Journal of Traditional Chinese Medicine ›› 2025, Vol. 45 ›› Issue (2): 376-384.DOI: 10.19852/j.cnki.jtcm.2025.02.012
• Original articles • Previous Articles Next Articles
Network pharmacology approach to unveiling the mechanism of berberine in the amelioration of morphine tolerance
HAN Shuai1,2,3, Du Zhikang1,2, WANG Zirui1,2, HUANG Tianfeng3, GE Yali3, SHI Jianwen4,5( ), GAO Ju3(
), GAO Ju3( )
)
- 1 Institute of Translational Medicine, Medical College, Yangzhou University, Yangzhou 225009, China
2 Jiangsu Key Laboratory of Integrated Traditional Chinese and Western Medicine for Prevention and Treatment of Senile Diseases, Yangzhou University, Yangzhou 225009, China
3 Northern Jiangsu People's Hospital Affiliated to Yangzhou University, Yangzhou 225009, China
4 Peking University People's Hospital, Qingdao 266111, China
5 Women and Children's Hospital, Qingdao University, Qingdao 266034, China
-
Received:2023-12-15Accepted:2024-05-23Online:2025-04-15Published:2025-03-10 -
Contact:Prof. GAO Ju, Northern Jiangsu People's Hospital Affiliated to Yangzhou University, Yangzhou 225009, China. doctor2227@163.com; Prof. SHI Jianwen, Peking University People's Hospital, Qingdao; Women and Children's Hospital, Qingdao University, Qingdao 266034, China. javen0523@163.com, Telephone: +86-15618135070; +86-18553263171 -
Supported by:Natural Science Foundation-funded Project: Study on the Mechanism of Mechanical Stress Sensing Element Piezo Type Mechanosensitive Ion Channel Component 2 Interacting with Nuclear Receptor Subfamily 4 Group A Member 2 Mediating Traumatic Brain Injury(82172190)
Cite this article
HAN Shuai, Du Zhikang, WANG Zirui, HUANG Tianfeng, GE Yali, SHI Jianwen, GAO Ju. Network pharmacology approach to unveiling the mechanism of berberine in the amelioration of morphine tolerance[J]. Journal of Traditional Chinese Medicine, 2025, 45(2): 376-384.
share this article
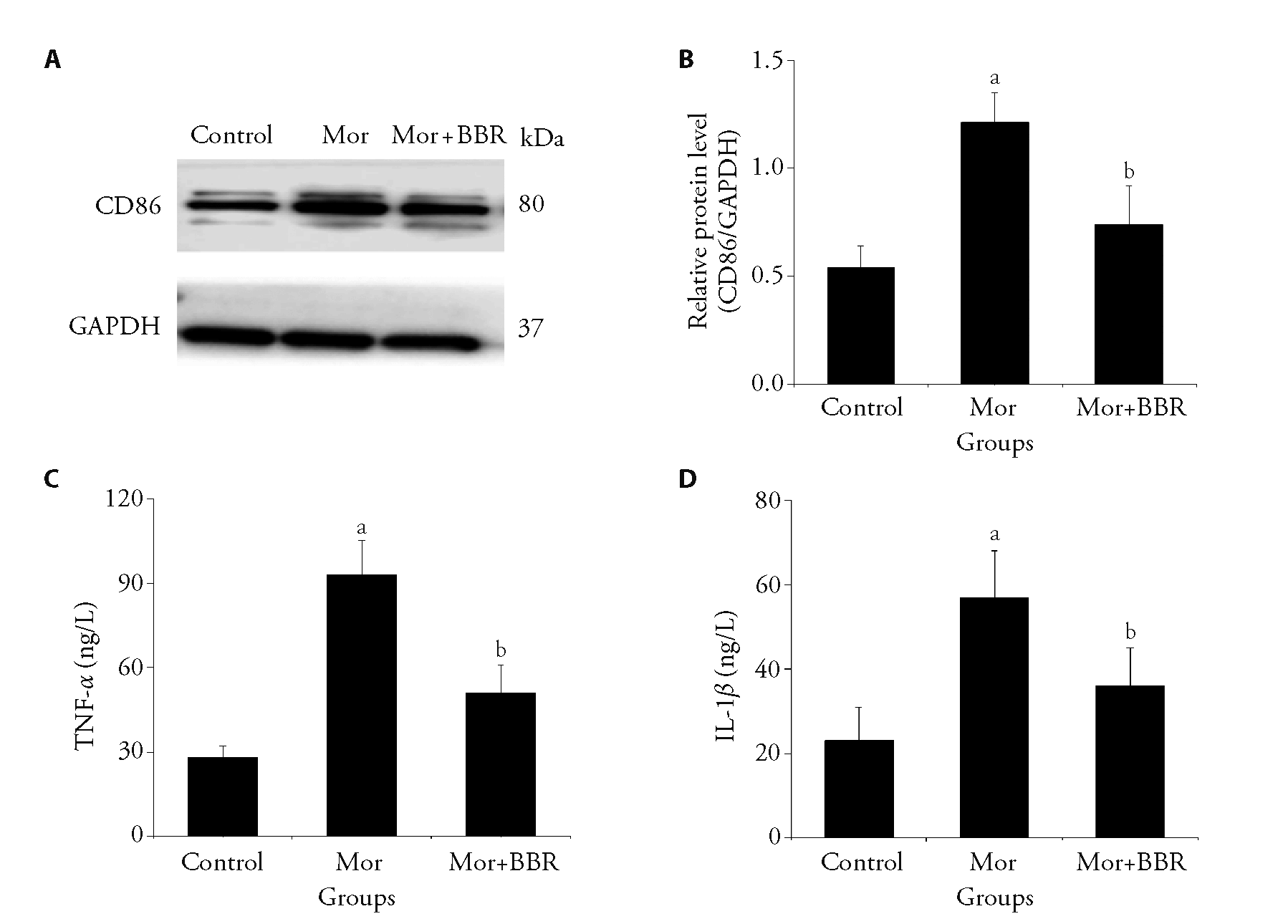
Figure 1 Inhibitory effect of berberine on spinal dorsal horn microglial activation and inflammatory factors in morphine-tolerant mice A: the protein expression of CD86 was detected by western blot; B: the relative expression of CD86 level was quantified; C: the production of TNF-α was detected by ELISA assays; D: the production of IL-1β was detected by ELISA assays. Mice were pretreated with BBR (5.0 mg/kg) i.p. and then injected with morphine (10.0 mg/kg) s.c. 30 min later, this was performed once per day for eight consecutive days. On day nine, except for the control group, each group was injected with morphine 10.0 mg/kg. i.p.: intraperitoneal, s.c.: subcutaneous, BBR: berberine; Mor: morphine; TNF-α: tumor necrosis factor-alpha; IL-β: Interleukin-β; ELISA: enzymelinked immunosorbent assay. All values are expressed as the mean ± standard deviation (n = 6). One-way analysis of variance followed by Dunnett's multiple comparison for (B, C, D). aP < 0.01 compared with the control group; bP < 0.01 compared with the morphine group.
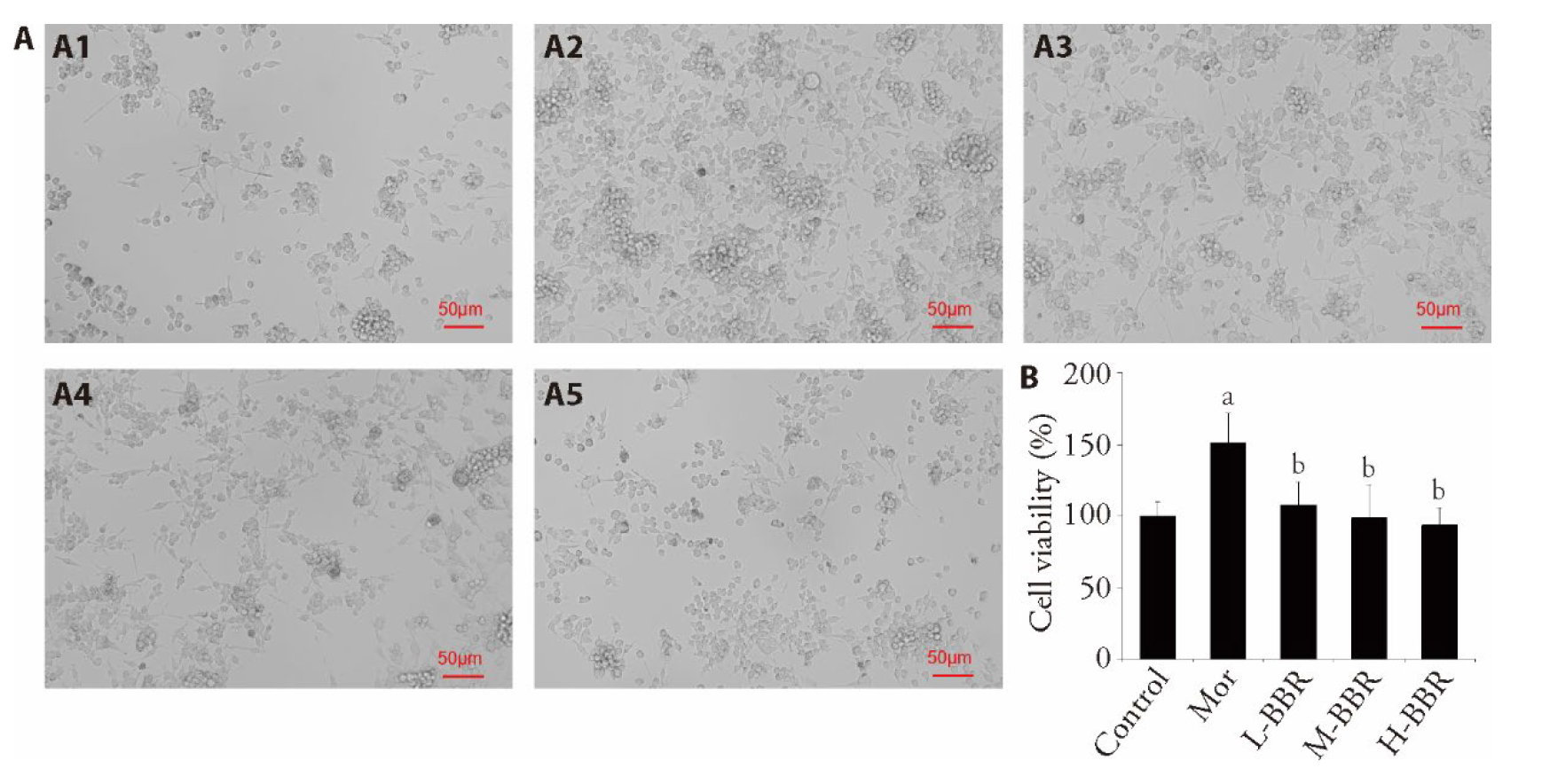
Figure 2 Inhibitory effect of berberine on morphine-mediated the activation of brain vascular 2 (BV2) cells A: effect of BBR on morphine-induced proliferation of BV2 cells (× 200). Scale bar: 50 μm. For A1: control group, A2: morphine group (200 μmol/L), and morphine (200 μmol/L) combined with BBR groups based on BBR dose (A3: 5.0, A4: 10.0, and A5: 20.0 μmol/L); B: the cell viability was evaluated by Cell Counting Kit-8 assay. BV2 cells were treated with BBR (5, 10, and 20 μmol/L) 2 h prior to morphine (200 μmol/L) induction. BBR: berberine; Mor: morphine; L-BBR: BBR dose 5.0 μmol/L; M-BBR: BBR dose 10.0 μmol/L; H-BBR: BBR dose 20.0 μmol/L. All values are expressed as the mean ± standard deviation (n = 6). One-way analysis of variance followed by Dunnett's multiple comparison for B. aP < 0.001 compared with the control group; bP < 0.01 compared with the morphine group.
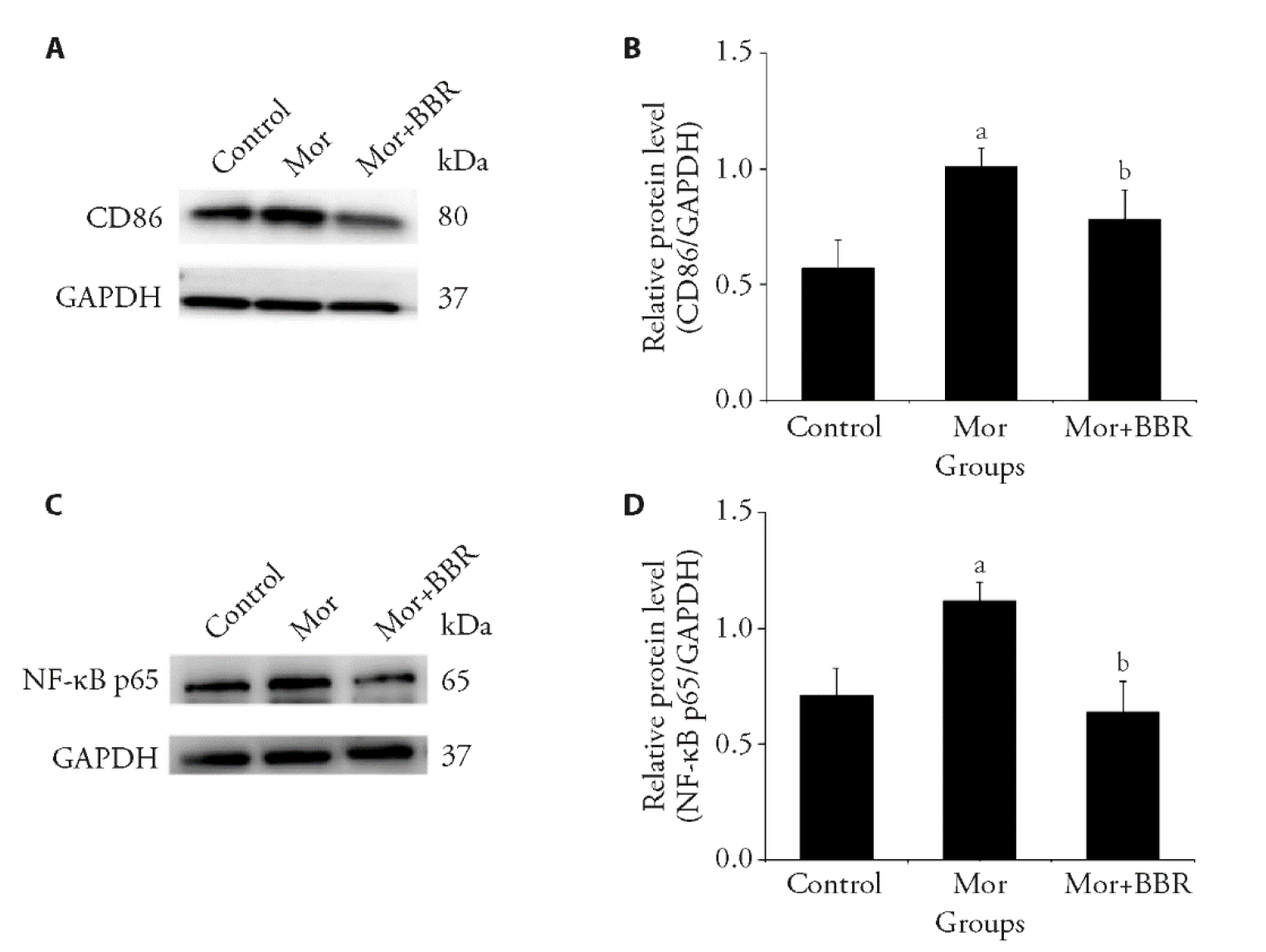
Figure 3 Inhibitory effect of berberine on morphine induced activation of brain vascular 2 (BV2) cells A: the protein expression of CD86 was detected by western blot; B: the relative expression of CD86 level was quantified; C: the protein expression of NF-κB p65 was detected by Western blot; D: the relative expression of NF-κB p65 level was quantified. BV2 cells were treated with BBR (20 μmol/L) prior to morphine (200 μmol/L) induction. BBR: berberine dose 20 μmol/L; Mor: morphine dose 200 μmol/L; CD86: Cluster of Differentiation 86; NF-κB: nuclear factor kappa B. All values are expressed as the mean ± standard deviation (n = 6). One-way analysis of variance followed by Dunnett's multiple comparison for (B, D). aP < 0.05 compared with the control group; bP < 0.05 compared with the morphine group.

Figure 4 Inhibitory effect of berberine on morphine-mediated apoptosis and oxidative stress in brain vascular 2 (BV2) cells A: effect of BBR on apoptosis in BV2 cells induced by morphine (× 200). Scale bar: 50 μm. A1: control group, A2: morphine group (200 μmol/L), and morphine (200 μmol/L) combined with BBR groups based on BBR dose (A3: 5.0, A4: 10.0, and A5: 20.0 μmol/L). B: effect of BBR on ROS in BV2 cells induced by morphine (× 200). Scale bar: 50 μm. B1: control group, B2: morphine group (200 μmol/L), and morphine (200 μmol/L) combined with BBR groups based on BBR dose (B3: 5.0, B4: 10.0, and B5: 20.0 μmol/L). C: BV2 cells were treated with BBR (20 μmol/L) prior to morphine (200 μmol/L) induction. The protein expression of Caspase-3, Bax and Bcl-2 were detected by Western blot; D: the relative expression of Caspase-3 level was quantified; E: the relative expression of Bax level was quantified; F: the relative expression of Bcl-2 level was quantified. BBR: berberine dose 20 μmol/L; Mor: morphine dose 200 μmol/L; ROS: reactive oxygen species; Bcl-2: B-cell lymphoma 2. All values are expressed as the mean ± standard deviation (n = 6). One-way analysis of variance followed by Dunnett's multiple comparison for (D, E, F). aP < 0.05, cP < 0.01 compared with the control group; bP < 0.05 compared with the morphine group.
| 1. | Jiao Y, Gao P, Dong L, et al. Molecular identification of bulbospinal on neurons by GPER which drives pain and morphine tolerance. J Clin Invest 2022; e154588. |
| 2. | Gao ZS. Berberine-loaded M2 macrophage-derived exosomes for spinal cord injury therapy. Acta Biomater 2021; 126: 211-23. |
| 3. |
Zhang Q, Ren Y, Mo Y, et al. Inhibiting Hv1 channel in peripheral sensory neurons attenuates chronic inflammatory pain and opioid side effects. Cell research 2022; 32: 461-76.
DOI PMID |
| 4. |
Jokinen V. Differential spinal and supraspinal activation of glia in a rat model of morphine tolerance. Neuroscience 2018; 375: 10-24.
DOI PMID |
| 5. | Tu HA, Chu HC, Guan S, et al. The role of the M1/M2 microglia in the process from cancer pain to morphine tolerance. Tissue Cell 2021; 68: 101438. |
| 6. | Martyn JAJ, Mao J, Bittner EA. Opioid tolerance in critical illness. N Engl J Med 2019; 380: 365-78. |
| 7. |
Wang CG, Wang QQ, Lou YT, et al. Salidroside attenuates neuroinflammation and improves functional recovery after spinal cord injury through microglia polarization regulation. J Cell Mol Med 2018; 22: 1148-66.
DOI PMID |
| 8. | Zhang Q, Wang XB, Cao SJ, et al. Berberine represses human gastric cancer cell growth in vitro and in vivo by inducing cytostatic autophagy via inhibition of MAPK/mTOR/p70S6K and Akt signaling pathways. Biomed Pharmacother 2020; 128: 110245. |
| 9. | Chen J, Wang G, Sun T, et al. Involvement of TCF7L2 in generation of morphine-induced antinociceptive tolerance and hyperalgesia by modulating TLR4/NF-κB/NLRP3 in microglia. Toxicol Appl Pharmacol 2021; 416: 115458. |
| 10. | Ouyang H, Zhang J, Chi D, et al. The YTHDF1-TRAF 6 pathway regulates the neuroinflammatory response and contributes to morphine tolerance and hyperalgesia in the periaqueductal gray. J Neuroinflammation 2022; 19: 310. |
| 11. | Zhang B, Wang X, Li S. An integrative platform of TCM network pharmacology and its application on a herbal formula, Qing-Luo-Yin. Evid Based Complement Alternat Med 2013; 2013: 456747. |
| 12. | Li S, Zhang B. Traditional Chinese Medicine network pharmacology: theory, methodology and application. Chin J Nat Med 2013; 11: 110-20. |
| 13. |
Fengtao P, Kesong LI, Yi Z, et al. Efficacy of Lushi Runzao decoction on ameliorating Sjogren's syndrome: a network pharmacology and experimental verification-based study. J Tradit Chin Med 2023; 43: 751-9.
DOI |
| 14. | Shang L, Wang Y, Li J, et al. Mechanism of Sijunzi decoction in the treatment of colorectal cancer based on network pharmacology and experimental validation. J Ethnopharmacol 2023; 302: 115876. |
| 15. | Zhou W, Zhang H, Wang X, et al. Network pharmacology to unveil the mechanism of Moluodan in the treatment of chronic atrophic gastritis. Phytomedicine 2022; 95: 153837. |
| 16. | Zhou W, Yang K, Zeng J, et al. FordNet: recommending Traditional Chinese Medicine formula via deep neural network integrating phenotype and molecule. Pharmacol Res 2021; 173: 105752. |
| 17. |
Shuai H, Hua M, Tingting J, et al. Inhibitory effect of berberine on morphine tolerance and hyperalgesia in mice. J Tradit Chin Med 2023; 43: 915-24.
DOI |
| 18. |
Guan S, Jin TT, Han S, et al. Dihydroartemisinin alleviates morphine-induced neuroinflammation in BV2 cells. Bioengineered 2021; 12: 9401-10.
DOI PMID |
| 19. |
Guan S, Miao F, Wang D, et al. Corilagin attenuates morphine-induced BV2 microglial activation and inflammation via regulating TLR2-mediated endoplasmic reticulum stress. J Toxicol Sci 2023; 48: 387-98.
DOI PMID |
| 20. |
Kaski SW, White AN, Gross JD, et al. Preclinical testing of nalfurafine as an opioid-sparing adjuvant that potentiates analgesia by the mu opioid receptor-targeting agonist morphine. J Pharmacol Exp Ther 2019; 371: 487-99.
DOI PMID |
| 21. | Borrelli EP, Morphis B, Youssef R, et al. Concurrent utilization of prescription opioids and non-opioid controlled substances: rhode island prescription drug monitoring program, 2018. R I Med J 2020; 103: 53-8. |
| 22. | Li X, Li H, Wang T, et al. Network pharmacology-based analysis of the mechanism of Saposhnikovia divaricata for the treatment of type Ⅰ allergy. Pharm Biol 2022; 60: 1224-36. |
| 23. | Zhao W, Shen F, Yao J, et al. Angiotensin Ⅱ receptor type 1 blocker candesartan improves morphine tolerance by reducing morphine-induced inflammatory response and cellular activation of BV2 cells via the PPARγ/AMPK signaling pathway. Mol Med Rep 2022; 26: 318. |
| 24. |
Khorsandi L, Orazizadeh M, Niazvand F, et al. Quercetin induces apoptosis and necroptosis in MCF-7 breast cancer cells. Bratisl Lek Listy 2017; 118: 123-8.
DOI PMID |
| 25. | Qu Y, Li X, Xu F, et al. Kaempferol alleviates murine experimental colitis by restoring gut microbiota and inhibiting the LPS-TLR4-NF-κB axis. Front Immunol 2021; 12: 679897. |
| 26. |
Cicero AF, Baggioni A. Berberine and its role in chronic disease. Adv Exp Med Biol 2016; 928: 27-45.
PMID |
| 27. |
Magdy S, Gamal M, Samir NF, et al. IκB kinase inhibition remodeled connexins, pannexin-1, and excitatory amino-acid transporters expressions to promote neuroprotection of galantamine and morphine. J Cell Physiol 2021; 236: 7516-32.
DOI PMID |
| 28. | Wang W, Ning JZ, He Y, et al. Unveiling the mechanism of Astragalus membranaceus in the treatment of gastrointestinal cancers based on network pharmacology. Eur J Integr Med 2020; 40: 101249. |
| 29. | Suski M, Bujak-Gizycka B, Madej J, et al. Co-administration of dextromethorphan and morphine:reduction of post-operative pain and lack of influence on morphine metabolism. Basic Clin Pharmacol Toxicol 2010; 107: 680-4. |
| 30. |
Säwe J. High-dose morphine and methadone in cancer patients. Clinical pharmacokinetic considerations of oral treatment. Clinical pharmacokinetics 1986; 11: 87-106.
PMID |
| 31. |
Staahl C, Upton R, Foster DJ, et al. Pharmacokinetic-pharmacodynamic modeling of morphine and oxycodone concentrations and analgesic effect in a multimodal experimental pain model. J Clin Pharmacol 2008; 48: 619-31.
DOI PMID |
| 32. |
Zelcer N, van de Wetering K, Hillebrand M, et al. Mice lacking multidrug resistance protein 3 show altered morphine pharmacokinetics and morphine-6-glucuronide antinociception. Proc Natl Acad Sci USA 2005; 102: 7274-9.
DOI PMID |
| 33. | Lim SY, Cengiz P. Opioid tolerance and opioid-induced hyperalgesia: Is TrkB modulation a potential pharmacological solution? Neuropharmacology 2022; 220: 109260. |
| 34. |
Bai L, Zhai C, Han K, et al. Toll-like receptor 4-mediated nuclear factor-κB activation in spinal cord contributes to chronic morphine-induced analgesic tolerance and hyperalgesia in rats. Neurosci Bull 2014; 30: 936-48.
DOI PMID |
| 35. | Oeckinghaus A, Ghosh S. The NF-kappaB family of transcription factors and its regulation. Cold Spring Harb Perspect Biol 2009; 1: a000034. |
| 36. | Ju M, Liu B, He H, et al. MicroRNA-27a alleviates LPS-induced acute lung injury in mice via inhibiting inflammation and apoptosis through modulating TLR4/MyD88/NF-κB pathway. Cell Cycle 2018; 17: 2001-18. |
| 37. |
Shafie A, Moradi F, Izadpanah E, et al. Neuroprotection of donepezil against morphine-induced apoptosis is mediated through toll-like receptors. Eur J Pharmacol 2015; 764: 292-7.
DOI PMID |
| 38. | Zeng XS, Geng WS, Wang ZQ, et al. Morphine addiction and oxidative stress: the potential effects of thioredoxin-1. Front Pharmacol 2020; 11: 82. |
| 39. |
Jang EY, Yang CH, Hedges DM, et al. The role of reactive oxygen species in methamphetamine self-administration and dopamine release in the nucleus accumbens. Addict Biol 2017; 22: 1304-15.
DOI PMID |
| 40. | Skrabalova J, Drastichova Z, Novotny J. Morphine as a potential oxidative stress-causing agent. Mini Rev Org Chem 2013; 10: 367-72. |
| 41. |
Ma J, Yuan X, Qu H, et al. The role of reactive oxygen species in morphine addiction of SH-SY5Y cells. Life Sci 2015; 124: 128-35.
DOI PMID |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||

