Journal of Traditional Chinese Medicine ›› 2025, Vol. 45 ›› Issue (2): 266-271.DOI: 10.19852/j.cnki.jtcm.20240927.001
• Original articles • Previous Articles Next Articles
Effects of Huluan decotion (护卵汤) on cyclophosphamide-induced autoimmune premature ovarian failure in murine models
FENG Guiling1,2( ), ZHOU Xiaolin3, SHEN Chengwan1,2, LI Panxiao4, ABULIZI ·Abudula1,2
), ZHOU Xiaolin3, SHEN Chengwan1,2, LI Panxiao4, ABULIZI ·Abudula1,2
- 1 College of Medicial science, Ningde Normal university, Ningde 352000, China
2 Fujian Key Laboratory of Toxicology and Pharmacotoxicology, Ningde 352000, China
3 Nanyang Medical College, Nanyang 473000, China
4 Department of Intensive care, Ningde Normal College affiliated Ningde Hospital, Ningde 352000, China
-
Received:2024-01-12Accepted:2024-04-08Online:2025-04-15Published:2024-09-27 -
Contact:FENG Guiling, College of Medicial science, Ningde Normal university, Ningde Fujian Province 352000, China; Fujian Key Laboratory of Toxicology and Pharmacotoxicology, Ningde City Fujian Province 352000, China. 1069026322@qq.com, Telephone: +86-13903771390 -
Supported by:Fujian Natural Science Foundation Project: Study on Potential Protein Targets of Huluan Decotion in the Intervention of Premature Ovarian Failure(2021J011173);Major Project Cultivation Plan Project of Ningde Normal University: the Effect of Huluan Decotion on the Decreased Ovarian Reserve Function Induced by Cyclophosphamide is Studied based on Forkhead box L2(2019ZDK06)
Cite this article
FENG Guiling, ZHOU Xiaolin, SHEN Chengwan, LI Panxiao, ABULIZI ·Abudula. Effects of Huluan decotion (护卵汤) on cyclophosphamide-induced autoimmune premature ovarian failure in murine models[J]. Journal of Traditional Chinese Medicine, 2025, 45(2): 266-271.
share this article
| Group | n | FSH (ng/mL) | LH (IU/L) | E2 (pd/mL) | AMH (ng/mL) |
|---|---|---|---|---|---|
| Con | 10 | 12.52±2.55a | 2.86±0.58a | 1166.55±214.55a | 0.28±0.04a |
| Mol | 10 | 31.10±5.28b | 5.02±0.86b | 698.80±156.32b | 0.08±0.03b |
| HLD-L | 10 | 17.36±3.67a | 3.55±0.48c | 704.89±156.04 | 0.13±0.05a |
| HLD-M | 10 | 16.15±4.22a | 3.46±0.56a | 960.10±173.28a | 0.23±0.04a |
| HLD-H | 10 | 11.21±3.76a | 3.13±0.68a | 965.31±148.67a | 0.24±0.04a |
| P | 10 | 20.90±4.16a | 3.48±0.74a | 902.81±187.33a | 0.24±0.06a |
Table 1 Serum levels of FSH, LH, and E2 in each group
| Group | n | FSH (ng/mL) | LH (IU/L) | E2 (pd/mL) | AMH (ng/mL) |
|---|---|---|---|---|---|
| Con | 10 | 12.52±2.55a | 2.86±0.58a | 1166.55±214.55a | 0.28±0.04a |
| Mol | 10 | 31.10±5.28b | 5.02±0.86b | 698.80±156.32b | 0.08±0.03b |
| HLD-L | 10 | 17.36±3.67a | 3.55±0.48c | 704.89±156.04 | 0.13±0.05a |
| HLD-M | 10 | 16.15±4.22a | 3.46±0.56a | 960.10±173.28a | 0.23±0.04a |
| HLD-H | 10 | 11.21±3.76a | 3.13±0.68a | 965.31±148.67a | 0.24±0.04a |
| P | 10 | 20.90±4.16a | 3.48±0.74a | 902.81±187.33a | 0.24±0.06a |
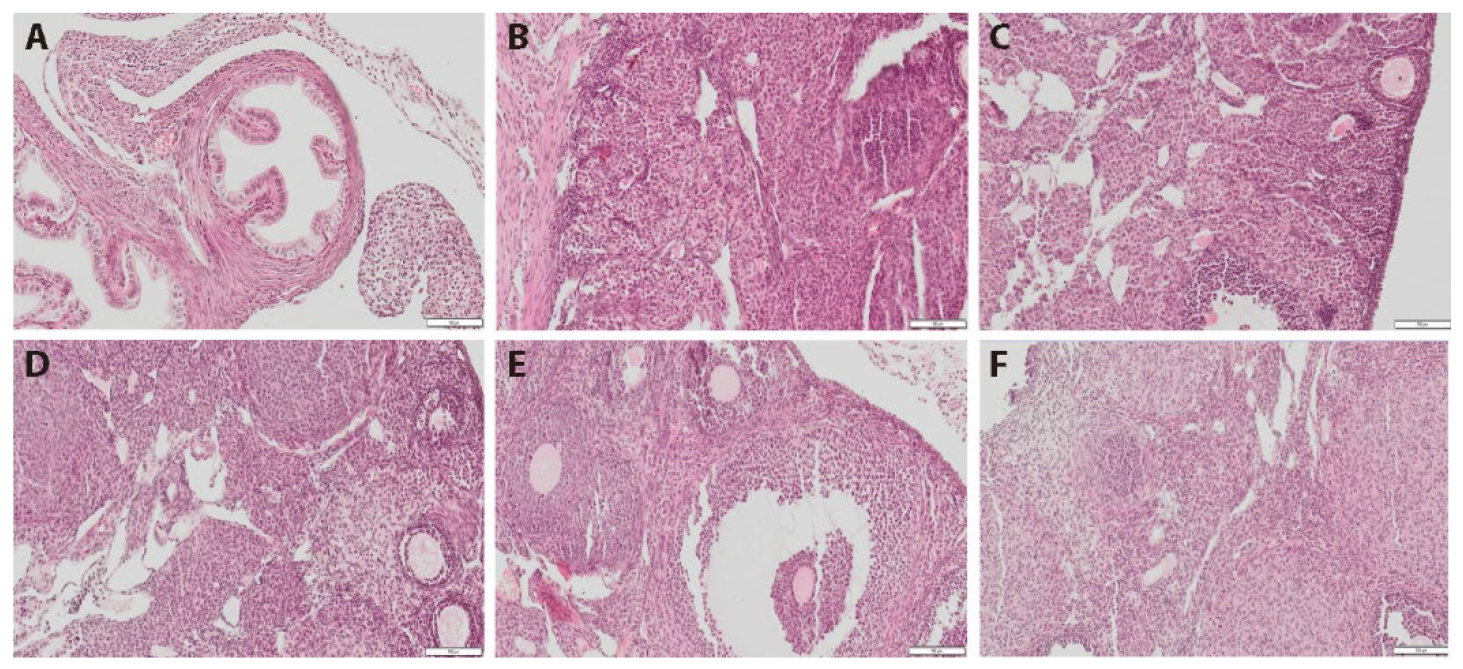
Figure 1 HE stained ovarian sections of each group A: control group; B: model group; C: premarin group; D: low of HLD groups; E: moderate of HLD groups; F: high dose of HLD group. Mice in the model, premarin, and HLD groups were administered a loading dose of CTX (100 mg·kg-1·d-1) followed by daily injections for 2 weeks, and then, positive group treated with premarin (0.03 mg/kg) once daily for 4 weeks. Control and model group received the same volume of distilled water, low (2 g/kg), moderate (5 g/kg, the dose used in vivo in human studies), and high (10 g/kg) dose of HLD groups treated by gastrogavage once daily for 4 weeks. Stained with HE, the numbers of primary and secondary follicles increased significantly compared with those in the model group. HLD: Huluan decotion; CTX: cyclophosphamide; HE: hematoxylin-eosin. Magnification × 200. Scale bar: 200 μm.
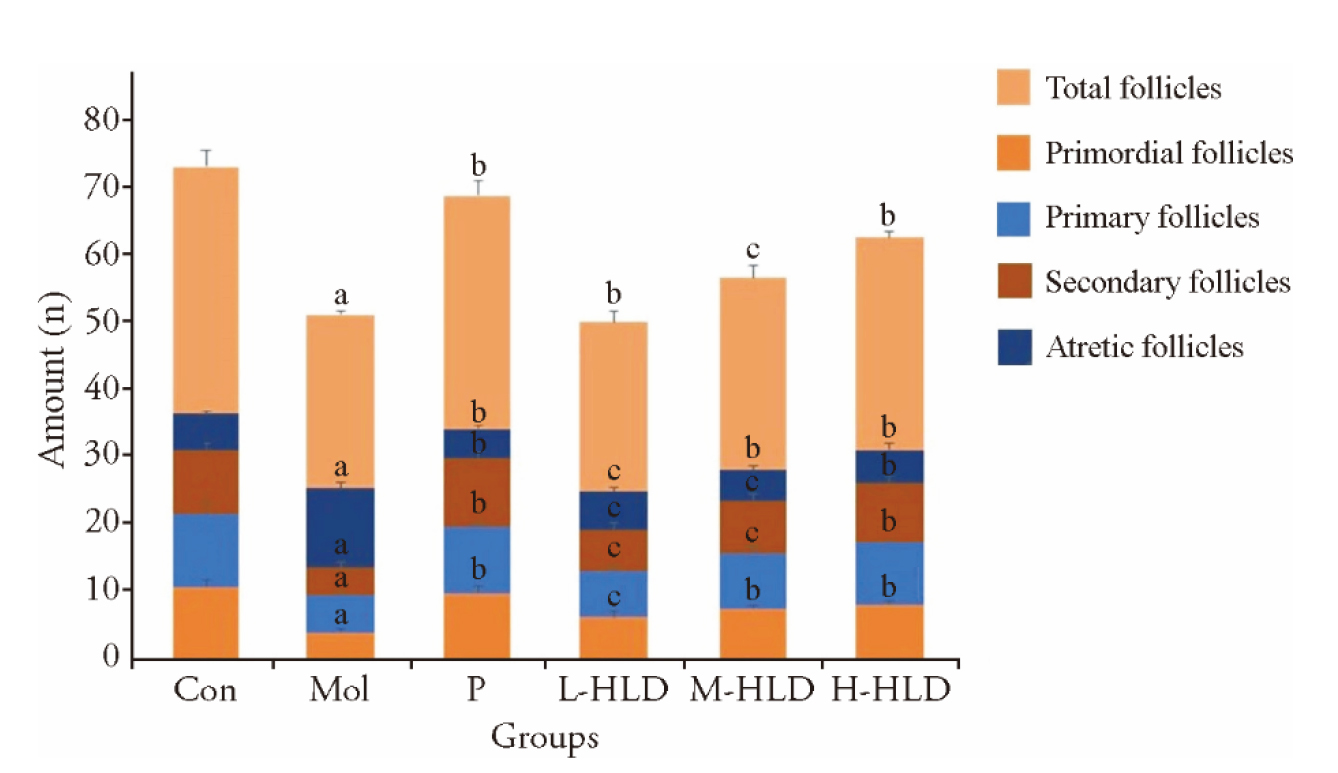
Figure 2 Comparison of the number of follicles at different levels in each group of mice Con: control group; Mol: model group; P: premarin group; L-HLD, M-HLD and H-HLD represent low, moderate, and high dose of Huluan decotion groups, respectively. Mice in the model, premarin, and HLD groups were administered a loading dose of CTX (100 mg·kg-1·d-1) followed by daily injections for 2 weeks, and then, positive group treated with premarin (0.03 mg/kg) once daily for 4 weeks. Control and model group received the same volume of distilled water, low (2 g/kg), moderate (5 g/kg, the dose used in vivo in human studies), and high (10 g/kg) dose of HLD groups treated by gastrogavage once daily for 4 weeks. The numbers of primary and secondary follicles in the Mol group decreased significantly compared with those in the con group, while treatment with HLD or premarin, the numbers of primary and secondary follicles increased significantly compared with those in the model group. HLD: Huluan decotion; CTX: cyclophosphamide. Data are presented as the mean ± standard deviation using one-way analysis of variance (n = 10). aP < 0.01 vs group Control; bP < 0.01 vs group MOL; cP < 0.05 vs group Mol.

Figure 3 Immunohistochemical analysis of the distinct presence of FOXL2 in the oophorons of each group A: control group; B: model group; C: premarin group; D: low dose of HLD group; E: moderate dose of HLD group; F: high dose of HLD group. Mice in the model, premarin, and HLD groups were administered a loading dose of CTX (100 mg·kg-1·d-1) followed by daily injections for 2 weeks, and then, positive group treated with premarin (0.03 mg/kg) once daily for 4 weeks. Control and model group received the same volume of distilled water, low (2 g/kg), moderate (5 g/ kg, the dose used in vivo in human studies), and high (10 g/ kg) dose of HLD groups treated by gastrogavage once daily for 4 weeks. Immunohistochemical staining of ovarian sections unveiled a decrease in FOXL2 expression within the model group, whereas an upsurge was observed in mice treated with HLD-M, HLD-H, and Premarin. HLD: Huluan decotion; CTX: cyclophosphamide; FOXL2: Forkhead Box L2. Magnification × 200. Scale bar: 200 μm.
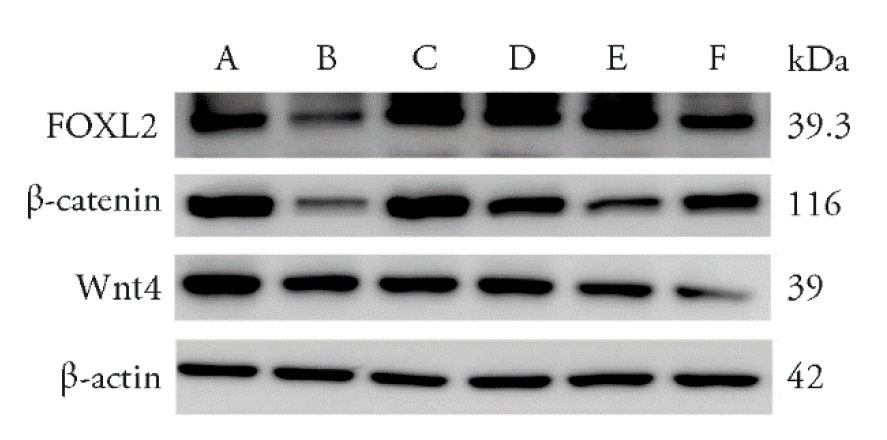
Figure 4 Expression Wnt4, β- catenin and FOXL2 proteins in the ovaries of mice in each group. A: control group; B: model group; C: premarin group; D: low dose of HLD group; E: moderate dose of HLD group; F: high dose of HLD group. Mice in the model, premarin, and HLD groups were administered a loading dose of CTX (100 mg·kg-1·d-1) followed by daily injections for 2 weeks, and then, positive group treated with premarin (0.03 mg/kg) once daily for 4 weeks. Control and model group received the same volume of distilled water, low (2 g/kg), moderate (5 g/kg, the dose used in vivo in human studies), and high (10 g/kg) dose of HLD groups treated by gastrogavage once daily for 4 weeks. Wnt4, β-catenin, and FOXL2 expression were detected by Western blot analysis. Wnt4, β-catenin, and FOXL2 levels in ovarian tissue decrease in the model group compared to the control group, while administration of HLD or Premarin elevated levels of these proteins. HLD: Huluan decotion; CTX: cyclophosphamide; FOXL2: Forkhead Box L2; Wnt4: WNT family member 4.
| 1. | Chon Sj, Umair Z, Yoon Ms. Premature ovarian insufficiency: past, present, and puture. Front Cell Dev Biol 2021; 9: 672890. |
| 2. |
De Vos M, Devroey P, Fauser Bc. Primary ovarian insufficiency. Lancet 2010; 376: 911- 21.
DOI PMID |
| 3. | Zhang Cr, Zhu Wn, Tao W, et al. Moxibustion against cyclophosphamide-induced premature ovarian failure in rats through inhibiting NLRP3-/Caspase-1-/GSDMD-Dependent pyroptosis. Evid Based Complement Alternat Med 2021; 2021: 8874757. |
| 4. |
Bellusci G, Mattiello L, Iannizzotto V, et al. Kinase-independent inhibition of cyclophosphamide-induced pathways protects the ovarian reserve and prolongs fertility. Cell Death Dis 2019; 10: 726.
DOI PMID |
| 5. |
Melekoglu R, Ciftci O, Eraslan S, Cetin A, Basak N. Beneficial effects of curcumin and capsaicin on cyclophosphamide-induced premature ovarian failure in a rat model. J Ovarian Res 2018; 11: 33.
DOI PMID |
| 6. | Maas A. Hormone therapy and cardiovascular disease: Benefits and harms. Best Pract Res Clin Endocrinol Metab 2021; 35: 101576. |
| 7. | Farquhar C, Marjoribanks J, Lethaby A, Suckling JA, Lamberts Q. Long term hormone therapy for perimenopausal and postmenopausal women. Cochrane Database Syst Rev 2017; 104: 93-5. |
| 8. |
Shareghi-Oskoue O, Aghebati-Maleki L, Yousefi M. Transplantation of human umbilical cord mesenchymal stem cells to treat premature ovarian failure. Stem Cell Res Ther 2021; 12: 454.
DOI PMID |
| 9. | Liu X, Song Y, Zhou F, et al. Network and experimental pharmacology on mechanism of Si-Wu-tang improving ovarian function in a mouse model of premature ovarian failure induced by cyclophosphamide. J Ethnopharmacol 2023; 301: 115842. |
| 10. | Zheng S, Ma M, Chen Y, Li M. Effects of quercetin on ovarian function and regulation of the ovarian PI3K/Akt/FoxO3a signalling pathway and oxidative stress in a rat model of cyclophosphamide-induced premature ovarian failure. Basic Clin Pharmacol Toxicol 2022; 130: 240-53. |
| 11. | Guo SJ. Yang Jin's experience of treating infertility and crafts with Shuangbu decoction. Global Tradit Chin Med 2016; 9: 831-3. |
| 12. | Feng GL, Zhang LH, Zhou XL, et al. Efficacy of Bushen Jianpi prescription on autoimmune premature ovarian failure in mice. J Tradit Chin Med 2017; 37: 667-74. |
| 13. | Feng GL, Zhou XL. Effect of Bushen Jianpi recipe on autoimmune premature ovarian failure of mice. J Tradit Chin Med 2016; 57: 71-5. |
| 14. | Zhang XL, Li J, Zhou XL, Chen QL. Effect of Er-Xian decoction on autoimmune premature ovarian failure in mice. J Tradit Chin Med 2021; 41: 725-31. |
| 15. | Wang ZY, Li MZ, Li WJ, Ouyang JF, Gou XJ, Huang Y. Mechanism of action of Daqinjiao decoction in treating cerebral small vessel disease explored using network pharmacology and molecular docking technology. Phytomedicine 2023; 108: 154538. |
| 16. | Myers M, Britt KL, Wreford NG, Ebling FJ, Kerr JB. Methods for quantifying follicular numbers within the mouse ovary. Reproduction 2004; 127: 569-80. |
| 17. | Liu W, Cao JL, Ouyang JF, et al. Chaihu and Longgu Muli decoction demonstrate neuroprotective effects in the rat model of Parkinson's disease with depression by regulating the AMPK/mTOR signaling pathway. Phytomedicine Plus 2023; 3: 100428. |
| 18. | Yang X, Yang L. Current understanding of the genomic abnormities in premature ovarian failure: chance for early diagnosis and management. Front Med (Lausanne) 2023; 10: 1194865. |
| 19. |
Han M, Cheng H, Wang J, et al. Abnormal aggregation of myeloid-derived suppressor cells in a mouse model of cyclophosphamide-induced premature ovarian failure. Gynecol Endocrinol 2019; 35: 985-90.
DOI PMID |
| 20. |
Farhat SA, Jabbari F, Jabbari P, Rezaei N. Targeting signaling pathways involved in primordial follicle growth or dormancy: potential application in prevention of follicular loss and infertility. Expert Opin Biol Ther 2022; 22: 871-81.
DOI PMID |
| 21. |
Zhang C, Xu X. Advancement in the treatment of diminished ovarian reserve by traditional Chinese and Western medicine. Exp Ther Med 2016; 11: 1173-76.
PMID |
| 22. |
Han Y, Wang T, Sun S, Zhai Z, Tang S. Cloning of the promoter region of a human gene, FOXL2, and its regulation by STAT3. Mol Med Rep 2017; 16: 2856-62.
DOI PMID |
| 23. | Chen H, Chen Q, Zhu Y, et al. MAP3K1 Variant causes hyperactivation of Wnt4/β-Catenin/FOXL2 signaling contributing to 46,XY Disorders/Differences of sex development. Front Genet 2022; 13: 736988. |
| 24. |
Gustin SE, Hogg K, Stringer JM, et al. WNT/β-catenin and p27/FOXL2 differentially regulate supporting cell proliferation in the developing ovary. Dev Biol 2016; 412: 250-60.
DOI PMID |
| 25. | Zhang P, Li T, Wu X, et al. Oxidative stress and diabetes: antioxidative strategies. Front Med 2020; 14: 583-600. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||

