 321-30.
DOI PMID |
| 56. |
Würtz P, Soininen P, Kangas AJ, et al. Branched-chain and aromatic amino acids are predictors of insulin resistance in young adults. Diabetes Care 2013; 36: 648-55.
DOI PMID |
| 57. | Xu F, Tavintharan S, Sum CF, Woon K, Lim SC, Ong CN. Metabolic signature shift in type 2 diabetes mellitus revealed by mass spectrometry-based metabolomics. J Clin Endocrinol Metab 2013; 98: E1060-5. |
| 58. | Harris RA, Joshi M, Jeoung NH. Mechanisms responsible for regulation of branched-chain amino acid catabolism. Biochem Biophys Res Commun 2004; 313: 391-6. |
| 59. |
Adeva MM, Calviño J, Souto G, Donapetry C. Insulin resistance and the metabolism of branched-chain amino acids in humans. Amino Acids 2012; 43: 171-81.
DOI PMID |
| 60. |
Lian K, Du C, Liu Y, et al. Impaired adiponectin signaling contributes to disturbed catabolism of branched-chain amino acids in diabetic mice. Diabetes 2015; 64: 49-59.
DOI PMID |
| 61. | Shamshoum H, Vlavcheski F, MacPherson REK, Tsiani E. Rosemary extract activates AMPK, inhibits mTOR and attenuates the high glucose and high insulin-induced muscle cell insulin resistance. Appl Physiol Nutr Metab 2021; 46: 819-27. |
| 62. | Guo S, Wang G, Yang Z. Ligustilide alleviates the insulin resistance, lipid accumulation, and pathological injury with elevated phosphorylated AMPK level in rats with diabetes mellitus. J Recept Signal Transduct Res 2021; 41: 85-92. |
| 63. | Xie T, So WY, Li XY, Leung PS. Fibroblast growth factor 21 protects against lipotoxicity-induced pancreatic β-cell dysfunction via regulation of AMPK signaling and lipid metabolism. Clin Sci (Lond) 2019; 133: 2029-44. |
| 64. | Wang Y, Rijal B, Xu M, et al. Renal denervation improves vascular endothelial dysfunction by inducing autophagy via AMPK/mTOR signaling activation in a rat model of type 2 diabetes mellitus with insulin resistance. Acta Diabetol 2020; 57: 1227-43. |
| 65. | Jung TW, Lee SH, Kim HC, et al. METRNL attenuates lipid-induced inflammation and insulin resistance via AMPK or PPARδ-dependent pathways in skeletal muscle of mice. Exp Mol Med 2018; 50: 1-11. |
| 66. | Melnik BC. Milk exosomal miRNAs: potential drivers of AMPK-to-mTORC1 switching in β-cell de-differentiation of type 2 diabetes mellitus. Nutr Metab (Lond) 2019; 16: 85. |
| 67. |
Jaafar R, Tran S, Shah AN, et al. MTORC1 to AMPK switching underlies β-cell metabolic plasticity during maturation and diabetes. J Clin Invest 2019; 129: 4124-37.
DOI PMID |
| 68. | Li S. Network pharmacology evaluation method guidance-draft. Shi Jie Zhong Yi Yao 2021; 7: 165-6 + 146-54. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||

