Journal of Traditional Chinese Medicine ›› 2025, Vol. 45 ›› Issue (2): 385-398.DOI: 10.19852/j.cnki.jtcm.2025.02.004
• Original articles • Previous Articles Next Articles
Integrating network pharmacology and experimental validation to uncover the synergistic effects of Huangqi (Radix Astragali Mongolici)-Ezhu (Rhizoma Curcumae Phaeocaulis) with 5-fluorouracil in colorectal cancer models
TAN Xiying1, GU Ruxin2,3, TAO Jing4, ZHANG Yu3, SUN RuiQian5, YIN Gang5, ZHANG Shuo6, TANG Decai5( )
)
- 1 Department of Pharmacy, Affiliated Hospital of Nanjing University of Chinese Medicine, Nanjing 210029, China
2 Department of Pain Management, Affiliated Drum Tower Hospital, Medical School of Nanjing University, Nanjing, 210008, China
3 School of Pharmacy, Nanjing Medical University, Nanjing 211166, China
4 School of Basic Medicine and Clinical Pharmacy, China Pharmaceutical University, Nanjing 210009, China
5 School of Integrated Chinese and Western Medicine, Nanjing University of Chinese Medicine, Nanjing 210023, China
6 Department of Pharmacy, Nantong Hospital of Traditional Chinese Medicine, Nantong, 226001, China
-
Received:2023-12-22Accepted:2024-05-15Online:2025-04-15Published:2025-03-10 -
Contact:TANG Decai, School of Integrated Chinese and Western Medicine, Nanjing University of Chinese Medicine, Nanjing 210023, China. talknow@njucm.edu.cn, Telephone: +86-13813929742 -
About author:TAN Xiying and GU Ruxin are co-first authors and contributed equally to this work -
Supported by:National Natural Science Foundation of China Youth Found Project: Anti-Colorectal Cancer Metastasis Mechanism of Huangqi (Radix Astragali Mongolici)-Ezhu (Rhizoma Curcumae Phaeocaulis) According to the Hypoxia-Inducible Factor 2 Alpha/β-Catenin Cross-Talk Which Influence the Colon Tumor Stem Cells in Hypoxia Microenvironment(82003961);National Natural Science Foundation of China Youth Found Project: the Mechanism of the Compatibility of Huangqi (Radix Astragali Mongolici) and Ezhu (Rhizoma Curcumae Phaeocaulis) on the Early Metastasis of Hepatocellular Carcinoma Mediated by Cancer Associated Fibroblasts(82104408);Science Foundation of China Project: Involvement of Hypoxia Inducible Factor-1α Signal in Huangqi (Radix Astragali Mongolici)-Ezhu (Rhizoma Curcumae Phaeocaulis) Combination Induced Remolding of Tumor Hypoxic Microenvironment(82074035);Science and Technology Development Project of Traditional Chinese Medicine in Jiangsu Province: Mechanism of Huangqi (Radix Astragali Mongolici)-Ezhu (Rhizoma Curcumae Phaeocaulis) Herb Pair on the Inhibition of Colon Cancer Metastasis Through the Wnt/β-catenin Pathway(YB201921)
Cite this article
TAN Xiying, GU Ruxin, TAO Jing, ZHANG Yu, SUN RuiQian, YIN Gang, ZHANG Shuo, TANG Decai. Integrating network pharmacology and experimental validation to uncover the synergistic effects of Huangqi (Radix Astragali Mongolici)-Ezhu (Rhizoma Curcumae Phaeocaulis) with 5-fluorouracil in colorectal cancer models[J]. Journal of Traditional Chinese Medicine, 2025, 45(2): 385-398.
share this article

Figure 1 HQEZ significantly increased sensitivity of colorectal cancer to 5-FU in vivo and in vitro A: measure of tumor volume; B: increase of tumor volume (n = 5-7); C: at the end of the animal experiment, the tumors were collected for photograph. C1: tumors from mice of control group; C2: tumors from mice of 5-FU group; C3: tumors from HQEZ group, C4: tumors from combined group 5-FU combined with HQEZ group (n = 5-7). And the final weight of tumors from every group (C5). D: in EdU incorporation experiment, HQEZ significantly enhanced the inhibition of 5-FU on the growth of HCT116 by immunofluorescence staining. D1: EdU signaling from control group; D2: DAPI which stains the nucleus of control group; D3: merge image of D1 and D2; D4: EdU signaling from 5-FU (0.18 μg/mL, 48 h) treated group; D5: DAPI which stains the nucleus of 5-FU treated group; D6: merge image of D4 and D5, D7: EdU signaling from 5-FU (0.18 μg/mL, 48 h) combined with HQEZ (30 mg/mL, 48 h) group; D8: DAPI which stains the nucleus of 5-FU (0.18 μg/mL, 48 h) combined with HQEZ (30 mg/mL, 48 h) group; D9: merge image of D7 and D8 (n = 5, repeated 3 times); D10: the rate of EdU positive cells. HQEZ: Huangqi (Radix Astragali Mongolici)-Ezhu (Rhizoma Curcumae Phaeocaulis); 5-FU: 5-Fluorouracil; i.p.: intraperitoneal; p.o.: peros; EdU: 5-Ethynyl-2’- deoxyuridine. Control group (PBS, i.p. every two days and p.o. daily), 5-FU group (30 mg/kg every two days, i.p.), HQEZ group (10 g·kg-1·d-1, p.o.), combined group (5-FU, 30 mg/kg every two days, i.p.; HQEZ, 10 g·kg-1·d-1, p.o.). Data are presented as mean ± standard deviation. aP < 0.05 and bP < 0.001, compared with the Control group. cP < 0.001, compared with the 5-FU group, differences were evaluated by one-way analysis of variance, bar = 50 μm.
| Cluster | Score | Nodes | Edges | Gene Symbol |
|---|---|---|---|---|
| 1 | 5.429 | 8 | 19 | MMP9, MPO, TLR9, MMP2, KDR, IGF1R, TLR4, PPARG |
| 2 | 4.8 | 6 | 12 | TYMS, AKT1, ESR2, SRC, PTGS2, EP300 |
| 3 | 3.5 | 5 | 7 | TOP2A, DHFR, DPYD, ABCC1, TOP1 |
| 4 | 3.5 | 5 | 7 | ABCB1, MTHFR, SLC19A1, EGFR, ABCG2 |
Table 1 Cluster information of the protein-protein interaction network
| Cluster | Score | Nodes | Edges | Gene Symbol |
|---|---|---|---|---|
| 1 | 5.429 | 8 | 19 | MMP9, MPO, TLR9, MMP2, KDR, IGF1R, TLR4, PPARG |
| 2 | 4.8 | 6 | 12 | TYMS, AKT1, ESR2, SRC, PTGS2, EP300 |
| 3 | 3.5 | 5 | 7 | TOP2A, DHFR, DPYD, ABCC1, TOP1 |
| 4 | 3.5 | 5 | 7 | ABCB1, MTHFR, SLC19A1, EGFR, ABCG2 |
| Active ingredient | Target name | PDB ID | Binding energy (kcal/mol) |
|---|---|---|---|
| Curcumin | EGFR | 1M17 | -2.16 |
| Folate | TYMS | 1JU6 | -1.98 |
| PTGS2 | 5IKR | -1.1 | |
| Isorhamnetin | ABCB1 | 6QEX | -1.6 |
| AKT1 | 6HHI | -3.59 | |
| Kaempferol | EGFR | 1M17 | -2.06 |
| ABCB1 | 6QEX | -3.05 | |
| PTGS2 | 5IKR | -3.92 | |
| AKT1 | 6HHI | -5.01 | |
| Quercetin | EGFR | 1M17 | -2.44 |
| ABCB1 | 6QEX | -1.34 | |
| AKT1 | 6HHI | -3.72 |
Table 2 Cluster information of the protein-protein interaction network
| Active ingredient | Target name | PDB ID | Binding energy (kcal/mol) |
|---|---|---|---|
| Curcumin | EGFR | 1M17 | -2.16 |
| Folate | TYMS | 1JU6 | -1.98 |
| PTGS2 | 5IKR | -1.1 | |
| Isorhamnetin | ABCB1 | 6QEX | -1.6 |
| AKT1 | 6HHI | -3.59 | |
| Kaempferol | EGFR | 1M17 | -2.06 |
| ABCB1 | 6QEX | -3.05 | |
| PTGS2 | 5IKR | -3.92 | |
| AKT1 | 6HHI | -5.01 | |
| Quercetin | EGFR | 1M17 | -2.44 |
| ABCB1 | 6QEX | -1.34 | |
| AKT1 | 6HHI | -3.72 |

Figure 2 Administration of HQEZ inhibited hub targets and FOXO pathway in HCT116 in vitro A: in CCK8 assay, HQEZ only significantly inhibited the proliferation of HCT116 in a relatively high concentration (n = 3). B: after 48 h, the influence of different concentration of HQEZ on the mRNA levels of target genes according to network pharmacology were evaluated by qPCR assay. B1: the influence of TYMS; B2: the influence of MDR1; B3: the influence of Bcl-2; B4: the influence of EGFR. C: after 48 hours, the protein levels of Hub targets from network pharmacology were evaluated by Western-blot assay and the bands of were showed. (n = 3). D: after 48 h, the protein levels of Hub targets from network pharmacology were evaluated by western-blot assay which were administrated with HQEZ dose-dependently. D1: the protein levels of BNIP3 were downregulated; D2: the protein levels of PEPCK were not significantly influenced; D3: the protein levels of CCND1 were not significantly influenced; D4: the protein levels of Catalase were significantly downregulated (n = 3). HCT116 cells were treated with different concentrations of HQEZ including 0, 5, 10, 20 and 40 mg/mL for 48 h. TYMS: thymidylate synthetase; MDR1: multidrug resistance protein 1; Bcl-2: B-cell lymphoma-2; EGFR: epidermal growth factor receptor; BNIP3: BCL2/Adenovirus E1B 19 kDa Protein-Interacting Protein 3; PEPCK: phosphoenolpyruvate carboxykinase 2; CCND1: Cyclin D1. Data are presented as mean ± standard deviation. aP < 0.05, bP < 0.01 and cP < 0.001, compared with the control group, differences were evaluated by one-way analysis of variance.
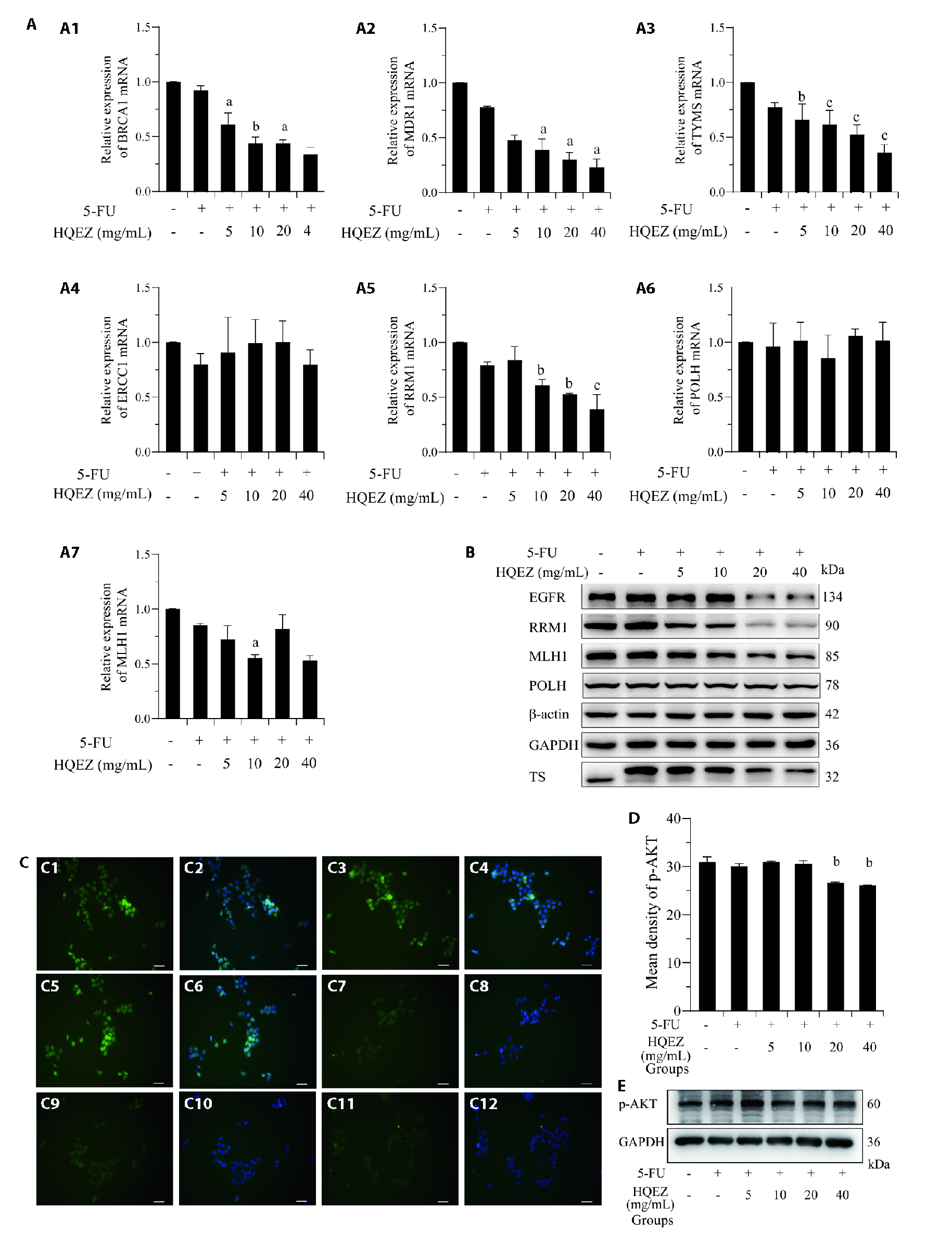
Figure 3 The combination therapy of HQEZ with 5-FU significantly down-regulated 5-FU resistance targets in HCT116 in vitro A: the influence of 5-FU (0.18 μg/mL) combined with different concentration of HQEZ on the transcription level of target genes in HCT116 cells after 48 h by qPCR assay. A1: BRCA1; A2: MDR1; A3: TYMS; A4: ERCC1; A5: RRM1; A6: POLH; A7: co MLH1; (n = 3). B: HQEZ decreased expression of protein from 5-FU resistance-related genes by western blot assay (n = 3). C: by immunofluorescence staining, combined treatment of 5-FU (0.18 μg/mL) and different concentration of HQEZ significantly increased the inhibition of p-AKT in HCT116 cells after 48 h. (n = 3). C1: p-AKT signaling from control group; C2: p-AKT merged with DAPI signaling from control group; C3: p-AKT signaling from 5-FU group; C4: p-AKT merged with DAPI signaling from 5-FU group; C5: p-AKT signaling from 5-FU combined HQEZ group; C6: p-AKT merged with DAPI signaling from 5-FU combined HQEZ (5 mg/mL) group; C7: p-AKT signaling from 5-FU combined HQEZ (10 mg/mL) group; C8: p-AKT merged with DAPI signaling from 5-FU combined HQEZ (10 mg/mL) group; C9: p-AKT signaling from 5-FU combined HQEZ (20 mg/mL) group; C10: p-AKT merged with DAPI signaling from 5-FU combined HQEZ (20 mg/mL) group; C11: p-AKT signaling from 5-FU combined HQEZ (40 mg/mL) group; C12: p-AKT merged with DAPI signaling from 5-FU combined HQEZ (40 mg/mL) group. D: the immunofluorescence density of p-AKT of C. E: the downregulation of p-AKT was also confirmed by western-blot. The concentration of 5-FU is 0.18 μg/mL and the cells were treated for 48 h. HCT116 cells were treated with 5-FU (0.18 μg/mL) and different concentrations of HQEZ, including 0 mg/mL, 5 mg/mL, 10 mg/mL, 20 mg/mL and 40 mg/mL for 48 h. HQEZ: Huangqi (Radix Astragali Mongolici)-Ezhu (Rhizoma Curcumae Phaeocaulis); 5-FU: 5-Fluorouracil; BRCA1: Breast and Ovarian Cancer Susceptibility Protein 1; MDR1: multidrug resistance protein 1; TYMS: thymidylate synthetase; ERCC1: Excision Repair Cross-Complementation Group 1; RRM1: ribonucleotide reductase catalytic subunit M1; POLH: DNA Polymerase Eta; MLH1: MutL Homolog 1; EGFR: epidermal growth factor receptor; GAPDH: glyceraldehyde-3-phosphate dehydrogenase; TS: thymidylate synthetase; p-AKT: phospho- protein kinase B; DAPI: 4',6-Diamidino-2-phenylindole dilactate. Data are presented as mean ± standard deviation. aP < 0.05, bP < 0.01 and cP < 0.001, compared with the Control group, differences were evaluated by one-way analysis of variance, bar = 20 μm.

Figure 4 Combination administration of HQEZ inhibited drug resistance targets relating to 5-FU in vivo and important candidate targets are upregulated in colorectal cancer patients according to TCGA database A: western-blot analysis of expression of genes in tumors from tumor-bearing mice after treatments; B: results of western-blot analysis of expression of genes in tumors from tumor-bearing mice after treatments; B1: MDR1 was downregulated in the combined group; B2: MLH1 was downregulated in the combined group; B3: EGFR was downregulated in the combined group; B4: RRM1 was downregulated in the combined group; B5: POLH was downregulated in the combined group; B6: p-AKT was downregulated in the combined group; B7: p53 was downregulated in the combined group; B8: Bcl-2 was downregulated in the combined group; B9: TYMS was downregulated in the combined group (n = 3). C: qPCR analysis of expression of genes in tumors from tumor-bearing mice after treatments; C1: Bcl-2 was downregulated in the combined group; C2: MDR1 was downregulated in the combined group; C3: EGFR was downregulated in the combined group; C4: RRM1 was downregulated in the combined group; C5: MLH1 was downregulated in the combined group; C6: POLH was downregulated in the combined group; C7: TYMS was downregulated in the combined group (n = 3). D: gene expressions in colorectal cancer patients according to TCGA database; D1: upregulated expressions of MDR1; D2: upregulated expressions of POLH; D3: upregulated expressions of TYMS; D4: upregulated expressions of RRM1; D5: upregulated expressions of MLH1. Mice were treated with phosphate-buffered saline (PBS, i.p. every two days and p.o. daily), 5-FU alone (30 mg/kg every two days, i.p. HQEZ alone (10 g·kg-1·d-1, p.o.) and 5-FU (30 mg/kg every two days, i.p.) combined with HQEZ (10 g·kg-1·d-1, p.o.). HQEZ: Huangqi (Radix Astragali Mongolici)-Ezhu (Rhizoma Curcumae Phaeocaulis); 5-FU: 5-Fluorouracil; MDR1: multidrug resistance protein 1; TYMS: thymidylate synthetase; RRM1: ribonucleotide reductase catalytic subunit M1; POLH: DNA Polymerase Eta; MLH1: MutL Homolog 1; p-AKT: phospho- protein kinase B; Bcl-2: B-cell lymphoma-2; p53: Tumor Suppressor P53. Data are presented as mean ± standard deviation. aP < 0.05, dP < 0.01 and eP < 0.001, compared with the control group. cP < 0.05 and bP < 0.01, compared with the 5-FU group. fP < 0.01 and gP < 0.001, compared with the normal group, differences were evaluated by one-way analysis of variance.
| 1. |
Dekker E, Tanis PJ, Vleugels JLA, Kasi PM, Wallace MB. Colorectal cancer. Lancet 2019; 394: 1467-80.
DOI PMID |
| 2. | Lichtenstern CR, Ngu RK, Shalapour S, Karin M. Immunotherapy, inflammation and colorectal cancer. Cells 2020; 9: 618. |
| 3. | Li J, Yuan Y, Yang F, et al. Expert consensus on multidisciplinary therapy of colorectal cancer with lung metastases (2019 edition). J Hematol Oncol 2019; 12: 16. |
| 4. |
Ganesh K, Stadler ZK, Cercek A, et al. Immunotherapy in colorectal cancer: rationale, challenges and potential. Nat Rev Gastroenterol Hepatol 2019; 16: 361-75.
DOI PMID |
| 5. | Liang T, Tong W, Ma S, Chang P. Standard therapies: solutions for improving therapeutic effects of immune checkpoint inhibitors on colorectal cancer. Oncoimmunology 2020; 9: 1773205. |
| 6. | Wensink E, Bond M, Kucukkose E, et al. A review of the sensitivity of metastatic colorectal cancer patients with deficient mismatch repair to standard-of-care chemotherapy and monoclonal antibodies, with recommendations for future research. Cancer Treat Rev 2021; 95: 102174. |
| 7. | Vodenkova S, Buchler T, Cervena K, et al. 5-fluorouracil and other fluoropyrimidines in colorectal cancer: past, present and future. Pharmacol Ther 2020; 206: 107447. |
| 8. |
Marques RP, Duarte GS, Sterrantino C, et al. Triplet (FOLFOXIRI) versus doublet (FOLFOX or FOLFIRI) backbone chemotherapy as first-line treatment of metastatic colorectal cancer: a systematic review and Meta-analysis. Crit Rev Oncol Hematol 2017; 118: 54-62.
DOI PMID |
| 9. | Glimelius B, Stintzing S, Marshall J, Yoshino T, de Gramont A. Metastatic colorectal cancer: advances in the folate-fluoropyrimidine chemotherapy backbone. Cancer Treat Rev 2021; 98: 102218. |
| 10. | Blondy S, David V, Verdier M, Mathonnet M, Perraud A, Christou N. 5-Fluorouracil resistance mechanisms in colorectal cancer: from classical pathways to promising processes. Cancer Sci 2020; 111: 3142-54. |
| 11. | Azwar S, Seow HF, Abdullah M, Faisal Jabar M, Mohtarrudin N. Recent updates on mechanisms of resistance to 5-Fluorouracil and reversal strategies in colon cancer treatment. Biology (Basel) 2021; 10: 854. |
| 12. |
Johnson JJ, Mukhtar H. Curcumin for chemoprevention of colon cancer. Cancer Lett 2007; 255: 170-81.
DOI PMID |
| 13. |
Chen M, May BH, Zhou IW, Xue CC, Zhang AL. FOLFOX 4 combined with herbal medicine for advanced colorectal cancer: a systematic review. Phytother Res 2014; 28: 976-91.
DOI PMID |
| 14. | Ong SKL, Shanmugam MK, Fan L, et al. Focus on formononetin: anticancer potential and molecular targets. Cancers (Basel) 2019; 11: 611. |
| 15. | Chen P, Li X, Zhang R, et al. Combinative treatment of beta-elemene and cetuximab is sensitive to KRAS mutant colorectal cancer cells by inducing ferroptosis and inhibiting epithelial-mesenchymal transformation. Theranostics 2020; 10: 5107-19. |
| 16. | Hashem S, Nisar S, Sageena G, et al. Therapeutic effects of curcumol in several diseases; an overview. Nutr Cancer 2021; 73: 181-95. |
| 17 |
Cheng X, Lu Y. A review of capecitabine-based adjuvant therapy for gastric cancer in the Chinese population. Future Oncol 2018; 14: 771-9.
DOI PMID |
| 18. |
Hopkins AL. Network pharmacology. Nat Biotechnol 2007; 25: 1110-11.
DOI PMID |
| 19. |
Shannon P, Markiel A, Ozier O, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res 2003; 13: 2498-504.
DOI PMID |
| 20. |
Yu G, Wang LG, Han Y, He QY. ClusterProfiler: an R package for comparing biological themes among gene clusters. OMICS 2012; 16: 284-7.
DOI PMID |
| 21. |
Forli S, Huey R, Pique ME, Sanner MF, Goodsell DS, Olson AJ. Computational protein-ligand docking and virtual drug screening with the AutoDock suite. Nat Protoc 2016; 11: 905-19.
DOI PMID |
| 22. | Sun RL, Tang DC, Gu JF. Study on intervention effect of Astragali Radix-Curcumae Rhizoma on growth and metastasis of colon cancer in orthotopic transplantation mice model of colon cancer. Zhong Guo Zhong Yao Za Zhi 2021; 46: 2267-75. |
| 23. | Ianevski A, Giri AK, Aittokallio T. SynergyFinder 2.0: visual analytics of multi-drug combination synergies. Nucleic Acids Res 2020; 48: W488-93. |
| 24. | Tan X, Xu M, Liu F, et al. Antimetastasis Effect of Astragalus membranaceus-Curcuma zedoaria via beta-catenin mediated CXCR4 and EMT signaling pathway in HCT116. Evid Based Complement Alternat Med 2019; 2019: 9692350. |
| 25. | Liu Y, Wang Y, Li X, et al. FOXO3a in cancer drug resistance. Cancer Lett 2022; 540: 215724. |
| 26. |
Prossomariti A, Piazzi G, Alquati C, Ricciardiello L. Are Wnt/beta-Catenin and PI3K/AKT/mTORC1 distinct pathways in colorectal cancer? Cell Mol Gastroenterol Hepatol 2020; 10: 491-506.
DOI PMID |
| 27. | Lou JS, Yao P, Tsim KWK. Cancer treatment by using Traditional Chinese Medicine: probing active compounds in anti-multidrug resistance during drug therapy. Curr Med Chem 2018; 25: 5128-41. |
| 28. |
Wang K, Chen Q, Shao Y, et al. Anticancer activities of TCM and their active components against tumor metastasis. Biomed Pharmacother 2021; 133: 111044.
DOI PMID |
| 29. | Pricci M, Girardi B, Giorgio F, et al. Curcumin and colorectal cancer: from basic to clinical evidences. Int J Mol Sci 2020; 21: 2364. |
| 30. | Reyes-Farias M, Carrasco-Pozo C. The anti-cancer effect of quercetin: molecular implications in cancer metabolism. Int J Mol Sci 2019; 20: 3177. |
| 31. |
Ashrafizadeh M, Tavakol S, Ahmadi Z, Roomiani S, Mohammadinejad R, Samarghandian S. Therapeutic effects of kaempferol affecting autophagy and endoplasmic reticulum stress. Phytother Res 2020; 34: 911-23.
DOI PMID |
| 32. | Gao Q, Li XX, Xu YM, et al. IRE1alpha-targeting downregulates ABC transporters and overcomes drug resistance of colon cancer cells. Cancer Lett 2020; 476: 67-74. |
| 33. | Morano F, Corallo S, Lonardi S, et al. Negative hyperselection of patients with RAS and BRAF wild-type metastatic colorectal cancer who received Panitumumab-based maintenance therapy. J Clin Oncol 2019; 37: 3099-110. |
| 34. |
Koumarianou A, Tzeveleki I, Mekras D, et al. Prognostic markers in early-stage colorectal cancer: significance of TYMS mRNA expression. Anticancer Res 2014; 34: 4949-62.
PMID |
| 35. |
He Y, Gao M, Tang H, Cao Y, Liu S, Tao Y. Metabolic intermediates in tumorigenesis and progression. Int J Biol Sci 2019; 15: 1187-99.
DOI PMID |
| 36. | Cernigliaro C, D'Anneo A, Carlisi D, et al. Ethanol-mediated stress promotes autophagic survival and aggressiveness of colon cancer cells via activation of Nrf2/HO-1 pathway. Cancers (Basel) 2019; 11: 505. |
| 37. |
LaRue H, Ayari C, Bergeron A, Fradet Y. Toll-like receptors in urothelial cells--targets for cancer immunotherapy. Nat Rev Urol 2013; 10: 537-45.
DOI PMID |
| 38. |
Karthika C, Hari B, Rahman MH, et al. Multiple strategies with the synergistic approach for addressing colorectal cancer. Biomed Pharmacother 2021; 140: 111704.
DOI PMID |
| 39. | Xie P, Mo JL, Liu JH, et al. Pharmacogenomics of 5-fluorouracil in colorectal cancer: review and update. Cell Oncol (Dordr) 2020; 43: 989-1001. |
| 40. |
Lam SW, Guchelaar HJ, Boven E. The role of pharmacogenetics in capecitabine efficacy and toxicity. Cancer Treat Rev 2016; 50: 9-22.
DOI PMID |
| 41. |
Nagarajan S, Bedi U, Budida A, et al. BRD4 promotes p63 and GRHL3 expression downstream of FOXO in mammary epithelial cells. Nucleic Acids Res 2017; 45: 3130-45.
DOI PMID |
| 42. | Planchard D. Adjuvant osimertinib in EGFR-mutated non-small-cell lung cancer. N Engl J Med 2020; 383: 1780-82. |
| 43. |
Gelsomino F, Barbolini M, Spallanzani A, Pugliese G, Cascinu S. The evolving role of microsatellite instability in colorectal cancer: a review. Cancer Treat Rev 2016; 51: 19-26.
DOI PMID |
| 44. | Lin S, Yue J, Guan X, et al. Polymorphisms of MTHFR and TYMS predict capecitabine-induced hand-foot syndrome in patients with metastatic breast cancer. Cancer Commun (Lond) 2019; 39: 57. |
| 45. | Yu KD, Ye FG, He M, et al. Effect of adjuvant paclitaxel and carboplatin on survival in women with triple-negative breast cancer: a phase 3 randomized clinical trial. JAMA Oncol 2020; 6: 1390-96. |
| 46. | Ghafouri-Fard S, Abak A, et al. 5-Fluorouracil: a narrative review on the role of regulatory mechanisms in driving resistance to this chemotherapeutic agent. Front Oncol 2021; 11: 658636. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||

