Journal of Traditional Chinese Medicine ›› 2024, Vol. 44 ›› Issue (5): 963-973.DOI: 10.19852/j.cnki.jtcm.2024.05.004
Previous Articles Next Articles
Role of toll-like receptor 4/mutant myeloid differentiation primary response 88/nuclear factor kappa-B mediated inflammation in diabetes mellitus with Northwest dryness syndrome
DENG Deqiang1,2, XIAO Yan1,2( ), MA Dan1,2, QIU Jinling1,2, HAO Congli1,2, WANG Di1,2, ZHANG Miao1,2
), MA Dan1,2, QIU Jinling1,2, HAO Congli1,2, WANG Di1,2, ZHANG Miao1,2
- 1 State Key Laboratory of Pathogenesis, Prevention and Treatment of High Incidence Diseases in Central Asia, Xinjiang Medical University, Urumqi 830054, China
2 Department of Endocrinology, Traditional Chinese Medicine Hospital of Urumqi, Urumqi 830099, China
-
Received:2023-06-15Accepted:2023-11-20Online:2024-10-15Published:2024-09-11 -
Contact:XIAO Yan, Department of Endocrinology, Traditional Chinese Medicine Hospital of Urumqi, Urumqi 830099, China. 411753009@qq.com Telephone: +86-18139686625 -
Supported by:Correlation Study on Chronic Inflammation Mediated by Toll-like Receptor 4/Mutant Myeloid Differentiation Primary Response 88/Nuclear Factor Kappa-B Signaling Pathway(SKL-HIDCA-2021-ZY5)
Cite this article
DENG Deqiang, XIAO Yan, MA Dan, QIU Jinling, HAO Congli, WANG Di, ZHANG Miao. Role of toll-like receptor 4/mutant myeloid differentiation primary response 88/nuclear factor kappa-B mediated inflammation in diabetes mellitus with Northwest dryness syndrome[J]. Journal of Traditional Chinese Medicine, 2024, 44(5): 963-973.
share this article
| Group | n | Before high-fat diet | High-fat diet for 2 weeks | High-fat diet for 4 weeks | 2 weeks after the intervention | 4 weeks after the intervention | 6 weeks after the intervention | 8 weeks after the intervention |
|---|---|---|---|---|---|---|---|---|
| Control | 6 | 4.60±0.28 | 4.60±0.33 | 4.62±0.21 | 4.68±0.17 | 4.58±0.17 | 4.58±0.15 | 4.70±0.18 |
| T2DM | 6 | 4.68±0.33 | 6.07±0.37ab | 6.17±0.26ab | 18.00±0.94abcd | 19.37±1.17abcde | 20.15±1.27abcde | 20.53±0.75abcde |
| Northwest dryness syndrome+T2DM | 6 | 4.72±0.29 | 6.05±0.62ab | 6.22±0.31ab | 18.48±0.98abcd | 19.22±0.90abcd | 21.97±0.96abcdefg | 24.55±1.37abcdefgi |
| Internal dampness syndrome+ 2DM | 6 | 4.67±0.23 | 6.23±0.54ab | 6.32±0.29ab | 17.95±0.91abcd | 19.28±0.83abcde | 20.66±0.79abcdegh | 21.90±0.81abcdefghi |
Table 1 Analysis of fasting blood glucose in rats (mmol/L,
| Group | n | Before high-fat diet | High-fat diet for 2 weeks | High-fat diet for 4 weeks | 2 weeks after the intervention | 4 weeks after the intervention | 6 weeks after the intervention | 8 weeks after the intervention |
|---|---|---|---|---|---|---|---|---|
| Control | 6 | 4.60±0.28 | 4.60±0.33 | 4.62±0.21 | 4.68±0.17 | 4.58±0.17 | 4.58±0.15 | 4.70±0.18 |
| T2DM | 6 | 4.68±0.33 | 6.07±0.37ab | 6.17±0.26ab | 18.00±0.94abcd | 19.37±1.17abcde | 20.15±1.27abcde | 20.53±0.75abcde |
| Northwest dryness syndrome+T2DM | 6 | 4.72±0.29 | 6.05±0.62ab | 6.22±0.31ab | 18.48±0.98abcd | 19.22±0.90abcd | 21.97±0.96abcdefg | 24.55±1.37abcdefgi |
| Internal dampness syndrome+ 2DM | 6 | 4.67±0.23 | 6.23±0.54ab | 6.32±0.29ab | 17.95±0.91abcd | 19.28±0.83abcde | 20.66±0.79abcdegh | 21.90±0.81abcdefghi |
| Group | n | GHbA1c (ng/mL) | Insulin (ng/mL) | HOMA-IR |
|---|---|---|---|---|
| Control | 6 | 11.18±0.83 | 9.66±0.54 | 2.02±0.14 |
| T2DM | 6 | 24.34±1.98a | 25.53±2.36a | 23.32±2.48a |
| Northwest dryness syndrome+T2DM | 6 | 41.13±1.37ab | 32.09±1.71ab | 35.06±3.36ab |
| Internal dampness syndrome+T2DM | 6 | 32.24±3.10abc | 29.74±1.36abc | 28.98±2.37abc |
Table 2 Changes of GHbA1c, insulin, and insulin resistance in rats (
| Group | n | GHbA1c (ng/mL) | Insulin (ng/mL) | HOMA-IR |
|---|---|---|---|---|
| Control | 6 | 11.18±0.83 | 9.66±0.54 | 2.02±0.14 |
| T2DM | 6 | 24.34±1.98a | 25.53±2.36a | 23.32±2.48a |
| Northwest dryness syndrome+T2DM | 6 | 41.13±1.37ab | 32.09±1.71ab | 35.06±3.36ab |
| Internal dampness syndrome+T2DM | 6 | 32.24±3.10abc | 29.74±1.36abc | 28.98±2.37abc |
| Group | n | Fasting blood glucose (mmol/L) | Blood glucose at 30 min (mmol/L) | Blood glucose at 60 min (mmol/L) | Blood glucose at 120 min (mmol/L) | Glucose AUC (mg·h-1·dL-1) |
|---|---|---|---|---|---|---|
| Control | 6 | 4.67±0.15 | 8.50±0.30d | 6.67±0.15 | 5.07±0.15e | 12.95±0.20 |
| T2DM | 6 | 20.53±0.67a | 26.73±1.12ad | 24.70±0.56ac | 22.57±1.06aef | 48.31±1.54a |
| Northwest dryness syndrome+T2DM | 6 | 25.43±0.71ab | 28.80±1.40abd | 32.13±1.45abde | 26.70±1.67abef | 58.21±2.79ab |
| Internal dampness syndrome+T2DM | 6 | 21.50±0.60ac | 25.13±0.93acd | 29.50±1.15 abcde | 24.03±0.87acdf | 52.08±1.91ac |
Table 3 Analysis of glucose tolerance in rats (
| Group | n | Fasting blood glucose (mmol/L) | Blood glucose at 30 min (mmol/L) | Blood glucose at 60 min (mmol/L) | Blood glucose at 120 min (mmol/L) | Glucose AUC (mg·h-1·dL-1) |
|---|---|---|---|---|---|---|
| Control | 6 | 4.67±0.15 | 8.50±0.30d | 6.67±0.15 | 5.07±0.15e | 12.95±0.20 |
| T2DM | 6 | 20.53±0.67a | 26.73±1.12ad | 24.70±0.56ac | 22.57±1.06aef | 48.31±1.54a |
| Northwest dryness syndrome+T2DM | 6 | 25.43±0.71ab | 28.80±1.40abd | 32.13±1.45abde | 26.70±1.67abef | 58.21±2.79ab |
| Internal dampness syndrome+T2DM | 6 | 21.50±0.60ac | 25.13±0.93acd | 29.50±1.15 abcde | 24.03±0.87acdf | 52.08±1.91ac |
| Group | n | Fasting blood glucose (mmol/L) | Blood glucose at 30 min (mmol/L) | Blood glucose at 60 min (mmol/L) | Blood glucose at 120 min (mmol/L) | Insulin AUC (mg·h-1·dL-1) |
|---|---|---|---|---|---|---|
| Control | 6 | 4.60±0.10 | 2.50±0.10d | 2.93±0.15 | 3.80±0.20 | 4.82±0.06 |
| T2DM | 6 | 21.37±1.00a | 20.50±0.56a | 24.10±1.81ade | 22.50±0.96a | 33.27±1.54a |
| Northwest dryness syndrome + T2DM | 6 | 25.17±0.75ab | 22.47±0.85ad | 27.10±1.57abe | 25.33±0.76abe | 37.41±0.90ab |
| Internal dampness syndrome + T2DM | 6 | 21.53±0.74ac | 20.80±0.56a | 24.43±1.25acde | 22.13±0.93acf | 33.53±1.07ac |
Table 4 Analysis of insulin tolerance in rats (
| Group | n | Fasting blood glucose (mmol/L) | Blood glucose at 30 min (mmol/L) | Blood glucose at 60 min (mmol/L) | Blood glucose at 120 min (mmol/L) | Insulin AUC (mg·h-1·dL-1) |
|---|---|---|---|---|---|---|
| Control | 6 | 4.60±0.10 | 2.50±0.10d | 2.93±0.15 | 3.80±0.20 | 4.82±0.06 |
| T2DM | 6 | 21.37±1.00a | 20.50±0.56a | 24.10±1.81ade | 22.50±0.96a | 33.27±1.54a |
| Northwest dryness syndrome + T2DM | 6 | 25.17±0.75ab | 22.47±0.85ad | 27.10±1.57abe | 25.33±0.76abe | 37.41±0.90ab |
| Internal dampness syndrome + T2DM | 6 | 21.53±0.74ac | 20.80±0.56a | 24.43±1.25acde | 22.13±0.93acf | 33.53±1.07ac |
| Group | n | ALT (U/L) | AST (U/L) | TC (mmol/L) | TG (mmol/L) | LDL (mmol/L) | HDL (mmol/L) |
|---|---|---|---|---|---|---|---|
| Control | 6 | 25.05±1.65 | 19.09±1.96 | 6.03±0.60 | 1.47±0.11 | 2.52±0.16 | 1.61±0.06 |
| T2DM | 6 | 42.37±3.07a | 33.78±2.19a | 14.16±1.54a | 4.65±0.58a | 5.89±0.41a | 1.19±0.06a |
| Northwest dryness syndrome + T2DM | 6 | 60.34±3.18ab | 51.66±2.61ab | 25.56±1.77ab | 7.87±0.50ab | 8.23±0.59ab | 0.56±0.07ab |
| Internal dampness syndrome + T2DM | 6 | 51.90±3.82abc | 45.71±4.62ab | 18.72±1.24abc | 5.21±0.46ac | 6.80±0.51ac | 0.87±0.08abc |
Table 5 Changes of liver function (ALT and AST) and blood lipid (TC, TG, LDL, and HDL) levels in rats (
| Group | n | ALT (U/L) | AST (U/L) | TC (mmol/L) | TG (mmol/L) | LDL (mmol/L) | HDL (mmol/L) |
|---|---|---|---|---|---|---|---|
| Control | 6 | 25.05±1.65 | 19.09±1.96 | 6.03±0.60 | 1.47±0.11 | 2.52±0.16 | 1.61±0.06 |
| T2DM | 6 | 42.37±3.07a | 33.78±2.19a | 14.16±1.54a | 4.65±0.58a | 5.89±0.41a | 1.19±0.06a |
| Northwest dryness syndrome + T2DM | 6 | 60.34±3.18ab | 51.66±2.61ab | 25.56±1.77ab | 7.87±0.50ab | 8.23±0.59ab | 0.56±0.07ab |
| Internal dampness syndrome + T2DM | 6 | 51.90±3.82abc | 45.71±4.62ab | 18.72±1.24abc | 5.21±0.46ac | 6.80±0.51ac | 0.87±0.08abc |

Figure 1 Pathological changes of pancreas, liver, perirenal adipose and epididymal adipose A: HE staining detection of the lesions of the pancreas in the four groups (magnification × 400). A1: control group; A2: T2DM group; A3: Northwest dryness group; A4: internal dampness group. B: HE staining detection of the lesions of the liver in the four groups (magnification × 400). B1: control group; B2: T2DM group; B3: Northwest dryness group; B4: internal dampness group.C: HE staining detection of the lesions of the perirenal adipose in the four groups (magnification × 400). C1: control group; C2: T2DM group; C3: Northwest dryness group; C4: internal dampness group. D: HE staining detection of the lesions of the epididymal adipose in the four group (magnification × 400). D1: control group; D2: T2DM group; D3: Northwest dryness group; D4: internal dampness group. E: PAS staining detection of the liver glycogen content in the four groups (magnification × 400). E1: control group; E2: T2DM group; E3: Northwest dryness group; E4: internal dampness group. Control group: rats were not treated; T2DM group: T2DM model was established; Northwest dryness syndrome + T2DM group: Northwest dryness syndrome and T2DM model were established; Internal dampness syndrome + T2DM group: Internal dampness syndrome and T2DM model were established. HE: hematoxylin-eosin; T2DM: type 2 diabetes mellitus.
| Group | n | Body weight (g) | Pancreas (g) | Organ-to-weight ratio (%) | Liver (g) | Organ-to-weight ratio (%) | Perirenal adipose (g) | Organ-to-weight ratio (%) | Epididymal adipose (g) | Organ-to-weight ratio (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Control | 6 | 421.44± 13.82 | 1.28± 0.07 | 0.30± 0.02 | 17.86± 1.09 | 4.24± 0.15 | 4.40± 0.18 | 1.05± 0.07 | 2.0±0.10 | 0.47± 0.04 |
| T2DM | 6 | 292.38± 16.43a | 0.73± 0.04a | 0.25± 0.03 | 11.92± 0.79a | 4.08± 0.08a | 2.32± 0.15a | 0.79±0.06 | 1.19±0.11a | 0.41± 0.04 |
| Northwest dryness syndrome+T2DM | 6 | 266.84± 10.63ab | 0.58± 0.03a | 0.22± 0.01 | 10.24± 0.70ab | 3.83± 0.14ab | 1.02± 0.11ab | 0.38± 0.05ab | 0.64±0.09ab | 0.24± 0.03ab |
| Internal dampness syndrome+T2DM | 6 | 277.24± 18.29abc | 0.66± 0.06a | 0.24± 0.02 | 10.92± 0.89abc | 3.94± 0.13ab | 1.57± 0.13abc | 0.57± 0.07abc | 0.86±0.06a | 0.31± 0.02a |
Table 6 Analysis of organ-to-weight ratios in rats (
| Group | n | Body weight (g) | Pancreas (g) | Organ-to-weight ratio (%) | Liver (g) | Organ-to-weight ratio (%) | Perirenal adipose (g) | Organ-to-weight ratio (%) | Epididymal adipose (g) | Organ-to-weight ratio (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Control | 6 | 421.44± 13.82 | 1.28± 0.07 | 0.30± 0.02 | 17.86± 1.09 | 4.24± 0.15 | 4.40± 0.18 | 1.05± 0.07 | 2.0±0.10 | 0.47± 0.04 |
| T2DM | 6 | 292.38± 16.43a | 0.73± 0.04a | 0.25± 0.03 | 11.92± 0.79a | 4.08± 0.08a | 2.32± 0.15a | 0.79±0.06 | 1.19±0.11a | 0.41± 0.04 |
| Northwest dryness syndrome+T2DM | 6 | 266.84± 10.63ab | 0.58± 0.03a | 0.22± 0.01 | 10.24± 0.70ab | 3.83± 0.14ab | 1.02± 0.11ab | 0.38± 0.05ab | 0.64±0.09ab | 0.24± 0.03ab |
| Internal dampness syndrome+T2DM | 6 | 277.24± 18.29abc | 0.66± 0.06a | 0.24± 0.02 | 10.92± 0.89abc | 3.94± 0.13ab | 1.57± 0.13abc | 0.57± 0.07abc | 0.86±0.06a | 0.31± 0.02a |
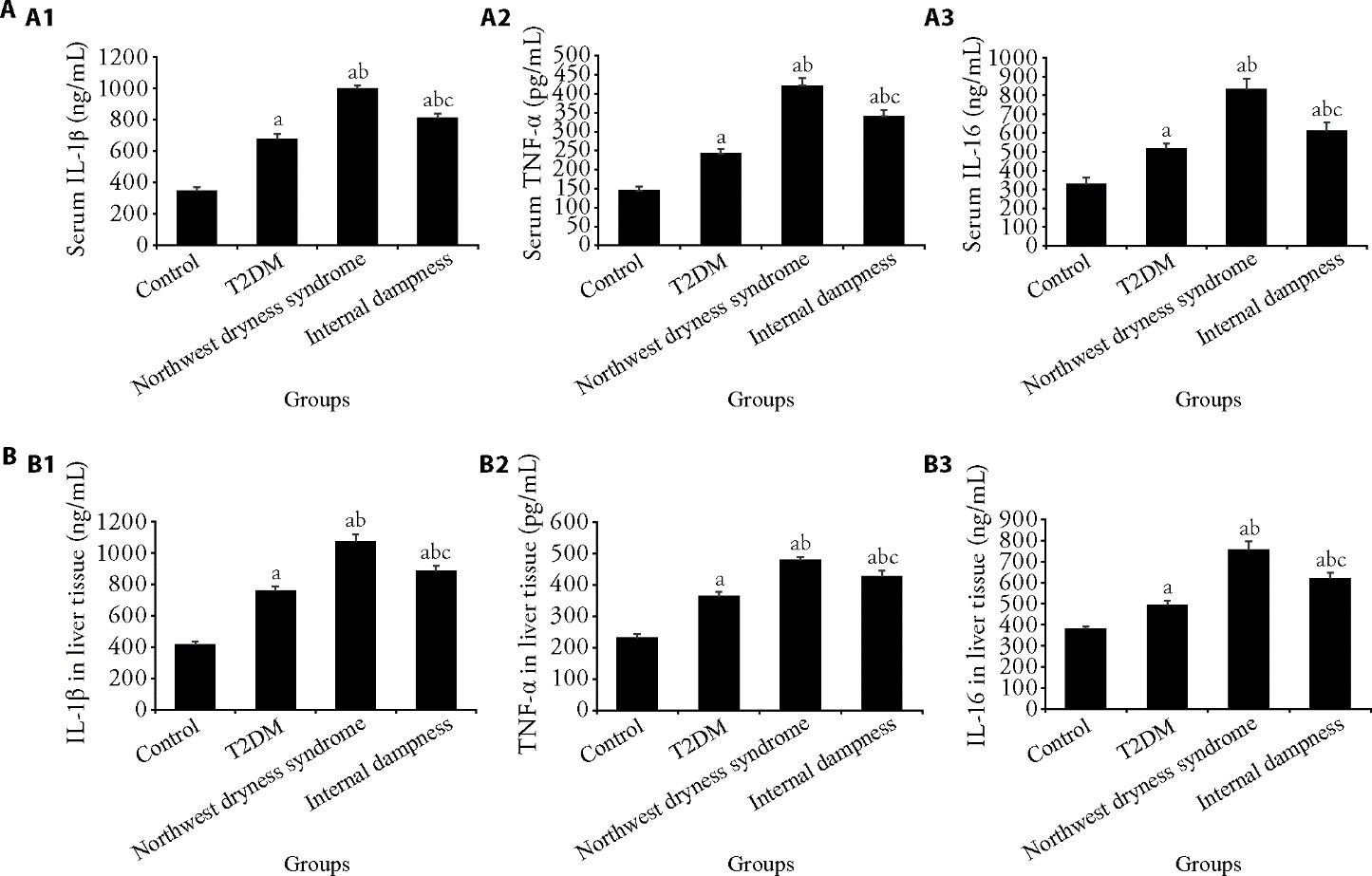
Figure 2 Changes in expression levels of IL-1β, TNF-α, and IL-16 in serum and liver tissues of rats by ELISA A: The levels of IL-1β, TNF-α, and IL-16 in serum of rats. A1: analysis of IL-1β expression; A2: analysis of TNF-α expression; A3: analysis of IL-16 expression. B: The levels of IL-1β, TNF-α, and IL-16 in liver tissues of rats. B1: analysis of IL-1β expression; B2: analysis of TNF-α expression; B3: analysis of IL-16 expression. Control group: rats were not treated (n = 6); T2DM group: T2DM model was established (n = 6); Northwest dryness syndrome + T2DM group: Northwest dryness syndrome and T2DM model were established (n = 6); Internal dampness syndrome + T2DM group: Internal dampness syndrome and T2DM model were established (n = 6). TNF-α: tumor necrosis factor-α; IL-1β: Interleukin 1 Beta; IL-6: Interleukin 16; T2DM: type 2 diabetes mellitus; ELISA: enzyme-linked immunosorbent assay. One-way analysis of variance and the least significance difference test were used for analysis. Data were expressed as mean ± standard deviation. Compared with the control group, aP<0.05; compared with the T2DM group, bP<0.05; and compared with the Northwest dryness group, cP<0.05.
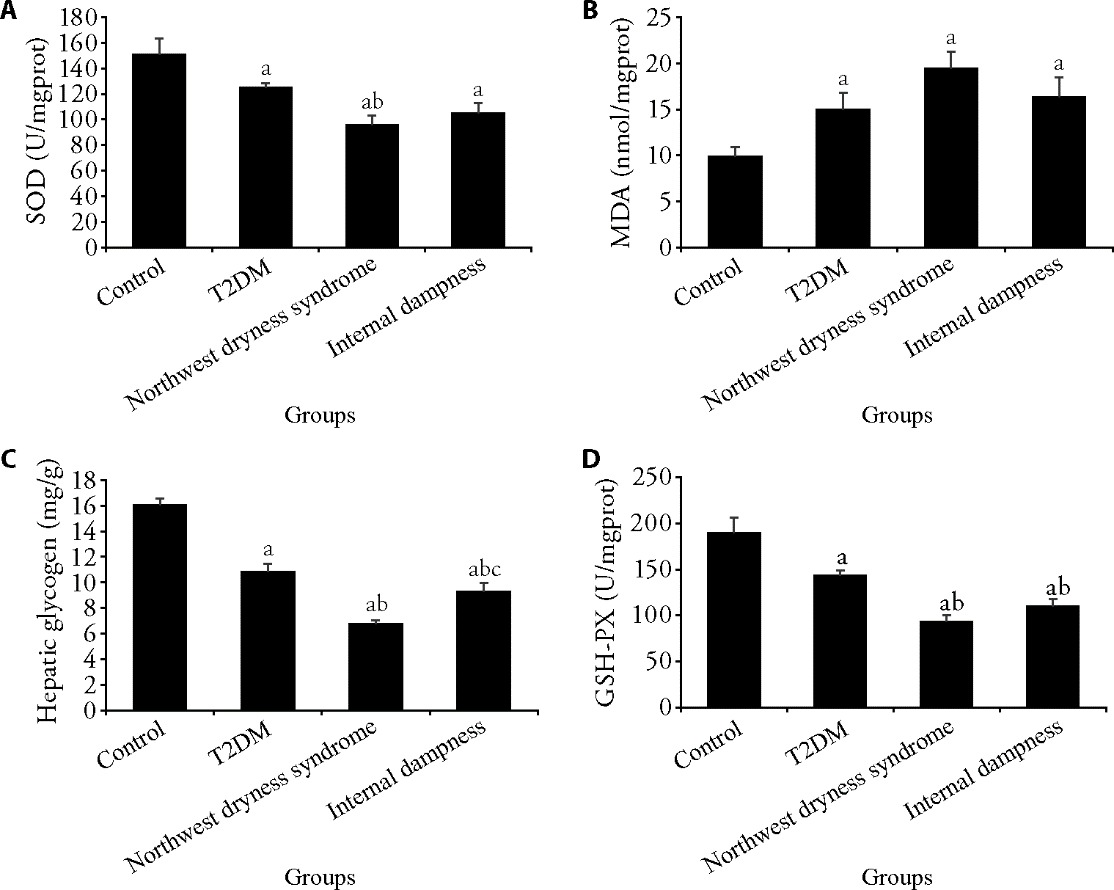
Figure 3 Changes in the levels of SOD, MDA, liver glycogen, and GSH-PX in rats A: SOD level in rats from each group; B: MDA level in rats from each group; C: liver glycogen level in rats from each group; D: GSH-PX level in rats from each group. T2DM: type 2 diabetes mellitus; Control group: rats were not treated (n = 6); T2DM group: T2DM model was established (n = 6); Northwest dryness syndrome + T2DM group: Northwest dryness syndrome and T2DM model were established (n = 6); Internal dampness syndrome + T2DM group: Internal dampness syndrome and T2DM model were established (n = 6). SOD: superoxide dismutase; MDA: malondialdehyde; GSH-PX: glutathione peroxidase. One-way analysis of variance and the least significance difference test were used for analysis. Data were expressed as mean ± standard deviation. Compared with the control group, aP < 0.05; compared with the T2DM group, bP < 0.05; and compared with the Northwest dryness group, cP<0.05.
| Group | n | IRF-5 (perirenal adipose tissue) | NF-κB p65 (perirenal adipose tissue) | IRF-5 (epididymal adipose tissue) | NF-κB p65 (epididymal adipose tissue) |
|---|---|---|---|---|---|
| Control | 6 | 1.00±0.00 | 1.00±0.00 | 1.00±0.00 | 1.00±0.00 |
| T2DM | 6 | 3.81±0.47a | 3.64±0.20a | 3.67±0.30a | 3.71±0.06a |
| Northwest dryness syndrome+T2DM | 6 | 10.78±1.58ab | 14.61±1.05ab | 11.16±0.74ab | 15.34±0.29ab |
| Internal dampness syndrome+T2DM | 6 | 6.46±0.64abc | 7.22±0.34abc | 6.66±0.33abc | 7.70±0.93abc |
Table 7 Analysis of mRNA expression levels of IRF-5 and NF-κB p65 in rats
| Group | n | IRF-5 (perirenal adipose tissue) | NF-κB p65 (perirenal adipose tissue) | IRF-5 (epididymal adipose tissue) | NF-κB p65 (epididymal adipose tissue) |
|---|---|---|---|---|---|
| Control | 6 | 1.00±0.00 | 1.00±0.00 | 1.00±0.00 | 1.00±0.00 |
| T2DM | 6 | 3.81±0.47a | 3.64±0.20a | 3.67±0.30a | 3.71±0.06a |
| Northwest dryness syndrome+T2DM | 6 | 10.78±1.58ab | 14.61±1.05ab | 11.16±0.74ab | 15.34±0.29ab |
| Internal dampness syndrome+T2DM | 6 | 6.46±0.64abc | 7.22±0.34abc | 6.66±0.33abc | 7.70±0.93abc |
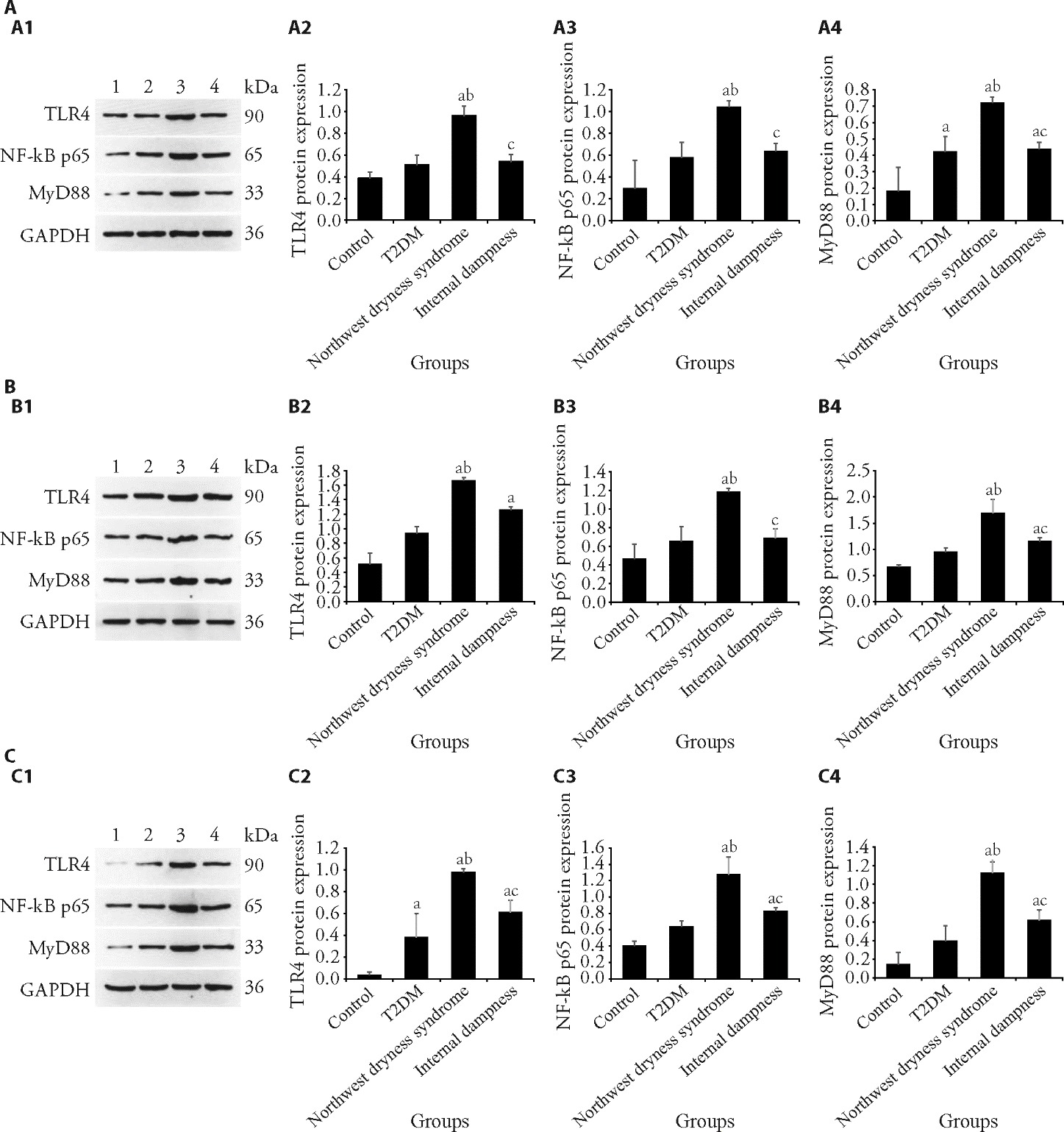
Figure 4 Changes in the protein expression levels of TLR4, NF-κB p65, and MyD88in the liver, perirenal adipose, and epididymal adipose tissues in rats A: TLR4, NF-κB p65, and MyD88 protein expression level in the liver tissue by Weston blot. A1: pictures of Western blot analysis of TLR4, NF-κB p65, and MyD88; A2: Western blot analysis of TLR4 expression; A3: Western blot analysis of NF-κB p65 MyD88 expression; A4: Western blot analysis of MyD88NF-κB p65 expression. B: TLR4, NF-κB p65, and MyD88 protein expression level in the perirenal adipose tissue by Weston blot. B1: pictures of Western blot analysis of TLR4, NF-κB p65, and MyD88; B2: Western blot analysis of TLR4 expression; B3: Western blot analysis of NF-κB p65 expression; B4: Western blot analysis of MyD88 expression. C: TLR4, NF-κB p65, and MyD88 protein expression level in the epididymal adipose tissue by Weston blot. C1: pictures of Western blot analysis of TLR4, NF-κB p65, and MyD88by Weston blot; C2: Western blot analysis of TLR4 expression; C3: Western blot analysis of NF-κB p65 expression; C4: Western blot analysis of MyD88 expression. Lane 1: Control; Lane 2: T2DM; Lane 3: northwest dryness syndrome; Lane 4: internal dampness. TLR4: toll-like receptor 4; MyD88: mutant myeloid differentiation primary response 88; NF-κB: nuclear factor kappa-B; T2DM: type 2 diabetes. Control group: rats were not treated (n = 6); T2DM group: T2DM model was established (n = 6); Northwest dryness syndrome + T2DM group: Northwest dryness syndrome and T2DM model were established (n = 6); Internal dampness syndrome + T2DM group: Internal dampness syndrome and T2DM model were established (n = 6). One-way analysis of variance and the least significance difference test were used for analysis. Data were expressed as mean ± standard deviation. Compared with the control group, aP<0.05; compared with the T2DM group, bP<0.05; and compared with the Northwest dryness group, cP<0.05.
| 1. | Ala M, Jafari RM, Dehpour AR. Diabetes mellitus and osteoporosis correlation: challenges and hopes. Curr Diabetes Rev 2020; 16: 984-1001. |
| 2. | Wang H, Li N, Chivese T, et al. IDF diabetes atlas: estimation of global and regional gestational diabetes mellitus prevalence for 2021 by international association of diabetes in pregnancy study group's criteria. Diabetes Res Clin Pract 2022; 183: 109050. |
| 3. | Li Y, Teng D, Shi X, et al. Prevalence of diabetes recorded in mainland China using 2018 diagnostic criteria from the American Diabetes Association: national cross sectional study. BMJ 2020; 369: m997. |
| 4. | Fu L, Yang Y, Zhang T. Research progress on immune patho-genesis of type 2 diabetes. Zhong Guo Tang Niao Bing Za Zhi 2021; 29: 393-6. |
| 5. | Hu Y. Research on the correlation between type 2 diabetes and northwest dryness syndrome and intervention treatment. Urumqi: Xinjiang Medical University; 2011: 1-56. |
| 6. | Wang Y, Wang X, Zhao C. Expression characteristics of serum interleukin-17A and interleukin-10 in patients with Northwest dryness syndrome. J Tradit Chin Med 2016; 57: 1049-52. |
| 7. | Zhou M. Overview of northwest dryness syndrome research. Shanghai Zhong Yi Yao Za Zhi 2005; 39: 43-5. |
| 8. | Zhou M, Tao P. Preliminary study on desert dryness syndrome- research on health care of desert oil workers. J Tradit Chin Med 1997; 38: 493-6. |
| 9. | Zhou M. Differentiation of pathogenic attribute of dryness. Xinjiang Zhong Yi Yao Za Zhi 2005; 23: 1-5. |
| 10. | Wang Y, Zhou M. Discussion on the characteristics of Wu Ju Tong's treatment of dryness - literature study on the treatment of Northwest dryness syndrome. Xinjiang Zhong Yi Yao Za Zhi 2006; 24: 1-3. |
| 11. | Jiang D, Zhou M. Discussion on the relationship between the etiology of dryness and cough-research on the etiology of Northwest dryness syndrome. Xinjiang Zhong Yi Yao 2006; 24: 7-9. |
| 12. | Shatanati, Sun H, Zhou M. Preliminary study on the relationship between environmental geographical factors and sub-health status -background study on Northwest dryness syndrome. Xinjiang Zhong Yi Yao 2006; 24: 74-6. |
| 13. | Hu Y, Huo B, Chen H, Zhou M. The influence of dryness on the pathogenesis of type 2 diabetes. Beijing Zhong Yi Yao Da Xue Xue Bao 2010; 17: 25-6. |
| 14. | Zhou M, Song X, Shan L, et al. Epidemiological investigation and analysis of Northwest dryness syndrome among different ethnic residents in Xinjiang. Xinjiang Yi Ke Da Xue Xue Bao 2006; 29: 1034-8. |
| 15. | Sharma D, Arora S, Banerjee A, Singh J. Improved insulin sensitivity in obese-diabetic mice via chitosan Nanomicelles mediated silencing of pro-inflammatory adipocytokines. Nanomedicine 2021; 33: 102357. |
| 16. | Xiang Q, Jiang W, Yu R, et al. The effect of Zuogui compound on intestinal inflammation and mucosal barrier in type 2 diabetes mice based on the theory of "spleen based deficiency". Zhong Guo Mian Yi Xue Za Zhi 2020; 36: 667-71-76. |
| 17. | Hu Y, Huo B, Zhou M. Observation on the therapeutic effect of Northwest dryness syndrome empirical prescriptions on type 2 diabetes. Liaoning Zhong Yi Yao Za Zhi 2012; 39: 1537-9. |
| 18. | Ma T, Huang X, Zheng H, et al. SFRP2 improves mitochondrial dynamics and mitochondrial biogenesis, oxidative stress, and apoptosis in diabetic cardiomyopathy. Oxid Med Cell Longev 2021; 2021: 9265016. |
| 19. |
Shao Q, Meng L, Lee S, et al. Empagliflozin, a sodium glucose co-transporter-2 inhibitor, alleviates atrial remodeling and improves mitochondrial function in high-fat diet/streptozotocin-induced diabetic rats. Cardiovasc Diabetol 2019; 18: 165.
DOI PMID |
| 20. | Wang L, Shi H, Zhou M. Observation on the duration of making animal model of Northwest dryness syndrome. Zhong Guo Zhong Yi Yao Za Zhi 2017; 32: 4585-8. |
| 21. | Zhou Y, Zhang J. Research progress on animal models of internal dampness syndrome. Liaoning Zhong Yi Yao Da Xue Za Zhi 2018; 20: 141-4. |
| 22. | Bao W, Sun H, Wu X, et al. Exploring anti-type 2 diabetes mellitus mechanism of Gegen Qinlian decoction by network pharmacology and experimental validation. Dis Markers 2022; 2022: 1927688. |
| 23. | Zhao W, Chen L, Zhou H, et al. Protective effect of carvacrol on liver injury in type 2 diabetic db/db mice. Mol Med Rep 2021; 24: 741. |
| 24. | Liu A, Ma L, Zhou G. Effect of Qinghao Biejia decoction and Northwest dryness syndrome empirical formula on perimenopausal anxiety rats with Yin deficiency and internal dryness. Zhong Guo Zhong Yi Ji Chu Yi Xue Za Zhi 2022; 28: 1085-8, 206. |
| 25. | Deng D, Zhang Y, Ma D, Dilare A. Effects of Xiaoke Jianpi Capsule in the treatment of type 2 diabetes IR and TNF-α and IL-6. Xinjiang Zhong Yi Yao 2018; 36: 7-10. |
| 26. | Zhu J, Sun L, Wang S, Zhang J, Wang M, Qiao F. Correlation between the serum interleukin-23, interleukin-17 and β cell function in adult patients with concealed autoimmune diabetes. Zhong Guo Yi Shi Za Zhi 2020; 22: 67-70. |
| 27. | Deng L, Zhao X, Chen M, et al. Study on the correlation between plasma adiponectin, visfatin, leptin and resistin levels and the incidence of colonic polyps in pre diabetes population. Zhong Guo Nei Fen Bi Dai Xie Za Zhi 2018; 34: 997-1002. |
| 28. | Wei Y, Li M, Yu J. Research progress on the relationship between chronic inflammation and insulin resistance. J Clin Pathol 2019; 39: 196-201. |
| 29. | Lin Y, Jin W. Expression of GLUT1/4 in different glucose level and its effect on migration inhibitory factor in patients with type 2 diabetes mellitus. Zhong Guo Tang Niao Bing Za Zhi 2016; 24: 60-3. |
| 30. | Crujeiras AB, Cordero P, Garcia-Diaz DF, Stachowska E, Gonzalez-Muniesa P. Molecular basis of the inflammation related to obesity. Oxid Med Cell Longev 2019; 2019: 5250816. |
| 31. | He F, Huang Y, Song Z, et al. Mitophagy-mediated adipose inflammation contributes to type 2 diabetes with hepatic insulin resistance. J Exp Med 2021; 218: e20201416. |
| 32. | He L, Chen S. Effects of metformin combined with glimepiride on glucose and lipid metabolism, hemorheology and oxidative stress in elderly patients with type 2 diabetes. Zhong Guo Lao Nian Xue Za Zhi 2020; 40: 2283-6. |
| 33. | Osorio FG, Barcena C, Soria-Valles C, et al. Nuclear lamina defects cause ATM-dependent NF-kappa B activation and link accelerated aging to a systemic inflammatory response. Genes Dev 2012; 26: 2311-24. |
| 34. |
Mitchell JP, Carmody RJ. NF-κB and the transcriptional control of inflammation. Int Rev Cell Mol Biol 2018; 335: 41-84.
DOI PMID |
| 35. | Deng D, Xu G, Zhou J, et al. Study on treatment of reinforcing spleen and dissipating dampness and promoting blood circulation (TRDP) on the function of pancreatic β cells in patients with type 2 diabetes mellitus. Liaoning Zhong Yi Yao Za Zhi 2015; 42: 1465-7. |
| Viewed | ||||||||||||||||||||||||||||||||||||||||||||||||||
|
Full text 52
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||
|
Abstract 37
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||

