Journal of Traditional Chinese Medicine ›› 2023, Vol. 43 ›› Issue (4): 667-675.DOI: 10.19852/j.cnki.jtcm.20230428.001
Previous Articles Next Articles
Qizhi Jiangtang capsule (芪蛭降糖胶囊) activates podocyte autophagy in diabetic kidney disease by inhibiting phosphatidylinositol 3-kinase/protein kinase B/mammalian target of rapamycin pathways
GUO Zhaoan1( ), SUN Lina2(
), SUN Lina2( ), LIU Yingying1, LI Ruifeng3, LIU Chong3, DIAO Ke4, SHI Jing3, SUN Jun3
), LIU Yingying1, LI Ruifeng3, LIU Chong3, DIAO Ke4, SHI Jing3, SUN Jun3
- 1 Department of Nephrology, Affiliated Hospital of Shandong University of Traditional Chinese Medicine, Jinan 250011, China
2 Department of Nephrology, Linyi People’s Hospital, Linyi 276003, China
3 Shandong University of Traditional Chinese Medicine, Jinan 250355, China
4 Shandong GuoxinYiyang Group Zibo Hospital, Zibo 255051, China
-
Received:2022-01-02Accepted:2022-07-27Online:2023-08-15Published:2023-04-28 -
Contact:SUN Lina, Department of Nephrology, Linyi People’s Hospital, Linyi 276003, China. hualuohuakai3@163.com. Telephone: +86-18763794562
GUO Zhaoan, Department of Nephrology, Affiliated Hospital of Shandong University of Traditional Chinese Medicine, Jinan 250011, China. gza63@163.com -
Supported by:National Natural Science Foundation of China Project: Experimental Research on Podocyte Autophagy of Diabetic Nephropathy Regulated by Qizhi Jiangtang Capusul(81874440);Natural Science Foundation of Shandong Province Project: Curcumin Ameliorates Diabetic Nephropathy via Regulating the Intestinal Barrier-Inflammation “cross-talk”(ZR2020QH063)
Cite this article
GUO Zhaoan, SUN Lina, LIU Yingying, LI Ruifeng, LIU Chong, DIAO Ke, SHI Jing, SUN Jun. Qizhi Jiangtang capsule (芪蛭降糖胶囊) activates podocyte autophagy in diabetic kidney disease by inhibiting phosphatidylinositol 3-kinase/protein kinase B/mammalian target of rapamycin pathways[J]. Journal of Traditional Chinese Medicine, 2023, 43(4): 667-675.
share this article
| Group | n | Urine protein (mg/24 h) | Scr(μmol/L) | BUN (mmol/L) |
|---|---|---|---|---|
| db/m | 10 | 80.5±11.9 | 52.3±10.3 | 5.6±1.4 |
| db/db | 10 | 153.7±11.2a | 131.8±13.2a | 12.4±1.2a |
| db/db+RAP | 10 | 126.6±9.5b | 89.4±12.8b | 7.5±1.1b |
| db/db+QZJT | 10 | 129.7±6.1b | 79.2±16.8b | 8.3±1.2b |
Table 1 Effects of QZJT on Urine protein, Scr and BUN in db/db mice ($\bar{x}±s$)
| Group | n | Urine protein (mg/24 h) | Scr(μmol/L) | BUN (mmol/L) |
|---|---|---|---|---|
| db/m | 10 | 80.5±11.9 | 52.3±10.3 | 5.6±1.4 |
| db/db | 10 | 153.7±11.2a | 131.8±13.2a | 12.4±1.2a |
| db/db+RAP | 10 | 126.6±9.5b | 89.4±12.8b | 7.5±1.1b |
| db/db+QZJT | 10 | 129.7±6.1b | 79.2±16.8b | 8.3±1.2b |
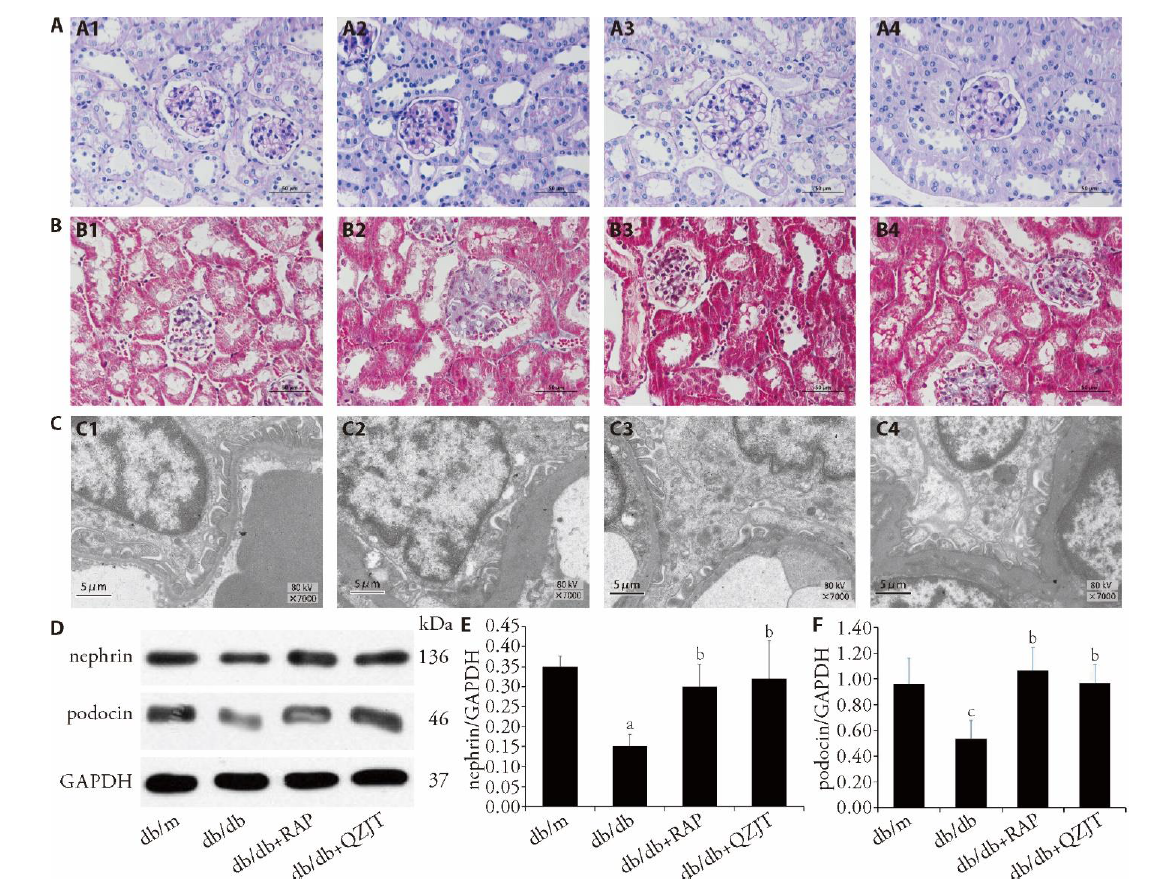
Figure 1 QZJT improves pathological damage and podocyte injury in db/db mice Renal tissues were observed by staining with PAS (A1-A4), and Masson (B1-B4) and photographed by a light microscope (× 400 magnification) in db/m, db/db, db/db + RAP, db/db + QZJT group, respectively. C1-C4: representative fields of podocyte foot processes under electron microscopy (scale bars: 5 μm) in db/m, db/db, db/db+RAP, db/db + QZJT group, respectively. D: Western blot of nephrin and podocin expression in the four groups. E: relative band intensities were used in order to quantify nephrin expression levels. F: relative band intensities were used in order to quantify podocin protein expression levels. Eight-week-old db/db mice were treated with or without QZJT or rapamycin. Db/m (control group group, n = 10), db/db (diabetic kidney disease group, n = 10), db/db+RAP (rapamycin (5 mg·kg-1·d-1), n = 10), db/db + QZJT group (QZJT 3.9 g/kg, n = 10). QZJT: Qizhi Jiangtang capsule; RAP: rapamycin; GAPDH: glyceraldehyde-3-phosphate dehydrogenase; PAS: periodic acid-Schiff; Masson: Masson's trichrome staining; EM: electron microscopy. Student t-test and one-way analysis of variance were used for comparison analysis. aP < 0.01, the db/db group vs the db/m group, bP < 0.05, the db/db group vs the db/db + RAP group or db/db + QZJT group, cP < 0.05, the db/db group vs the db/m group.

Figure 2 QZJT treatment induces podocyte autophagy in db/db mice A: autophagic vesicles (autophagosomes, arrows) of podocytes in the four groups were detected by transmission electron microscopy. Scale bar: 1 μm; B: the number of autophagic vesicles was detected by transmission electron microscopy in 10-15 randomly selected electron micrographs from the four groups; C, D: Western blot of LC3I, LC3II, beclin-1 expression in db/m, db/db, db/db + RAP, db/db + QZJT. E: quantitative analysis of LC3II/LC3I protein levels. Eight-week-old db/db mice were treated with or without QZJT or rapamycin. F: quantitative analysis of beclin-1 protein levels.Eight-week-old db/db mice were treated with or without QZJT or rapamycin. Db/m (control group group, n = 10), db/db (diabetic kidney disease group, n = 10), db/db + RAP (rapamycin (5 mg·kg-1·d-1), n = 10), db/db + QZJT group (QZJT 3.9 g/kg, n = 10). QZJT: Qizhi Jiangtang capsule; HG: high glucose; NG: normal glucose; RAP: rapamycin; LC3: light chain 3; AV: autophagic vacuole. Student t-test and one-way analysis of variance were used for comparison analysis. aP < 0.05, bP < 0.01, the db/db group vs the db/m group; cP < 0.05, the db/db group vs the db/db + RAP group or db/db + QZJT group.

Figure 3 QZJT treatment plays a protective role in podocytes exposed to HG by regulating autophagy A: representative western blotting images of nephrin and synaptopodin in NG, HG, HG + QZJT, HG + QZJT + 3MA groups; B: representative Western blotting images of LC3 II, LC3 I and beclin-1 in the four groups; C: quantitative analysis of nephrin expression levels; D: quantitative analysis of synaptopodin expression levels; E: quantitative analysis of LC3II/LC3I protein levels; F: quantitative analysis of beclin-1 protein levels; G1: autophagic vesicles (autophagosomes, arrows) of podocytes in NG group were detected by transmission electron microscopy; G2: autophagic vesicles (autophagosomes, arrows) of podocytes in HG group were detected by transmission electron microscopy; G3: autophagic vesicles (autophagosomes, arrows) of podocytes in HG + QZJT group were detected by transmission electron microscopy; G4: autophagic vesicles (autophagosomes, arrows) of podocytes in HG + QZJT + 3MA group were detected by transmission electron microscopy; H: the number of autophagic vesicles was detected by transmission electron microscopy in 10-15 randomly selected electron micrographs from each group. The differentiated podocytes were incubated with normal glucose (NG, 5.5 mM) or high glucose (HG, 35 mM). The cells in the QZJT groups were preconditioned with 10% drug-containing serum for 2 h, then cultured in high glucose for 48 h (HG + QZJT). The cells in the QZJT + 3MA group were pre-treated with 5 mM of 3MA for 1 h before QZJT treatment, then cultured in high glucose for 48 h (HG + QZJT + 3MA). QZJT: Qizhi Jiangtang capsule; HG: high glucose; NG: normal glucose; LC3: light chain 3; AV: autophagic vacuole; 3MA: 3 methyladenine. Student t-test and one-way analysis of variance were used for comparison analysis. aP < 0.01, the HG group vs the NG group; bP < 0.05, cP < 0.01, the HG group vs the HG + QZJT group or HG + QZJT + 3MA group; dP < 0.05, the HG group vs the NG group.

Figure 4 Effect of QZJT on PI3K/AKT pathway in db/db mice and HG-induced podocytes A: representative western blotting images of p-PI3K, p-AKT and p-mTOR in the four groups of mice; B: quantitative analysis of p-PI3K expression levels in the four groups of mice; C: quantitative analysis of p-AKT expression levels in the four groups of mice; D: quantitative analysis of p-mTOR expression levels in the four groups of mice. Eight-week-old db/db mice were treated with or without QZJT or rapamycin. Db/m (control group group, n = 10), db/db (diabetic kidney disease group, n = 10), db/db + RAP (rapamycin (5 mg·kg-1·d-1), n = 10), db/db + QZJT group (QZJT 3.9 g/kg, n = 10). QZJT: Qizhi Jiangtang capsule; HG: high glucose; NG: normal glucose; RAP: rapamycin; p-PI3K: phosphorylated phosphatidylinositol 3-kinase; p-AKT: phosphorylated protein kinase B; p-MTOR: phosphorylated mammalian target of rapamycin. Student t-test and one-way analysis of variance were used for comparison analysis. aP < 0.01, the db/db group vs the db/m group, bP < 0.01, cP < 0.05, the db/db group vs the db/db + RAP group or db/db + QZJT group; E: representative western blotting images of p-PI3K, p-AKT and p-mTOR in NG, HG, HG + QZJT, HG + QZJT + 3MA groups. F: relative band intensities were used in order to quantify protein p-PI3K expression levels; G: relative band intensities were used in order to quantify protein p-AKT expression levels; H: relative band intensities were used in order to quantify protein p-mTOR expression levels.The differentiated podocytes were incubated with normal glucose (NG, 5.5 mM) or high glucose (HG, 35 mM). The cells in the QZJT groups were preconditioned with 10% drug-containing serum for 2 h, then cultured in high glucose for 48 h (HG + QZJT). The cells in the QZJT + 3MA group were pre-treated with 5 mM of 3MA for 1 h before QZJT treatment, then cultured in high glucose for 48 h (HG + QZJT + 3MA). 3MA: 3 methyladenine. Student t-test and one-way analysis of variance were used for comparison analysis. dP < 0.01, eP < 0.05, the HG group vs the NG group; fP < 0.01, the HG group vs the HG + QZJT group or HG + QZJT + 3MA group.
| 1. | Collins AJ, Foley RN, Chavers B, et al. US renal data system 2013 Annual Data Report. Am J Kidney Dis 2014; 63: A7. |
| 2. |
Altintas MM, Reiser J. Podocytes: way to go. Am J Pathol 2019; 189: 226-8.
DOI PMID |
| 3. | Sai YP, Song YC, Chen XX, Luo X, Liu J, Cui WJ. Protective effect of astragalosides from radix astragali on adriamycin-induced podocyte injury. Exp Ther Med 2018; 15: 4485-90. |
| 4. |
Kim Y, Park CW. New therapeutic agents in diabetic nephropathy. Korean J Intern Med 2017; 32: 11-25.
DOI PMID |
| 5. |
Kawakami T, Gomez IG, Ren S, et al. Deficient autophagy results in mitochondrial dysfunction and FSGS. J Am Soc Nephrol 2015; 26: 1040-52.
DOI PMID |
| 6. |
Bork T, Liang W, Yamahara K, et al. Podocytes maintain high basal levels of autophagy independent of mtor signaling. Autophagy 2020; 16: 1932-48.
DOI URL |
| 7. | Lin TA, Wu VC, Wang CY. Autophagy in chronic kidney diseases. Cells 2019; 8. |
| 8. |
Tagawa A, Yasuda M, Kume S, et al. Impaired podocyte autophagy exacerbates proteinuria in diabetic nephropathy. Diabetes 2016; 65: 755-67.
DOI PMID |
| 9. |
Zhang Y, Wang Y, Luo M, et al. Elabela protects against podocyte injury in mice with streptozocin-induced diabetes by associating with the PI3K/Akt/mTOR pathway. Peptides 2019; 114: 29-37.
DOI PMID |
| 10. |
Zheng D, Tao M, Liang X, Li Y, Jin J, He Q. p66Shc regulates podocyte autophagy in high glucose environment through the Notch-PTEN-PI3K/Akt/mTOR pathway. Histol Histopathol 2020; 35: 405-15.
DOI PMID |
| 11. | Guo ZA, Yu CJ, Liu G, Meng FC, Li Y, Peng SL. Treatment of stage 3b diabetic kidney disease patients with macroalbuminuria by Qizhi Jiangtang capsule: a multicenter randomized control clinical study. Zhong Guo Zhong Xi Yi Jie He Za Zhi 2014; 34: 1047-52. |
| 12. | Li Y, Yu C, Guo Z, et al. Effect of Qizhi Jiangtang capsule on the ratio of intima/media thickness and inflammatory factors in renal small artery of diabetic nephropathy rats. Zhong Xi Yi Jie He Shen Bing Za Zhi 2013; 14: 858-63. |
| 13. | Guo Z, Meng F, Yu C. Effect of Qizhi Jiangtang capsule on function and structure of kidney in diabetic nephropathy rats. Zhong Guo Yi Yao Ke Xue 2015; 5: 31-6. |
| 14. |
Lavoz C, Matus YS, Orejudo M, et al. Interleukin-17A blockade reduces albuminuria and kidney injury in an accelerated model of diabetic nephropathy. Kidney Int 2019; 95: 1418-32.
DOI URL |
| 15. |
Thomas B. The global burden of diabetic kidney disease: time trends and gender gaps. Curr Diab Rep 2019; 19: 18.
DOI |
| 16. |
Wang B, Qian JY, Tang TT, et al. VDR/Atg3 axis regulates slit diaphragm to tight junction transition via p62-mediated autophagy pathway in diabetic nephropathy. diabetes 2021; 70: 2639-51.
DOI PMID |
| 17. | Zheng X, Feng Y, Han L. Clinical curative effect analysis of Qizhi Jiangtang capsule for diabetes nephrosis patients. Zhong Hua Zhong Yi Yao Xue Kan 2018; 36: 994-6. |
| 18. | Lu M, Wang P, Ge Y, et al. Activation of mineralocorticoid receptor by ecdysone, an adaptogenic and anabolic ecdysteroid, promotes glomerular injury and proteinuria involving overactive GSK3β pathway signaling. Sci Rep 2018; 8: 12225. |
| 19. | Dai H, Liu Q, Liu B. Research progress on mechanism of podocyte depletion in diabetic nephropathy. J Diabetes Res 2017; 2017: 2615286. |
| 20. |
Xuan C, Xi YM, Zhang YD, Tao CH, Zhang LY, Cao WF. Yiqi Jiedu Huayu decoction alleviates renal injury in rats with diabetic nephropathy by promoting autophagy. Front Pharmacol 2021; 12: 624404.
DOI URL |
| 21. |
Nagata M. Podocyte injury and its consequences. Kidney Int 2016; 89: 1221-30.
DOI URL |
| 22. |
Liang W, Yamahara K, Hernando-Erhard C, et al. A reciprocal regulation of spermidine and autophagy in podocytes maintains the filtration barrier. Kidney Int 2020; 98: 1434-48.
DOI PMID |
| 23. | Liu WJ, Huang WF, Ye L, et al. The activity and role of autophagy in the pathogenesis of diabetic nephropathy. Eur Rev Med Pharmacol Sci 2018; 22: 3182-9. |
| 24. |
Jin J, Hu K, Ye M, Wu D, He Q. Rapamycin reduces podocyte apoptosis and is involved in autophagy and mTOR/ P70S6K/4EBP1 signaling. Cell Physiol Biochem 2018; 48: 765-72.
DOI PMID |
| 25. |
Fantus D, Rogers NM, Grahammer F, Huber TB, Thomson AW. Roles of mTOR complexes in the kidney: implications for renal disease and transplantation. Nat Rev Nephrol 2016; 12: 587-609.
DOI PMID |
| 26. |
Xiao T, Guan X, Nie L, et al. Rapamycin promotes podocyte autophagy and ameliorates renal injury in diabetic mice. Mol Cell Biochem 2014; 394: 145-54.
DOI PMID |
| 27. |
Bachar-Wikstrom E, Wikstrom JD, Ariav Y, et al. Stimulation of autophagy improves endoplasmic reticulum stress-induced diabetes. Diabetes 2013; 62: 1227-37.
DOI PMID |
| 28. |
Al-Bari MAA, Xu P. Molecular regulation of autophagy machinery by mTOR-dependent and -independent pathways. Ann N Y Acad Sci 2020; 1467: 3-20.
DOI URL |
| 29. |
Yang F, Qu Q, Zhao C, et al. Paecilomyces cicadae-fermented radix astragali activates podocyte autophagy by attenuating PI3K/AKT/mTOR pathways to protect against diabetic nephropathy in mice. Biomed Pharmacother 2020; 129: 110479.
DOI PMID |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||

