Journal of Traditional Chinese Medicine ›› 2024, Vol. 44 ›› Issue (5): 896-905.DOI: 10.19852/j.cnki.jtcm.20240806.001
Previous Articles Next Articles
Actinidia chinensis polysaccharide interferes with the epithelial-mesenchymal transition of gastric cancer by regulating the nuclear transcription factor-κB pathway to inhibit invasion and metastasis
ZHANG Guangshun1,2, XU Xiaonan3,4,5, XU Chuyun6, LIAO Guanghui1,2, XU Hao3,4,5, LOU Zhaohuan1,2( ), ZHANG Guangji3,4,5(
), ZHANG Guangji3,4,5( )
)
- 1 School of Pharmaceutical Sciences, Zhejiang Chinese Medical University, Hangzhou 310053, China
2 Key Labortary of Blood-stasis-toxin Syndrome of Zhejiang Province, Hangzhou 310053, China
3 School of Basic Medical Sciences, Zhejiang Chinese Medical University, Hangzhou 310053, China
4 Key Labortary of Blood-stasis-toxin Syndrome of Zhejiang Province, Hangzhou 310053, China
5 Traditional Chinese Medicine "Preventing Disease" Wisdom Health Project Research Center of Zhejiang, Hangzhou 310053, China
6 the First Affiliated Hospital of Zhejiang Chinese Medical University, Hangzhou 310053, China
-
Received:2023-04-21Accepted:2023-08-29Online:2024-10-15Published:2024-08-06 -
Contact:Prof. ZHANG Guangji, School of Basic Medical Sciences, Zhejiang Chinese Medical University, Hangzhou 310053, China. zgj@zcmu.edu.cn Telephone: +86-13335812880; +86-13957152511; Prof. LOU Zhaohuan, School of Pharmaceutical Sciences, Zhejiang Chinese Medical University, Hangzhou 310053, China. zhaohuanlou@zcmu.edu.cn -
Supported by:Innovative Research and Application based on the Core Etiology and Pathogenesis of Different Diseases and the Same Treatment Plan based on "Blood Stasis, Toxin and Depression"(2019YFC1708700);Innovative Research and Application based on the Core Etiology and Pathogenesis of Different Diseases and the Same Treatment Plan based on "Blood Stasis, Toxin and Depression"(2019YFC1708701);Biological Basic Research on Gastric Cancer-Carcinoma Transformation and Intervention by Removing Blood Stasis and Detoxification Based on the Pathogenesis Theory of Blood Stasis and Toxin Interaction(82030119);Study on the Mechanism of Tanshinone Combined with Arsenic Trioxide Stasis and Poison to Regulate Macrophage Polarization and Improve the Inflammatory Microenvironment of Liver Cancer(81874455);Research on Innovative Traditional Chinese Medicine Drugs-preclinical Research on Anti-lung Cancer of Diterpenoid Tanshinone(2019C03072);Study on the Diagnosis and Treatment Program of Damp-heat Accumulation Type Colorectal Cancer Based on Correlation Analysis of Intestinal Microbiota- Traditional Chinese Medicine Syndrome Type(2020ZX005);Research on Quantitative Characterization of Preparation Process and Quality Standard Control Specification of Traditional Chinese Medicine Paste (Gao Zi Ji)(2019ZZ006);Study on the Molecular Mechanism of Actinidia Chinensis Polysaccharide Interfering with Epithelial Mesenchymal Transformation in Gastric Cancer by Inhibiting Nuclear Factor-κB Pathway(81273904);Study on the Mechanism of the Effective Components of Tengli Root Regulating Tumor-associated Fibroblasts to Improve the Tumor Microenvironment and Prevent the Metastasis of Gastric Cancer(2022JKZKTS16)
Cite this article
ZHANG Guangshun, XU Xiaonan, XU Chuyun, LIAO Guanghui, XU Hao, LOU Zhaohuan, ZHANG Guangji. Actinidia chinensis polysaccharide interferes with the epithelial-mesenchymal transition of gastric cancer by regulating the nuclear transcription factor-κB pathway to inhibit invasion and metastasis[J]. Journal of Traditional Chinese Medicine, 2024, 44(5): 896-905.
share this article

Figure 1 ACPS improved tumor inflammatory response A: histopathology of gastric cancer transplanted tumor; A1: representative picture of the model group; A2: 5-FU group; A3: ACPS-L group; A4: ACPS-H group; HE staining, × 200; B: serum levels of IL-6 in each group; C: serum levels of TGF-β in each group. 5-FU: 5-fluorouracil; ACPS: actinidia chinensis polysaccharide; ACPS-L: actinidia chinensis polysaccharide low-dose group (50 mg·kg-1·d-1); ACPS-H: actinidia chinensis polysaccharide high-dose group (100 mg·kg-1·d-1). The drug was given intraperitoneally once a day for 28 d. IL-6: interleukin-6; TGF-β: transforming growth factor beta. The statistical method used in this figure is one-way analysis of variance. Results are presented as mean ± standard deviation (n = 6). aP<0.01 model group vs normal group; bP<0.01 vs model group; cP<0.05 model group vs normal group.

Figure 2 Scratch experiment was used to evaluate the effect of ACPS on the migration of BGC-823 cells A: migration at time points of 0, 6, 24, 48 h in scratch experiment; A1: control group at 0 h post-administration; A2: control group at 6 h post-administration; A3: control group at 24 h post-administration; A4: control group at 48 h post-administration; A5: EGF group at 0 h post-administration (100 ng/mL); A6: EGF group at 6 h post-administration; A7: EGF group at 24 h post-administration; A8: EGF group at 48 h post-administration; A9: ACPS group at 0 h post-administration (0.1 mg/mL); A10: ACPS group at 6 h post-administration; A11: ACPS group at 24 h post-administration; A12: ACPS group at 48 h post-administration; A13: EGF (100 ng/mL) + ACPS (0.1 mg/mL) group at 0 h post-administration; A14: EGF + ACPS group at 6 h post-administration; A15: EGF + ACPS group at 24 h post-administration; A16: EGF + ACPS group at 48 h post-administration; B: mobility of each group was compared at 48 h post-administration. EGF: epidermal growth factor; ACPS: Actinidia chinensis polysaccharide. The scratch experiment photos were collected under a 10 μm mirror. The statistical method used in this figure is one-way analysis of variance. Results are presented as mean ± standard deviation (n = 3). aP<0.01 vs control group.

Figure 3 Immunohistochemistry was used to detect the expression of MMP9, N-cadherin and p-NF-κB p65 A: representative picture of MMP9 protein expression in each group by immunohistochemistry (DAB staining, × 200); A1: model group; A2: 5-FU group; A3: ACPS-L dose group; A4: ACPS-H dose group; B: representative picture of N-cadherin protein expression in each group by immunohistochemistry (DAB staining, × 200); B1: model group; B2: 5-FU group; B3: ACPS-L dose group; B4: ACPS-H dose group; C: representative picture of p-NF-κB p65 protein expression in each group by immunohistochemistry (DAB staining, × 200); C1: model group; C2: 5-FU group; C3: ACPS-L dose group; A4: ACPS-H dose group; D: mean OD values of MMP9 in each group were compared by immunohistochemistry; E: mean OD values of N-cadherin in each group were compared by immunohistochemistry; F: mean OD values of p-NF-κB p65 in each group were compared by immunohistochemistry. 5-FU: 5-fluorouracil; ACPS: actinidia chinensis polysaccharide; ACPS-L: actinidia chinensis polysaccharide low-dose group (50 mg·kg-1·d-1); ACPS-H: actinidia chinensis polysaccharide high-dose group (100 mg·kg-1·d-1). The drug was given intraperitoneally once a day for 28 d. MMP-9: matrix metallopeptidase 9; N-cadherin: neural cadherin; P-NFκB p65: phosphorylated nuclear factor kappa-light-chain-enhancer of activated B cells p65 subunit. The statistical method used in this figure is one-way analysis of variance. Results are presented as mean ± standard deviation (n = 6). aP<0.01 vs model group.
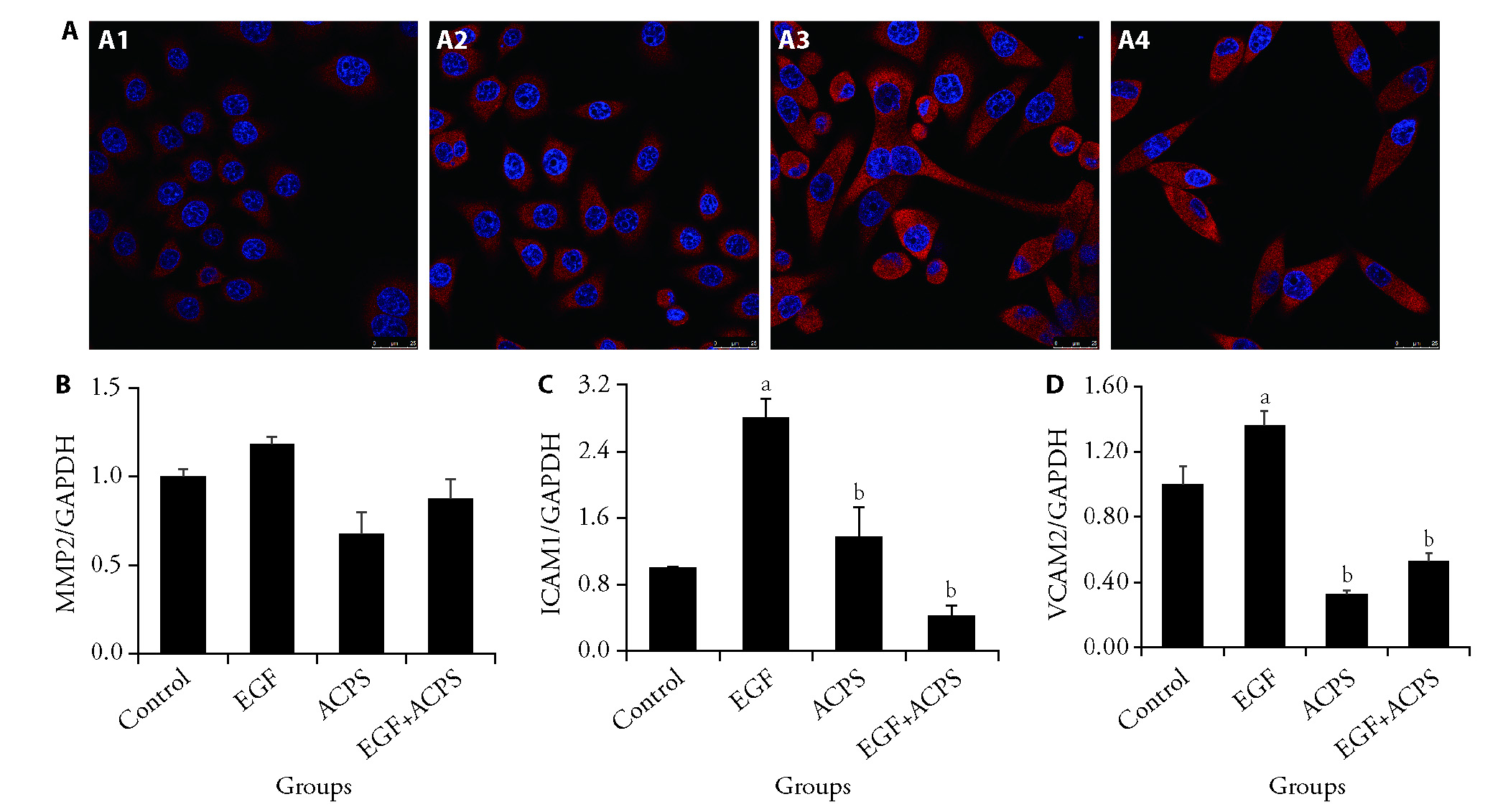
Figure 4 ACPS promoted p65-NF-κB nuclear transfer in BGC-823 cells and reduced the mRNA expression of MMP2, ICAM2, and VCAM1 A: expression of p65-NFκB was detected using confocal technology. A1: control group; A2: EGF group (100 ng/mL); A3: ACPS group (0.1 mg/mL); A4: EGF + ACPS group; Blue fluorescence represents nuclei stained with DAPI; The red fluorescence represents p65-NFκB; B: qPCR was used to detect MMP2 genes in each group standardized with GAPDH; C: qPCR was used to detect ICAM2 genes in each group standardized with GAPDH; D: qPCR was used to detect VCAM1 genes in each group standardized with GAPDH. Drug intervention for 48 h. p65-NFκB: p65 subunit of nuclear factor kappa-light-chain-enhancer of activated B cells; DAPI: 6-diamidino-2-phenylindole; qPCR: quantitative Polymerase chain reaction; MMP2: matrix metalloproteinase-2; ICAM2: intercellular adhesion molecule 2; VCAM1: vascular cell adhesion molecule 1; GAPDH: glyceraldehyde-3-phosphate dehydrogenase; EGF: epidermal growth factor; ACPS: actinidia chinensis polysaccharide. The scale bar of the above image is 25 μm. The statistical method used in this figure is one-way analysis of variance. Results are presented as mean ± standard deviation (n = 3). aP<0.01, EGF group vs Control group; bP<0.01, vs EGF group.
| 1. | Zang X, Jiang J, Gu J, et al. Circular RNA EIF4G 3 suppresses gastric cancer progression through inhibition of β-catenin by promoting δ-catenin ubiquitin degradation and upregulating SIK1. Mol Cancer 2022; 21: 141. |
| 2. |
Zhang Y, Liu C, Zhou L. Prognosis of gastric adenocarcinoma associated with girdin, Akt, and cortactin. Ann Saudi Med 2022; 42: 181-90.
DOI PMID |
| 3. | Zhou Y, Li X, Luo W, et al. Allicin in digestive system cancer: from biological effects to clinical treatment. Front Pharmacol 2022; 13: 903259. |
| 4. | Gao R, Lyu Y. Characterizing the Antitumor Effect of coptis chinensis and mume fructus against colorectal cancer based on pharmacological analysis. Evid Based Complement Alternat Med 2022; 2022: 9061752. |
| 5. | Cangiano LR, Henry DD, Ciriaco FM, et al. Triterpenes from Olea europaea modulate in vitro ruminal fermentation. Transl Anim Sci 2022; 6: txac056. |
| 6. | She Y, Zhao X, Wu P, et al. COL8A 1 Predicts the clinical prognosis of gastric cancer and is related to epithelial-mesenchymal transition. Biomed Res Int 2022; 2022: 7567447. |
| 7. |
Fu C, Wang J, Pallikkuth S, et al. EWI2 prevents EGFR from clustering and endocytosis to reduce tumor cell movement and proliferation. Cell Mol Life Sci 2022; 79: 389.
DOI PMID |
| 8. | Song J, Wei R, Huo S, et al. Metastasis related epithelial-mesenchymal transition signature predicts prognosis and response to immunotherapy in gastric cancer. Front Immunol 2022; 13: 920512. |
| 9. |
Sun Y, Du R, Shang Y, et al. Rho GTPase-activating protein 35 suppresses gastric cancer metastasis by regulating cytoskeleton reorganization and epithelial-to-mesenchymal transition. Bioengineered 2022; 13: 14605-15.
DOI PMID |
| 10. | Westrick RJ, Røjkjaer LP, Yang AY, et al. Deficiency of plasminogen activator inhibitor-2 results in accelerated tumor growth. J Thromb Haemost 2020; 18: 2968-75. |
| 11. | Wang Y, Liu F, Chen L, et al. Neutrophil extracellular traps (NETs) promote non-small cell lung cancer metastasis by suppressing lncRNA mir503HG to activate the NF-κB/NLRP 3 inflammasome pathway. Front Immunol 2022; 13: 867516. |
| 12. |
Mirzaei S, Saghari S, Bassiri F, et al. NF-κB as a regulator of cancer metastasis and therapy response: a focus on epithelial-mesenchymal transition. J Cell Physiol 2022; 237: 2770-95.
DOI PMID |
| 13. |
Huber MA, Azoitei N, Baumann B, et al. NF-kappaB is essential for epithelial-mesenchymal transition and metastasis in a model of breast cancer progression. J Clin Invest 2004; 114: 569-81.
DOI PMID |
| 14. |
Gao Z, Deng G, Li Y, et al. Actinidia chinensis Planch prevents proliferation and migration of gastric cancer associated with apoptosis, ferroptosis activation and mesenchymal phenotype suppression. Biomed Pharmacother 2020; 126: 110092.
DOI PMID |
| 15. |
Zeng Q, Nie X, Li L, et al. Salidroside promotes sensitization to doxorubicin in human cancer cells by affecting the PI3K/Akt/HIF signal pathway and inhibiting the expression of tumor-resistance-related proteins. J Nat Prod 2022; 85: 196-204.
DOI PMID |
| 16. | Lu X, Qian CN, Mu YG, et al. Serum CCL2 and serum TNF-α--two new biomarkers predict bone invasion, post-treatment distant metastasis and poor overall survival in nasopharyngeal carcinoma. Eur J Cancer 2011; 47: 339-46. |
| 17. | Xie F, Zhou X, Li H, et al. USP8 promotes cancer progression and extracellular vesicle-mediated CD8+ T cell exhaustion by deubi-quitinating the TGF-β receptor TβRII. EMBO J 2022; 41: e108791. |
| 18. | Jiang H, Ma P, Duan Z, et al. Ginsenoside Rh4 suppresses metastasis of gastric cancer via SIX1-dependent TGF-β/Smad2/3 signaling pathway. Nutrients 2022; 14: 1564. |
| 19. |
Cheng H, Chen L, Fang Z, et al. STIM2 promotes the invasion and metastasis of breast cancer cells through the NFAT1/TGF-β1 pathway. Cell Mol Biol (Noisy-le-grand) 2022; 67: 55-61.
DOI PMID |
| 20. |
Bailly M. Connecting cell adhesion to the actin polymerization machinery: vinculin as the missing link? Trends Cell Biol 2003; 13: 163-5.
PMID |
| 21. |
Chen H, Cohen DM, Choudhury DM, et al. Spatial distribution and functional significance of activated vinculin in living cells. J Cell Biol 2005; 169: 459-70.
DOI PMID |
| 22. | Chan LP, Tseng YP, Wang HC, et al. Growth regulated oncogene-α contribute to EMT/MMPs pathway by binding its receptors in head and neck squamous cell carcinoma. Life sciences 2022; 306: 120791. |
| 23. |
Alba J, Barcia R, Gutiérrez-Berzal J, et al. Could inhibition of metalloproteinases be used to block the process of metastasis? Cell Biochem Funct 2022; 40: 600-7.
DOI PMID |
| 24. |
Nishimura S, Yamamoto Y, Sugimoto A, et al. Lipocalin-2 negatively regulates epithelial-mesenchymal transition through matrix metalloprotease-2 downregulation in gastric cancer. Gastric cancer 2022; 25: 850-61.
DOI PMID |
| 25. | Yu C, Chen W, Cai Y, et al. The lncRNA ZNF667-AS 1 inhibits propagation, invasion, and angiogenesis of gastric cancer by silencing the expression of N-Cadherin and VEGFA. J Oncol 2022; 2022: 3579547. |
| 26. | Loh CY, Chai JY, Tang TF, et al. The E-cadherin and N-cadherin switch in epithelial-to-mesenchymal transition: signaling, therapeutic Implications, and Challenges. Cells 2019; 8: 1118. |
| 27. |
Chauhan A, Islam AU, Prakash H, et al. Phytochemicals targeting NF-κB signaling: potential anti-cancer interventions. J Pharm Anal 2022; 12: 394-405.
DOI PMID |
| 28. | Wang W, Zhou L, Sun Z, et al. Down-regulation of tripartite motif containing 59 (TRIM59) blocks the NF-κB signaling pathway and inhibits the invasion and migration of nasopharyngeal carcinoma cells. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 2022; 38: 419-24. |
| 29. |
Sha M, Ye J, Zhang LX, et al. Celastrol induces apoptosis of gastric cancer cells by miR-21 inhibiting PI3K/Akt-NF-κB signaling pathway. Pharmacology 2014; 93: 39-46.
DOI PMID |
| 30. | Liao X, Che X, Zhao W, et al. The β-adrenoceptor antagonist, propranolol, induces human gastric cancer cell apoptosis and cell cycle arrest via inhibiting nuclear factor κB signaling. Oncol Rep 2010; 24: 1669-76. |
| 31. | Sohma I, Fujiwara Y, Sugita Y, et al. Parthenolide, an NF-κB inhibitor, suppresses tumor growth and enhances response to chemotherapy in gastric cancer. Cancer Genomics Proteomics 2011; 8: 39-47. |
| 32. | Zhang S, Xie B, Wang L, et al. Macrophage-mediated vascular permeability via VLA4/VCAM1 pathway dictates ascites development in ovarian cancer. J Clin Invest 2021; 131; e140315. |
| 33. | Bronikowska J, Kłósek M, Janeczko T, et al. The modulating effect of methoxy-derivatives of 2'-hydroxychalcones on the release of IL-8, MIF, VCAM-1 and ICAM-1 by colon cancer cells. Biomed Pharmacother 2022; 145: 112428. |
| 34. |
Melero I, Gabari I, Corbí AL, et al. An anti-ICAM-2 (CD102) monoclonal antibody induces immune-mediated regressions of transplanted ICAM-2-negative colon carcinomas. Cancer Res 2002; 62: 3167-74.
PMID |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||

