Journal of Traditional Chinese Medicine ›› 2025, Vol. 45 ›› Issue (5): 970-978.DOI: 10.19852/j.cnki.jtcm.2025.05.004
• Original Articles • Previous Articles Next Articles
Exploring the therapeutic potential of Morus alba Linne extract in targeting localized adiposity
CHOI You Yeon1, JIN Seong chul1, KIM Mi Hye2, BAEK Hee Kyung1, KIM Dong Hyun1, OH Sung Hyuk1, YANG Woong Mo1( )
)
- 1 Department of Convergence Korean Medical Science, College of Korean Medicine, Kyung Hee University, Seoul 02447, Republic of Korea
2 College of Korean Medicine, Woosuk University, Wanju 555338, Republic of Korea
-
Received:2024-02-12Accepted:2024-06-15Online:2025-10-15Published:2025-09-15 -
Contact:YANG Woong Mo, Department of Convergence Korean Medical Science, College of Korean Medicine, Kyung Hee University, Seoul 02447, Republic of Korea. wmyang@khu.ac.kr, Telephone: +82+82-2-961-2209 -
Supported by:Korea Health Technology R&D Project through the National Research Foundation of Korea, funded by the Korean Government(Project Number: NRF-2019R1I1A2A01063598);Undergraduate Research Program of the College of Korean Medicine, Kyung Hee University, Republic of Korea, in 2023(Project Number: 2023)
Cite this article
CHOI You Yeon, JIN Seong chul, KIM Mi Hye, BAEK Hee Kyung, KIM Dong Hyun, OH Sung Hyuk, YANG Woong Mo. Exploring the therapeutic potential of Morus alba Linne extract in targeting localized adiposity[J]. Journal of Traditional Chinese Medicine, 2025, 45(5): 970-978.
share this article
| Category | Description | P value (< 0.05) | FDR value | Background genes | Common genes |
|---|---|---|---|---|---|
| GO biological process | Cellular response to insulin stimulus | 3.8E-18 | 1.3E-16 | 169 | 31 |
| Regulation of cellular response to insulin stimulus | 4.0E-10 | 6.6E-09 | 72 | 15 | |
| Regulation of insulin secretion | 2.5E-09 | 3.6E-08 | 182 | 21 | |
| Regulation of insulin receptor signalling pathway | 7.9E-09 | 1.1E-07 | 64 | 13 | |
| Insulin receptor signalling pathway | 2.9E-08 | 3.7E-07 | 87 | 14 | |
| KEGG pathways | Insulin signalling pathway | 9.3E-22 | 7.8E-21 | 133 | 32 |
| Regulation of lipolysis in adipocytes | 1.3E-09 | 3.7E-09 | 54 | 13 | |
| Insulin secretion | 1.3E-04 | 2.4E-04 | 82 | 9 |
Table 1 MAB target pathway based on GO process and KEGG 2021 human pathway
| Category | Description | P value (< 0.05) | FDR value | Background genes | Common genes |
|---|---|---|---|---|---|
| GO biological process | Cellular response to insulin stimulus | 3.8E-18 | 1.3E-16 | 169 | 31 |
| Regulation of cellular response to insulin stimulus | 4.0E-10 | 6.6E-09 | 72 | 15 | |
| Regulation of insulin secretion | 2.5E-09 | 3.6E-08 | 182 | 21 | |
| Regulation of insulin receptor signalling pathway | 7.9E-09 | 1.1E-07 | 64 | 13 | |
| Insulin receptor signalling pathway | 2.9E-08 | 3.7E-07 | 87 | 14 | |
| KEGG pathways | Insulin signalling pathway | 9.3E-22 | 7.8E-21 | 133 | 32 |
| Regulation of lipolysis in adipocytes | 1.3E-09 | 3.7E-09 | 54 | 13 | |
| Insulin secretion | 1.3E-04 | 2.4E-04 | 82 | 9 |

Figure 1 Effect of MAB on weight of inguinal fat tissues and the size of adipocytes in high-fat diet-induced obese mice A: weekly body weight changes in HFD-fed mice treated with MAB or saline. HFD: high-fat diet group, HFD + MAB injection: high-fat diet with MAB injection; B: gross morphology of inguinal fat pads after 18 injections of saline or MAB (100 μL per injection). B1: representative image of mouse dissection. White dotted line indicates the area of inguinal fat pads in the saline- and MAB-treated groups. scale bar = 0.5 cm, B2: quantification of inguinal fat weight relative to body weight; C: dual-energy X-ray absorptiometry analysis of body after 18 injections of saline or MAB (100 μL per injection). saline: treated with saline; MAB: treated with MAB. C1: representative dual-energy X-ray absorptiometry images showing fat (red), lean tissue (blue), and bone (white), Scale bar is 0.5 cm, C2: relative fat mass in the inguinal region after 18 injections of saline or MAB; D: histological assessment via hematoxylin and eosin staining visualized the reduction in adipocyte size after 18 injections of saline or MAB. D1: treated with saline, D2: treated with MAB; D3: quantification of average adipocyte diameter in saline- and MAB-treated groups. D4: fat diameter ratio in saline- and MAB-treated groups. Saline: treated with saline; MAB: treated with MAB. MAB: the water extract of Morus alba L. bark; HFD: high-fat diet; ANOVA: analysis of variance. Statistical significance was assessed using one-way ANOVA. Results are presented as mean ± standard deviation (n = 6). aP < 0.001, bP < 0.01, vs saline group.
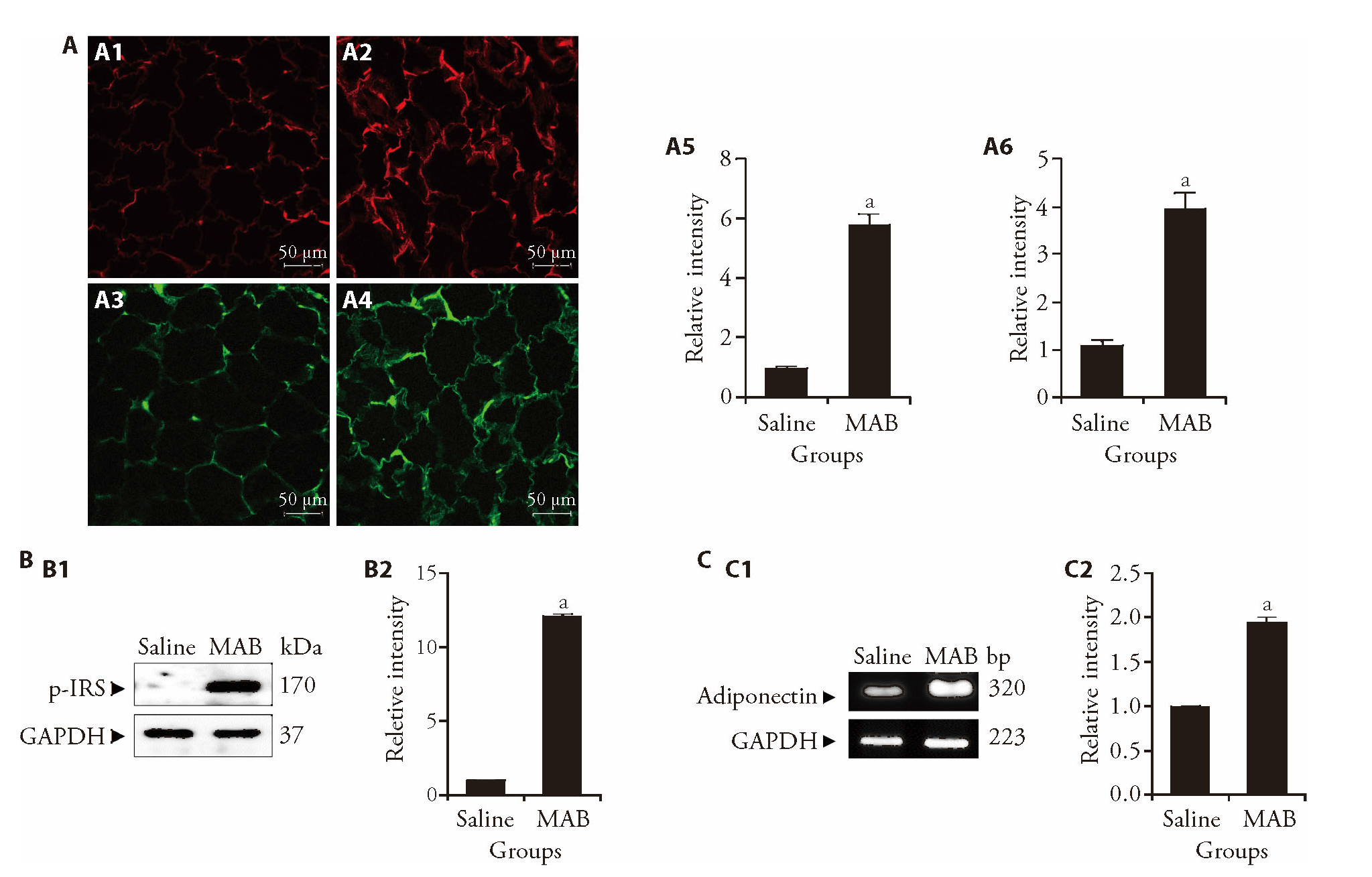
Figure 2 Effect of MAB on the expression of insulin signaling-related mediators in inguinal fat tissues of obese mice A: immunofluorescence detection of GLUT4 (upper panels, red) and phospho-IRS-1 (lower panels, green) in inguinal fat pads after 18 injections of saline or MAB (100 μL per injection). Fluorescence intensity was quantified in arbitrary units. A1: GLUT4 expression in the saline-treated group; A2: GLUT4 expression in the MAB-treated group; A3: phospho-IRS-1 expression in the saline-treated group; A4: phospho-IRS-1 expression in the MAB-treated group. Scale bar = 50 μm. A5: relative fluorescence intensity of GLUT4; A6: relative fluorescence intensity of phospho-IRS-1; B: protein expression of p-IRS-1 levels in inguinal fat pad after 18 injections of saline or MAB (100 μL per injection); B1: protein expression of phospho-IRS-1 detected by Western blot; B2: quantified relative intensity of phospho-IRS-1 normalized to the saline group, which was assigned a value of 1; C: mRNA expression of adiponectin in inguinal fat pad after 18 injections of saline or MAB (100 μL per injection); C1: mRNA expression of adiponectin detected by RT-PCR; C2: quantified relative intensity of adiponectin normalized to the saline group, which was assigned a value of 1. Saline: treated with saline; MAB: treated with MAB. MAB: the water extract of Morus alba L. bark; GLUT4: glucose transporter type 4; p-IRS-1: phosphorylated insulin receptor substrate-1; ANOVA: analysis of variance. Statistical significance was assessed using one-way ANOVA. Results are presented as mean ± standard deviation (n = 6). aP < 0.001, vs saline group.
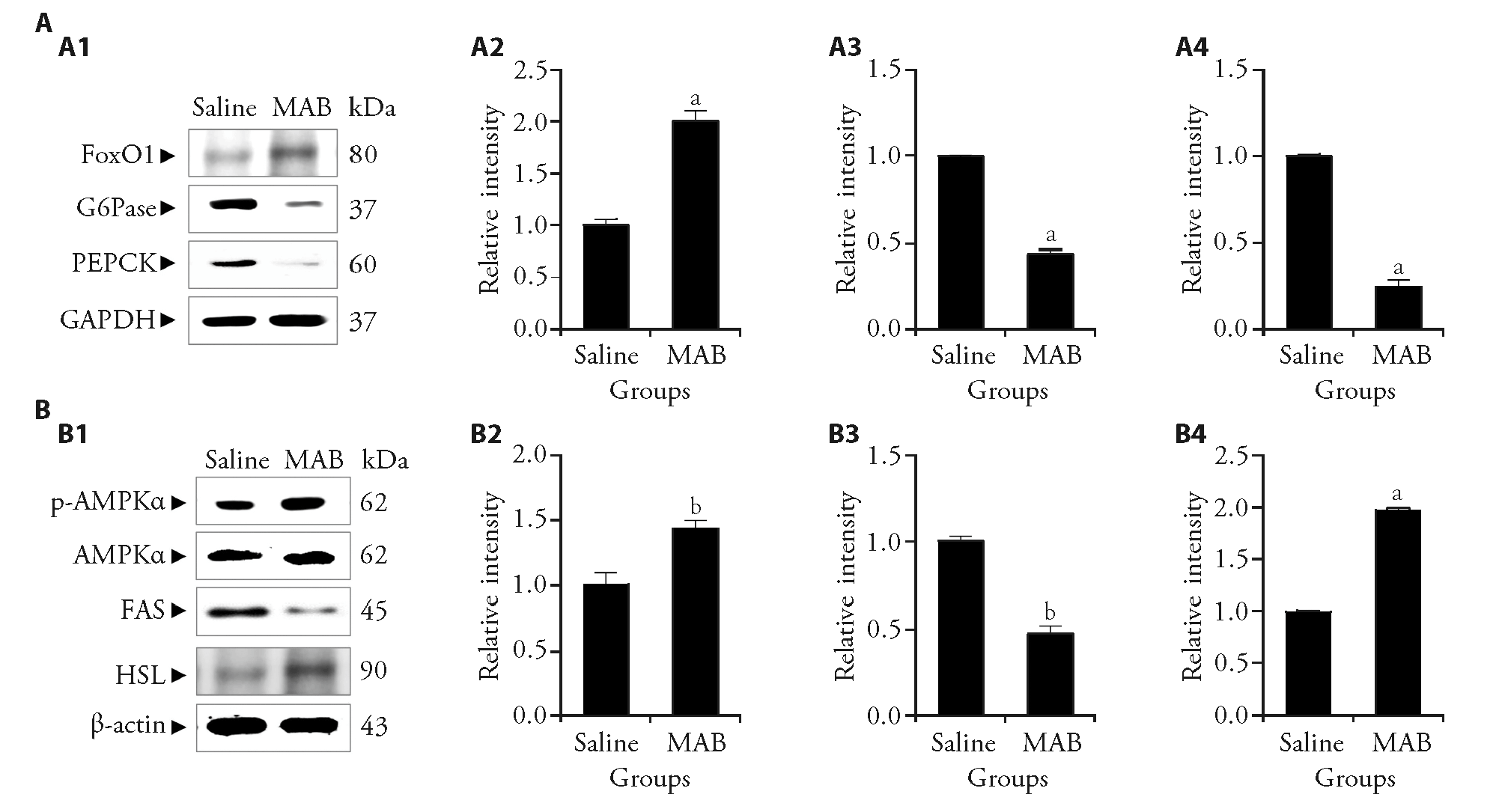
Figure 3 Effect of MAB on the expression of gluconeogenesis-mediators and AMPK-mediated FAS/HSL pathway in inguinal fat tissues of obese mice A: protein expression of gluconeogenesis-related markers in the inguinal fat pad following 18 injections of saline or MAB. A1: Western blot images showing the expression levels of FoxO1, G6Pase, and PEPCK in the inguinal fat pad of saline- and MAB-treated groups. GAPDH was used as a loading control; A2: relative intensity of FoxO1; A3: relative intensity of G6Pase; A4: relative intensity of PEPCK; B: protein expression of lipid metabolism-related markers in the inguinal fat pad following 18 injections of saline or MAB; B1: western blot images showing the expression of p-AMPKα, total AMPKα, FAS, and HSL in the inguinal fat pad; B2: relative intensity of p-AMPKα. AMPKα was used as a loading control; B3: relative intensity of FAS; B4: relative intensity of HSL. β-actin was used as a loading control. Protein expression was quantified as a relative value by normalizing it to the saline group, which was assigned a value of 1. Saline: treated with saline; MAB: treated with MAB. MAB: the water extract of Morus alba L. bark; FoxO1: forkhead box protein O1; G6Pase: glucose-6-phosphatase; PEPCK: phosphoenolpyruvate carboxykinase; GAPDH: glyceraldehyde-3-phosphate dehydrogenase; AMPK: adenosine 5‘-monophosphate-activated protein kinase; p-AMPK: phosphorylated AMPK; FAS: fatty acid synthase; HSL: hormone-sensitive lipase; ANOVA: analysis of variance. Results are presented as mean ± standard deviation (n = 6). Statistical significance was assessed using one-way ANOVA. aP < 0.001, b P < 0.01, vs saline group.
| 1. | Luo L, Liu M. Adipose tissue in control of metabolism. J Endocrinol 2016; 231: R77-99. |
| 2. |
Sbraccia P, D'Adamo M, Guglielmi V. Is type 2 diabetes an adiposity-based metabolic disease? From the origin of insulin resistance to the concept of dysfunctional adipose tissue. Eat Weight Disord 2021; 26: 2429-41.
DOI PMID |
| 3. |
Oikonomou EK, Antoniades C. The role of adipose tissue in cardiovascular health and disease. Nat Rev Cardiol 2019; 16: 83-99.
DOI PMID |
| 4. | Dilworth L, Facey A, Omoruyi F. Diabetes mellitus and its metabolic complications: the role of adipose tissues. Int J Mol Sci 2021; 22. |
| 5. | Gomez-Hernandez A, Beneit N, Diaz-Castroverde S, Escribano O. Differential role of adipose tissues in obesity and related metabolic and vascular complications. Int J Endocrinol 2016; 2016: 1216783. |
| 6. |
Wueest S, Schoenle EJ, Konrad D. Depot-specific differences in adipocyte insulin sensitivity in mice are diet- and function-dependent. Adipocyte 2012; 1: 153-6.
DOI PMID |
| 7. |
Noh Y, Heo CY. The effect of phosphatidylcholine and deoxycholate compound injections to the localized adipose tissue: an experimental study with a murine model. Arch Plast Surg 2012; 39: 452-6.
DOI PMID |
| 8. | Bramante CT, Lee CJ, Gudzune KA. Treatment of obesity in patients with diabetes. Diabetes Spectr 2017; 30: 237-43. |
| 9. | Choi WJ, Kim MH, Park N, Chung JY, Park SJ, Yang WM. Effect and mechanism of Magnolia officinalis pharmacopuncture for treating localized fat via network pharmacology and experimental study. Integr Med Res 2023; 12: 100948. |
| 10. | Lee H, Kim MH, Jin SC, Choi Y, Nam YK, Yang WM. LIPOSA pharmacopuncture, a new herbal formula, affects localized adiposity by regulating lipid metabolism in vivo. Exp Ther Med 2021; 22: 1290. |
| 11. | Tian S, Wang M, Liu C, Zhao H, Zhao B. Mulberry leaf reduces inflammation and insulin resistance in type 2 diabetic mice by TLRs and insulin signalling pathway. BMC Complement Altern Med 2019; 19: 326. |
| 12. |
Li HX, Jo E, Myung CS, Kim YH, Yang SY. Lipolytic effect of compounds isolated from leaves of mulberry (Morus alba L.) in 3T3-L1 adipocytes. Nat Prod Res 2018; 32: 1963-6.
DOI PMID |
| 13. | Chen C, Mohamad Razali UH, Saikim FH, Mahyudin A, Mohd Noor NQI. Morus alba L. plant: bioactive compounds and potential as a functional food ingredient. Foods 2021; 10: 689. |
| 14. |
Lim HJ, Jin HG, Woo ER, Lee SK, Kim HP. The root barks of Morus alba and the flavonoid constituents inhibit airway inflammation. J Ethnopharmacol 2013; 149: 169-75.
DOI PMID |
| 15. | Batiha GE, Al-Snafi AE, Thuwaini MM, et al. Morus alba: a comprehensive phytochemical and pharmacological review. Naunyn Schmiedebergs Arch Pharmacol 2023; 396: 1399-413. |
| 16. | Kim NY, Thomas SS, Hwang DI, Lee JH, Kim KA, Cha YS. Anti-obesity effects of Morus alba L. and aronia melanocarpa in a high-fat diet-induced obese C57BL/6J mouse model. Foods 2021; 10: 1914. |
| 17. | Ha MT, Seong SH, Nguyen TD, et al. Chalcone derivatives from the root bark of Morus alba L. act as inhibitors of PTP1B and alpha-glucosidase. Phytochemistry 2018; 155: 114-25. |
| 18. |
Yimam M, Jiao P, Hong M, et al. Morus alba, a medicinal plant for appetite suppression and weight loss. J Med Food 2019; 22: 741-51.
DOI PMID |
| 19. | Barreau C, Labit E, Guissard C, et al. Regionalization of browning revealed by whole subcutaneous adipose tissue imaging. Obesity (Silver Spring) 2016; 24: 1081-9. |
| 20. | Wondmkun YT. Obesity, insulin resistance, and type 2 diabetes: associations and therapeutic implications. Diabetes Metab Syndr Obes 2020; 13: 3611-6. |
| 21. |
Copps KD, White MF. Regulation of insulin sensitivity by serine/threonine phosphorylation of insulin receptor substrate proteins IRS1 and IRS2. Diabetologia 2012; 55: 2565-82.
DOI PMID |
| 22. | Chadt A, Al-Hasani H. Glucose transporters in adipose tissue, liver, and skeletal muscle in metabolic health and disease. Pflugers Arch 2020; 472: 1273-98. |
| 23. |
Ruan H, Dong LQ. Adiponectin signaling and function in insulin target tissues. J Mol Cell Biol 2016; 8: 101-9.
DOI PMID |
| 24. |
Chakrabarti P, Kandror KV. FoxO 1 controls insulin-dependent adipose triglyceride lipase (ATGL) expression and lipolysis in adipocytes. J Biol Chem 2009; 284: 13296-300.
DOI PMID |
| 25. | Shah AM, Wondisford FE. Tracking the carbons supplying gluconeogenesis. J Biol Chem 2020; 295: 14419-29. |
| 26. |
Reshef L, Hanson RW, Ballard FJ. A possible physiological role for glyceroneogenesis in rat adipose tissue. J Biol Chem 1970; 245: 5979-84.
PMID |
| 27. | Xu H, Lyu X, Guo X, et al. Distinct AMPK-mediated FAS/HSL pathway is implicated in the alleviating effect of nuciferine on obesity and hepatic steatosis in HFD-fed mice. Nutrients 2022; 14: 1898. |
| 28. |
Wueest S, Rapold RA, Schumann DM, et al. Deletion of Fas in adipocytes relieves adipose tissue inflammation and hepatic manifestations of obesity in mice. J Clin Invest 2010; 120: 191-202.
DOI PMID |
| 29. | Althaher AR. An overview of hormone-sensitive lipase (HSL). Sci World J 2022; 2022: 1964684. |
| 30. |
Habegger KM, Hoffman NJ, Ridenour CM, Brozinick JT, Elmendorf JS. AMPK enhances insulin-stimulated GLUT4 regulation via lowering membrane cholesterol. Endocrinology 2012; 153: 2130-41.
DOI PMID |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||

