Journal of Traditional Chinese Medicine ›› 2025, Vol. 45 ›› Issue (5): 1087-1097.DOI: 10.19852/j.cnki.jtcm.2025.05.015
• Original Articles • Previous Articles Next Articles
Therapeutic potential of Traditional Chinese Medicine Yisui Shengxue pills (益髓生血丸) to inhibit hypoxia-inducible factor-1alpha and general control nonderepressible 2 to regulate the post-chemotherapy immune response: integrating network pharmacology and experimental validation
QI Yafeng1, LIU Yu1, LIU Yeyuan1, LI Yangyang1, ZHANG Shangzu1, CHEN Yaping1, XU Qian1, HAO Guoxiong1, LIU Yongqi3, ZHANG Liying2( ), ZHANG Zhiming4(
), ZHANG Zhiming4( )
)
- 1 Clinical School of Traditional Chinese Medicine, Gansu University of Traditional Chinese Medicine, Lanzhou 730000, China
2 School of Integrative Chinese and Western Medicine, Gansu University of Traditional Chinese Medicine, Lanzhou 730000, China
3 School of Basic Medicine, Gansu University of Traditional Chinese Medicine, Lanzhou 730000, China
4 Department of Oncology, Gansu Provincial Hospital of Traditional Chinese Medicine, Lanzhou 730050, China
-
Received:2024-08-22Accepted:2024-12-25Online:2025-10-15Published:2025-09-15 -
Contact:Prof. ZHANG Liying, School of Integrative Chinese and Western Medicine, Gansu University of Traditional Chinese Medicine, Lanzhou 730000, China. zhangliying201212@163.com;
Prof. ZHANG Zhiming, Department of Oncology, Gansu Provincial Hospital of Traditional Chinese Medicine, Lanzhou 730000, China. zhangzhimingys@163.com,Telephone: +86-13669375649; 86-13099267516 -
Supported by:High-end Talents Undertake Provincial Science and Technology Plan Projects(Yangtze River Scholars Award Scheme; Gankeji 2021: reference 20-9);Gansu Province Integrated Traditional Chinese and Western Medicine Tumor Clinical Medical Research Center(Reference: 18JR2FA001)
Cite this article
QI Yafeng, LIU Yu, LIU Yeyuan, LI Yangyang, ZHANG Shangzu, CHEN Yaping, XU Qian, HAO Guoxiong, LIU Yongqi, ZHANG Liying, ZHANG Zhiming. Therapeutic potential of Traditional Chinese Medicine Yisui Shengxue pills (益髓生血丸) to inhibit hypoxia-inducible factor-1alpha and general control nonderepressible 2 to regulate the post-chemotherapy immune response: integrating network pharmacology and experimental validation[J]. Journal of Traditional Chinese Medicine, 2025, 45(5): 1087-1097.
share this article

Figure 1 The target pathways of YSSX for the treatment of post-chemotherapy immunosuppression were predicted by network pharmacology A: the targets of YSSX, targets of post-chemotherapy immunosuppression, and the intersection of active ingredient targets between different drugs; B: Interaction network map of PPI proteins of core targets in the first 60 core targets after immunosuppression following YSSX treatment with chemotherapy, and Cytohubba plugin screening and mapping of the core target network. B1: Interaction network map of PPI proteins of core targets in the first 60 core targets after immunosuppression following YSSX treatment with chemotherapy; B2: Cytohubba plugin screening and mapping of the core target network. C: depict GO functional enrichment analysis; D: depict KEGG pathway enrichment analysis. YSSX: Yishui Shengxue pills; PPI: protein-protein interaction; GO: gene ontology; KEGG: Kyoto Encyclopedia of Genes and Genomes.

Figure 2 Effects of YSSX on the overall health and immunity of mice that were immunosuppressed by different chemotherapy drugs A: changes in the weight of mice in each group; B: spleen thymus indices; B1: spleen indices; B2: thymus indices; C: changes in the peripheral white blood cells, lymphocytes, and neutrophils content of experimental mice; C1: white blood cells; C2: lymphocytes; C3: neutrophils; D: pathological changes in the thymus after HE staining (size: 20 μm); E: pathological changes in the spleen after HE staining (size: 20 μm); F: Pathological changes in the bone marrow after HE staining (size: 20 μm); D1, E1, F1: Ctrl group; D2, E2, F2: CBP group; D3, E3, F3: CBP+YSSX group; D4, E4, F4: 5-Fu group; D5, E5, F5: 5-Fu + YSSX group; D6, E6, F6: PTX group; D7, E7, F7: PTX + YSSX group. Ctrl group: received a normal diet and drinking water without any intervention; CBP group: received CBP intraperitoneally at a dose of 82.3 mg/kg for five consecutive days, followed by 0.2 mL/animal per day of normal saline for seven consecutive days after modelling; CBP + YSSX group: received CBP intraperitoneally at a dose of 82.3 mg/kg for five consecutive days, followed by the intragastric administration of YSSX at a dose of 1.05 g/kg for 7 d after modelling; PTX group: the mice received PTX at a dose of 41.6 mg/kg once every three days for a total of 14 d, and then received the intragastric administration of 0.2 mL/animal per day with normal saline for seven consecutive days; PTX + YSSX group: the mice were treated with PTX at a dose of 41.6 mg/kg once every three days for a total of 14 d, followed by the intragastric administration of YSSX at a dose of 1.05 g/kg for 7 d after modelling; 5-Fu group: the mice received 5-Fu at a dose of 182 mg/kg three times per week for 7 d, and then normal saline was administered by oral gavage at a dose of 0.2 mL/animal per day for 7 d after modelling; 5-Fu + YSSX group: the mice were treated with 5-Fu at a dose of 182 mg/kg three times per week for 7 d, followed by the intragastric administration of YSSX at a dose of 1.05 g/kg for 7 d after modeling. Ctrl: blank group; CBP: carboplatin group; 5-Fu: fluorouracil group; PTX: paclitaxel; YSSX: Yishui Shengxue pills; WBC: white blood cell; HE: hematoxylin-eosin. Statistical significance was assessed using one-way analysis of variance. All data were expressed as the mean ± standard deviation (n = 3). Compared with the blank group, aP < 0.05; compared with the CBP, bP < 0.05; compared with the 5-Fu group, cP < 0.05; compared with the PTX group dP < 0.05.

Figure 3 The expression of key targets and blood immunity indicators were detected by WB and qRT-PCR A: protein and gene transcription of HIF-1α, iNOS, GCN2, eIF2α, and ATF4 protein in MDSCs from the thymus, spleen, and bone marrow; B: mRNA expression of HIF-1α, GCN2 and eIF2α in MDSCs in the thymus; C: mRNA expression of HIF-1α, GCN2 and eIF2α in MDSCs in the spleen; D: mRNA expression of HIF-1α, GCN2 and eIF2α in MDSCs in the bone marrow; B1, C1, D1: HIF-1α; B2, C2, D2: GCN2; B3, C3, D3: eIF2α. Ctrl group: received a normal diet and drinking water without any intervention; CBP group: received CBP intraperitoneally at a dose of 82.3 mg/kg for five consecutive days, followed by 0.2 mL/animal per day of normal saline for seven consecutive days after modelling; CBP + YSSX group: received CBP intraperitoneally at a dose of 82.3 mg/kg for five consecutive days, followed by the intragastric administration of YSSX at a dose of 1.05 g/kg for 7 d after modelling; PTX group: the mice received PTX at a dose of 41.6 mg/kg once every three days for a total of 14 d, and then received the intragastric administration of 0.2 mL/animal per day with normal saline for seven consecutive days; PTX + YSSX group: the mice were treated with PTX at a dose of 41.6 mg/kg once every three days for a total of 14 d, followed by the intragastric administration of YSSX at a dose of 1.05 g/kg for 7 d after modelling; 5-Fu group: the mice received 5-Fu at a dose of 182 mg/kg three times per week for 7 d, and then normal saline was administered by oral gavage at a dose of 0.2 mL/animal per day for 7 d after modelling; 5-Fu + YSSX group: the mice were treated with 5-Fu at a dose of 182 mg/kg three times per week for 7 d, followed by the intragastric administration of YSSX at a dose of 1.05 g/kg for 7 d after modeling. Ctrl: blank group; CBP: carboplatin group; 5-Fu: fluorouracil group; PTX: paclitaxel; YSSX: Yishui Shengxue pills; HIF-1α: hypoxia-inducible factor 1-alpha; iNOS: inducible nitric oxide synthase; eIF2α: eukaryotic initiation factor 2 alpha; GCN2: general control nonderepressible 2; ATF4: activating transcription factor 4; WB: Western blotting; qRT-PCR: real-time quantitative reverse transcription-polymerase chain reaction; MDSCs: myeloid-derived suppressor cells. Statistical significance was assessed using one-way analysis of variance. All data were expressed as the mean ± standard deviation (n = 3). Compared with the blank group, aP < 0.05; compared with the CBP group, bP < 0.05; compared with the 5-Fu group, cP < 0.05; compared with the PTX group, dP < 0.05.
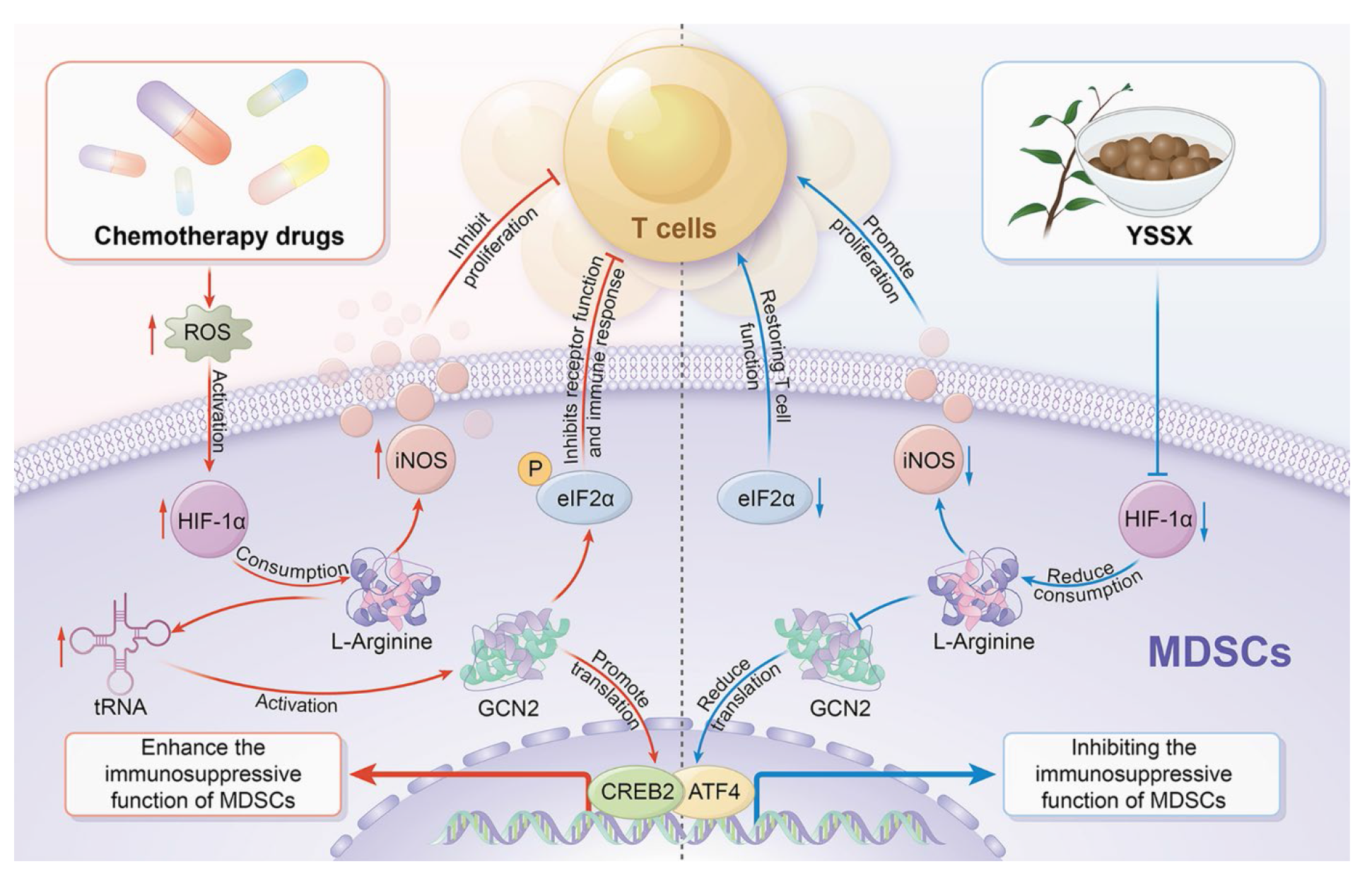
Figure 4 YSSX improves the modulation of MDSCs-mediated immunosuppression after chemotherapy Chemotherapeutic agents triggered ROS accumulation, recruiting and activating MDSCs, whose high expression of HIF-1α promoted L-arginine depletion, followed by the release of iNOS, affecting T cell proliferation. Meanwhile, GCN2 senses L-arginine depletion activation and promotes eIF2α phosphorylation, which in turn inhibits T cell immune response, and in turn promotes increased translation of CREB2/ATF4, enhancing MDSCs activity (left). After YSSX intervention, HIF-1α transcription was blocked, and then L-arginine depletion was reduced, iNOS production was decreased, and T-cell proliferation was enhanced. reduced L-arginine depletion, GCN2 activity was reduced, and eIF2α phosphorylation was inhibited, which promoted T-cells to perform their normal immune functions; at the same time, due to the reduction in GCN2 expression mediating the reduction of CREB2/ATF4 translation, the activity of MDSCs was also inhibited (right). YSSX: Yishui Shengxue pills; ATF4: activating transcription factor 4; eIF2α: eukaryotic translation initiation factor 2α; iNOS: inducible nitric oxide synthase; HIF-1α: hypoxia-inducible factor-1α; GCN2: general control nonderepressible 2; GCN2: general control nonderepressible 2; CREB2: cAMP response element-binding protein 2; ATF4: activating transcription factor 4. This image was created by Adobe Illustr (San Jose, CA, USA).
| 1. | Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics. CA Cancer J Clin 2023; 73: 17-48. |
| 2. |
Wilson BE, Jacob S, Yap ML, Ferlay J, Bray F, Barton MB. Estimates of global chemotherapy demands and corresponding physician workforce requirements for 2018 and 2040: a population-based study. Lancet Oncol 2019; 20: 769-80.
DOI PMID |
| 3. | Walsh RJ, Soo RA. Resistance to immune checkpoint inhibitors in non-small cell lung cancer: biomarkers and therapeutic strategies. Ther Adv Med Oncol 2020; 12: 1758835920937902. |
| 4. | Gao X, Sui H, Zhao S, Gao X, Su Y, Qu P. Immunotherapy targeting myeloid-derived suppressor cells (MDSCs) in tumor microenvironment. Front Immunol 2021; 11: 585214. |
| 5. | Philip M, Schietinger A. CD8+ T cell differentiation and dysfunction in cancer. Nat Rev Immunol 2022; 22: 209-23. |
| 6. | Semenza GL. Intratumoral hypoxia and mechanisms of immune evasion mediated by hypoxia-inducible factors. Physiology (Bethesda) 2021; 36: 73-83. |
| 7. |
Nakamura H, Makino Y, Okamoto K, et al. TCR engagement increases hypoxia-inducible factor-1 alpha protein synthesis via rapamycin-sensitive pathway under hypoxic conditions in human peripheral T cells. J Immunol 2005; 174: 7592-9.
DOI PMID |
| 8. | Halaby MJ, Hezaveh K, Lamorte S, et al. GCN2 drives macrophage and MDSC function and immunosuppression in the tumor microenvironment. Sci Immunol 2019; 4: eaax8189. |
| 9. | Zhang ZM, Wang X. A Traditional Chinese Medicine composition for treating myelosuppression leukopenia after chemotherapy. China patent CN110151965B. 2021 Sep 21. |
| 10. | Zhang ZM, Wang X. Packing box (YSSX). China patent CN305558663S. 2020 Jan 17. |
| 11. | Zhu Z, Feng YD, Zou YL, et al. Integrating serum pharmacochemistry, network pharmacology and untargeted metabolomics strategies to reveal the material basis and mechanism of action of Feining keli in the treatment of chronic bronchitis. J Ethnopharmacol 2024; 335: 118643. |
| 12. | Chee NT, Carriere CH, Miller Z, Welford S, Brothers SP. Activating transcription factor 4 regulates hypoxia inducible factor 1α in chronic hypoxia in pancreatic cancer cells. Oncol Rep 2023; 49: 14. |
| 13. |
Fletcher M, Ramirez ME, Sierra RA, et al. l-Arginine depletion blunts antitumor T-cell responses by inducing myeloid-derived suppressor cells. Cancer Res 2015; 75: 275-83.
DOI PMID |
| 14. |
Ramji DP, Foka P. CCAAT/enhancer-binding proteins: structure, function and regulation. Biochem J 2002; 365: 561-75.
DOI PMID |
| 15. | Ba H, Jiang R, Zhang M, et al. Suppression of transmembrane tumor necrosis factor alpha processing by a specific antibody protects against colitis-associated cancer. Front Immunol 2021; 12: 687874. |
| 16. | Jiang ZJ, Cai HF, Yuan C, et al. Spore Oil enhances the effect of cyclophosphamide inhibiting programmed death-1 and prolongs the survival of H22 tumor-bearing mice. J Tradit Chin Med 2024; 44: 652-9. |
| 17. |
Manepalli S, Gandhi JA, Ekhar VV, Asplund MB, Coelho C, Martinez LR. Characterization of a cyclophosphamide-induced murine model of immunosuppression to study Acinetobacter baumannii pathogenesis. J Med Microbiol 2013; 62: 1747-54.
DOI PMID |
| 18. | WU JY, Li H, Zhang XY, Gullen E, Chong G, Wang J. Efficacy of Yisui granule on myelodysplastic syndromes in SKM-1 mouse xenograft model through suppressing Wnt/β-catenin signaling pathway. J Tradit Chin Med 2024; 44: 78-87. |
| 19. |
Wigmore PM, Mustafa S, El-Beltagy M, Lyons L, Umka J, Bennett G. Effects of 5-FU. Adv Exp Med Biol 2010; 678: 157-64.
PMID |
| 20. | Eckert MA, Orozco C, Xiao J, Javellana M, Lengyel E. The effects of chemotherapeutics on the ovarian cancer microenvironment. Cancers (Basel) 2021; 13: 3136. |
| 21. | Chi D, Berchuck A, Dizon DS, Yashar CM. Principles and practice of gynecologic oncology. Alphen aan den Rijn: Wolters Kluwer, 2017: 174-82. |
| 22. |
Cui Z, Zhang J, Zhang J, Xu L. Evaluation of IgG, IgM, CD4+ and CD8+ T cells during neoadjuvant chemotherapy with Tezio and Apatinib in gastric cancer patients. Cell Mol Biol (Noisy-le-grand) 2020; 66: 113-8.
PMID |
| 23. | Kang K, Deng X, Xie W, Chen J, Lin H, Chen Z. Rhodotorula mucilaginosa ZTHY2 attenuates cyclophosphamide-induced immunosuppression in mice. Animals (Basel) 2023; 13: 3376. |
| 24. | Tcyganov EN, Hanabuchi S, Hashimoto A, et al. Distinct mechanisms govern populations of myeloid-derived suppressor cells in chronic viral infection and cancer. J Clin Invest 2021; 131: e145971. |
| 25. | Han J, Fei X, Sun N, Xing J, Cai E, Yang L. Effect of Ligustri Lucidi Fructus on myelosuppression in mice induced by cytoxan. Biomed Chromatogr 2023; 37: e5524. |
| 26. | Miyamoto T, Murakami R, Hamanishi J, et al. B7-H3 suppresses antitumor immunity via the CCL2-CCR2-M2 macrophage axis and contributes to ovarian cancer progression. Cancer Immunol Res 2022; 10: 56-69. |
| 27. |
Zhao Y, Shen XF, Cao K, et al. Dexamethasone-induced myeloid-derived suppressor cells prolong allo cardiac graft survival through iNOS- and glucocorticoid receptor-dependent mechanism. Front Immunol 2018; 9: 282.
DOI PMID |
| [1] | PEI Ke, LI Yong, LIN Zhe, LYU Guangfu. Mechanisms of Baishao (Radix Paeoniae Alba) and Gancao (Radix Glycyrrhizae) on major depressive disorder: network pharmacology and in vivo validation [J]. Journal of Traditional Chinese Medicine, 2025, 45(5): 1067-1077. |
| [2] | ZHANG Wei, REN Changhong, GAO Chen, XU Jun, WU Xiaodan, YANG Yong. Systematic understanding of mechanism of Shenfu decoction (参附汤) improve the prognosis of ischemic stroke using a network pharmacology and animal experiment approach [J]. Journal of Traditional Chinese Medicine, 2025, 45(5): 1078-1086. |
| [3] | HAO Shulan, NAN Peng, LIU Likun, LI Xiaoli, ZHONG Qiming, GAO Yu, WANG Xixing, NIE Yingfang. Effectiveness of Yiqi Chupi powder (益气除疲散) for alleviating cancer-related fatigue in patients following colorectal cancer surgery: a randomized controlled trial [J]. Journal of Traditional Chinese Medicine, 2025, 45(5): 1119-1126. |
| [4] | CHOI You Yeon, JIN Seong chul, KIM Mi Hye, BAEK Hee Kyung, KIM Dong Hyun, OH Sung Hyuk, YANG Woong Mo. Exploring the therapeutic potential of Morus alba Linne extract in targeting localized adiposity [J]. Journal of Traditional Chinese Medicine, 2025, 45(5): 970-978. |
| [5] | SONG Mingming, MEN Bo, CHEN Mei, LIU Rui, MO Hongping, ZHANG Da, PAN Tao, WEN Xudong. Exploration of the mechanism of Danggui Buxue decoction (当归补血汤) for the treatment of gastric ulcer based on network pharmacology, molecular docking, and in vivo experiment [J]. Journal of Traditional Chinese Medicine, 2025, 45(4): 806-816. |
| [6] | LI Yue, DENG Jinyan, PI Shanshan, ZHANG Yingjuan, ZHAO Dan, GUO Yi, YE Yong’an, ZAO Xiaobin, DU Hongbo. Weifuchun (胃复春 ) exerts therapeutic effects on gastric fundic gland polyps by promoting ferroptosis [J]. Journal of Traditional Chinese Medicine, 2025, 45(3): 618-627. |
| [7] | HUANG Jiaen, LUO Qing, DONG Gengting, PENG Weiwen, HE Jianhong, DAI Weibo. Xiahuo Pingwei San (夏藿平胃散) attenuated intestinal inflammation in dextran sulfate sodium-induced ulcerative colitis mice through inhibiting the receptor for advanced glycation end-products signaling pathway [J]. Journal of Traditional Chinese Medicine, 2025, 45(2): 311-325. |
| [8] | HAN Shuai, Du Zhikang, WANG Zirui, HUANG Tianfeng, GE Yali, SHI Jianwen, GAO Ju. Network pharmacology approach to unveiling the mechanism of berberine in the amelioration of morphine tolerance [J]. Journal of Traditional Chinese Medicine, 2025, 45(2): 376-384. |
| [9] | TAN Xiying, GU Ruxin, TAO Jing, ZHANG Yu, SUN RuiQian, YIN Gang, ZHANG Shuo, TANG Decai. Integrating network pharmacology and experimental validation to uncover the synergistic effects of Huangqi (Radix Astragali Mongolici)-Ezhu (Rhizoma Curcumae Phaeocaulis) with 5-fluorouracil in colorectal cancer models [J]. Journal of Traditional Chinese Medicine, 2025, 45(2): 385-398. |
| [10] | SHI Jinyu, PAN Fuwei, GE Haiya, YANG Zongrui, ZHAN Hongsheng. Mechanism of Qigu capsule (芪骨胶囊) as a treatment for sarcopenia based on network pharmacology and experimental validation [J]. Journal of Traditional Chinese Medicine, 2025, 45(2): 399-407. |
| [11] | HU Huiming, WENG Jiajun, TANG Fangrui, WANG Yaqi, FAN Shengxian, WANG Xuecheng, CUI Can, SHAO Feng, ZHU Yanchen. Hypolipidemic effect and mechanism of Hedan tablet (荷丹片) based on network pharmacology [J]. Journal of Traditional Chinese Medicine, 2025, 45(2): 408-421. |
| [12] | YUAN Jianan, CHENG Kunming, LI Chao, ZHANG Xiang, DING Zeyu, LI Bing, ZHENG Yongqiu. Atractylenolide I ameliorates post-infectious irritable bowel syndrome by inhibiting the polymerase I and transcript release factor and c-Jun N-terminal kinase/inducible nitric oxide synthase pathway [J]. Journal of Traditional Chinese Medicine, 2025, 45(1): 57-65. |
| [13] | YAN Kai, WANG Wei, WANG Yan, GAO Huijuan, FENG Xingzhong. Network pharmacology-based study on the mechanism of Tangfukang formula (糖复康方) against type 2 diabetes mellitus [J]. Journal of Traditional Chinese Medicine, 2025, 45(1): 76-88. |
| [14] | ZHU Peixuan, SU Zeqi, FAN Qiongyin, ZHANG Cai, WANG Ting. Network pharmacology and animal experiments revealed the protective effects of Guilong prescription (归龙方) on chronic prostatitis and its possible mechanisms [J]. Journal of Traditional Chinese Medicine, 2025, 45(1): 89-99. |
| [15] | ZHANG Wantong, YI Danhui, LU Fang, YANG Qiaoning, SHI Shuai, LI Qiuyan, WENG Weiliang, WANG Xujie, ZHU Baochen. Screening optimal target populations with symptomatic bradyarrhythmia for pharmacotherapy: a discriminant analysis pilot study [J]. Journal of Traditional Chinese Medicine, 2025, 45(1): 160-166. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||

