Journal of Traditional Chinese Medicine ›› 2023, Vol. 43 ›› Issue (4): 661-666.DOI: 10.19852/j.cnki.jtcm.2023.04.003
Previous Articles Next Articles
Synergistic effect of schizandrin A and DNase I knockdown on high glucose induced beta cell apoptosis by decreasing intracellular calcium concentration
ZHU Bin1( ), YU Ning2, WANG Lei3, TIAN Yue1, WU Mingfen1, ZHAO Zhigang1(
), YU Ning2, WANG Lei3, TIAN Yue1, WU Mingfen1, ZHAO Zhigang1( )
)
- 1 Department of Pharmacy, Beijing Tiantan Hospital, Capital Medical University, Beijing 100070, China
2 Accreditation Center of Traditional Chinese Medicine Physician of National Administration of Traditional Chinese Medicine, Beijing 100120, China
3 Department of Endocrinology, the Third Affiliated Hospital of Beijing University of Chinese Medicine, Beijing 100050, China
-
Received:2022-03-16Accepted:2022-07-08Online:2023-08-15Published:2023-07-03 -
Contact:ZHAO Zhigang, Department of Pharmacy, Beijing Tiantan Hospital Affiliated to Capital Medical University, Beijing 100070, China. 1022zzg@sina.com. Telephone: +86-10-59975442; +86-010-59976856
ZHU Bin, Department of Pharmacy, Beijing Tiantan Hospital Affiliated to Capital Medical University, Beijing 100070, China. zbtcm@163.com -
Supported by:Natural Science Foundation of China: the Protective Effect of Schizandrin A to β Cell Apoptosis by DNase I in Type 2 Diabetes(81803909)
Cite this article
ZHU Bin, YU Ning, WANG Lei, TIAN Yue, WU Mingfen, ZHAO Zhigang. Synergistic effect of schizandrin A and DNase I knockdown on high glucose induced beta cell apoptosis by decreasing intracellular calcium concentration[J]. Journal of Traditional Chinese Medicine, 2023, 43(4): 661-666.
share this article
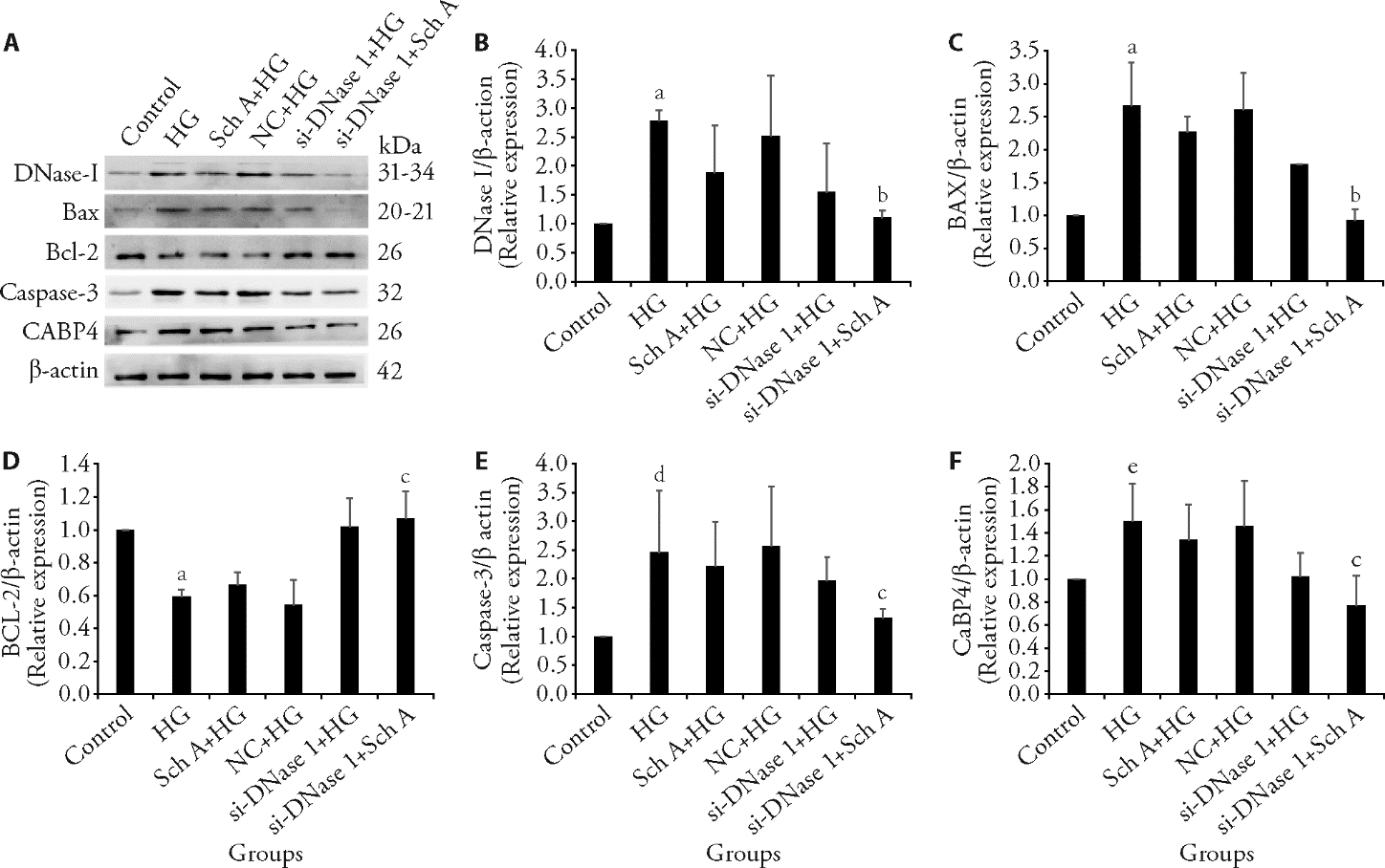
Figure 1 Analysis of apoptosis-associated protein level by Western blot A: immunoblot assay for determining apoptosis protein. B-F: quantitative analysis of immunoblotting results of DNase I, BAX, Bcl-2, Caspase-3, and CaBP4 in each group. Densitometry was used to compare the expression levels. β-actin was used as an internal loading control. HG: treated with 30 mmol/L high glucose; SchA + HG: treated with 9 μmol/L Sch A and 30 mmol/L high glucose for 48 h; NC: treated with negative control siRNA and 30 mmol/L high glucose; si-DNase I + HG: treated with DNase I siRNA and 30 mmol/L high glucose; si-DNase I + HG + SchA: treated with 9 μmol/L Sch A and DNase I siRNA under 30 mmol/L high glucose condition for 48 h; Control group: treated with equivalent volume of normal saline. DNase I: deoxyribonuclease I; Bax: Bcl-2-associated X protein; Bcl-2: B-cell lymphoma-2; CaBP4: calcium binding protein 4; Sch A: schizandrin A; HG: high glucose; NC: negative control. Data are expressed as the mean ± standard deviation (n = 3). aP < 0.05, dP < 0.001, eP < 0.01, compared with control group; bP < 0.05, cP < 0.01, compared with HG group.
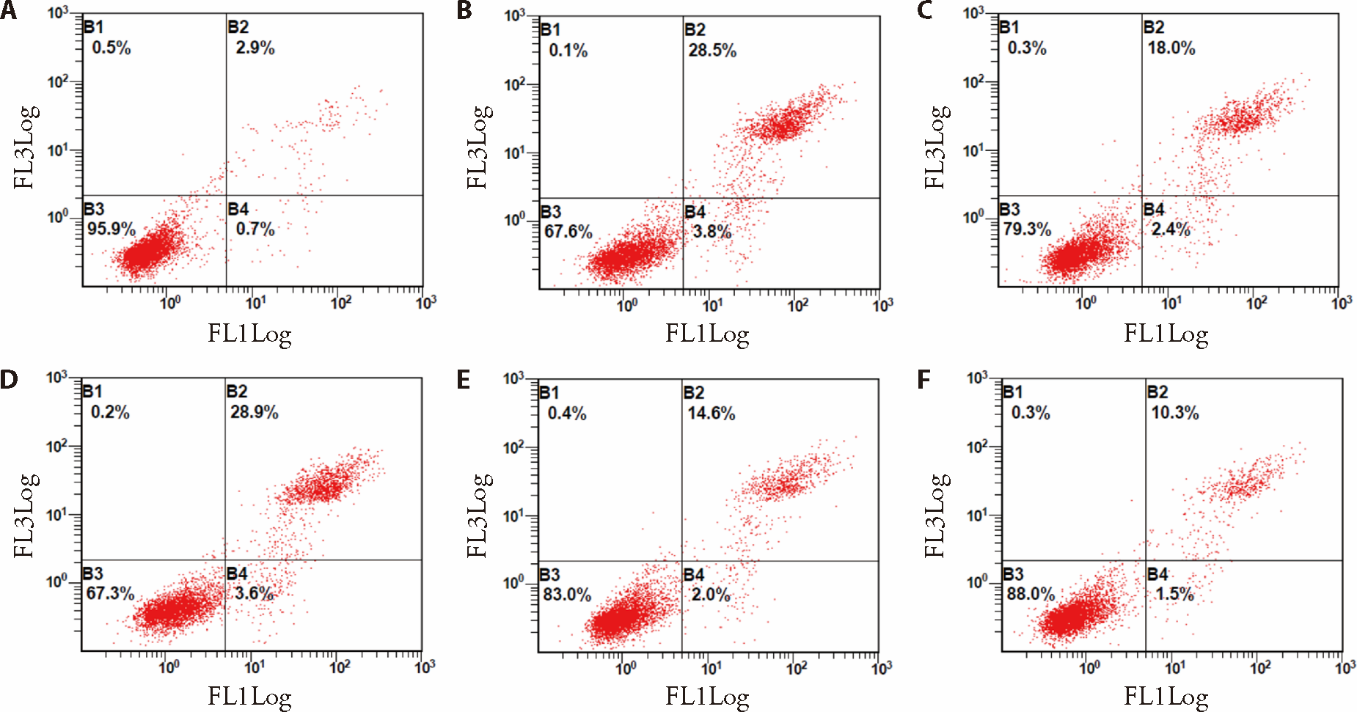
Figure 2 Cell apoptosis rate analyzed by flow cytometry analysis A: the control group; B the HG group; C: the Sch A + HG group; D: the NC + HG group; E: the si-DNase I + HG group; F: the si-DNase I + HG + Sch A group. HG: treated with 30 mmol/L high glucose; SchA + HG: treated with 9 μmol/L Sch A and 30 mmol/L high glucose for 48 h; NC: treated with negative control siRNA and 30 mmol/L high glucose; si-DNase I+HG: treated with DNase I siRNA and 30 mmol/L high glucose; si-DNase I + HG + SchA: co-treated with 9 μmol/L Sch A and DNase I siRNA under 30 mmol/L high glucose condition for 48 h; Control group: treated with equivalent volume of normal saline. DNase I: deoxyribonuclease I; Sch A: schizandrin A; HG: high glucose; NC: negative control.
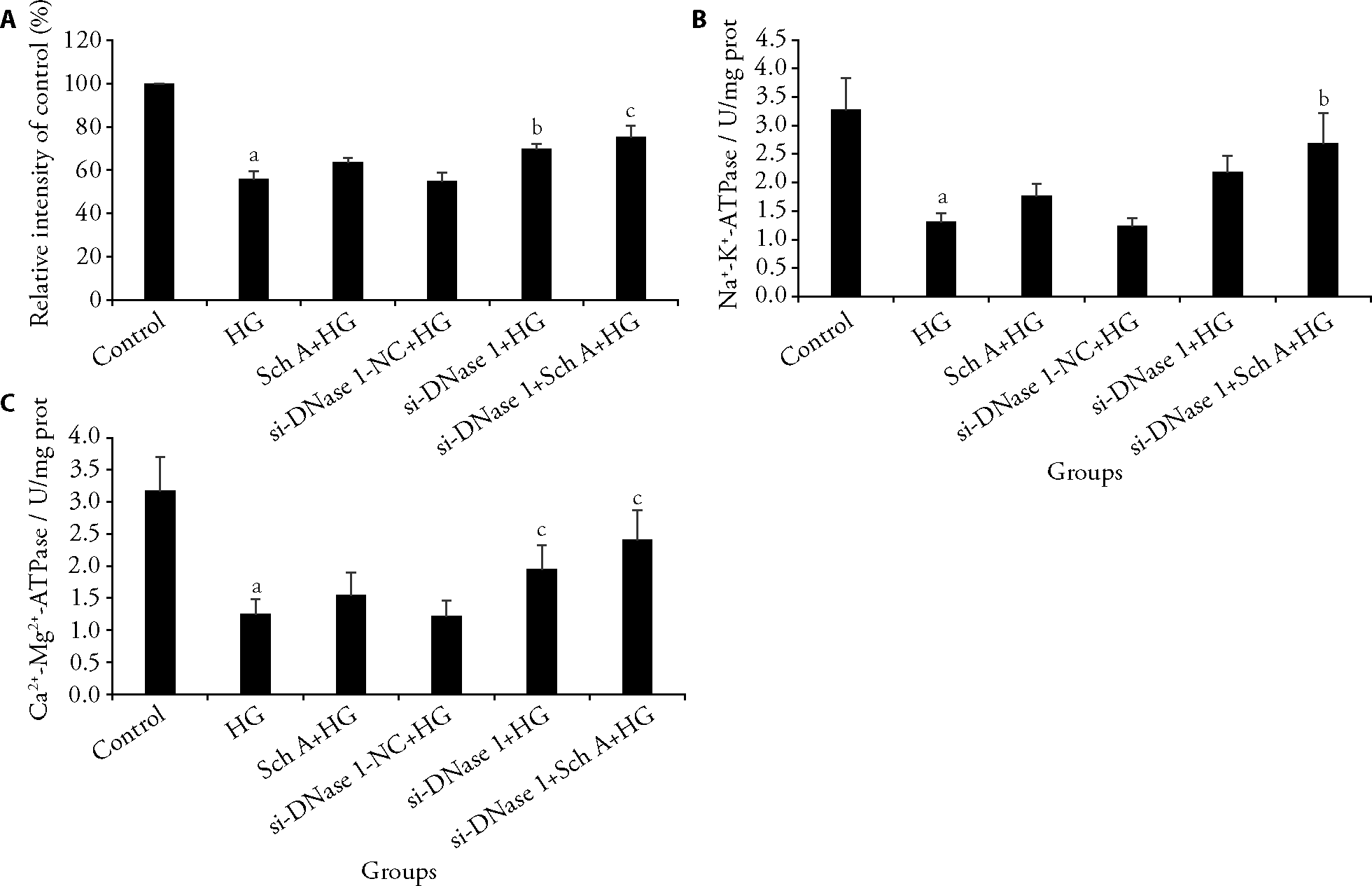
Figure 3 Level of cell membrane potential, Na+-K+-ATPase and Ca2+-Mg2+-ATPase activity A: the cell membrane potential; B: the Na+-K+-ATPase activity; C: the Ca2+-Mg2+-ATPase activity. HG: treated with 30 mmol/L high glucose; SchA + HG: treated with 9 μmol/L Sch A and 30 mmol/L high glucose for 48h; NC: treated with negative control siRNA and 30 mmol/L high glucose; si-DNase I + HG: treated with DNase I siRNA and 30 mmol/L high glucose; si-DNase I + HG + SchA: co-treated with 9 μmol/L Sch A and DNase I siRNA under 30 mmol/L high glucose condition for 48 h; Control group: treated with equivalent volume of normal saline. ATPase: adenosine triphosphatease; DNase I: deoxyribonuclease I; Sch A: schizandrin A; HG: high glucose; NC: negative control. Data are expressed as the mean ± standard deviation (n = 3). aP < 0.001, compared with control group; bP < 0.01, cP < 0.001, compared with HG group.
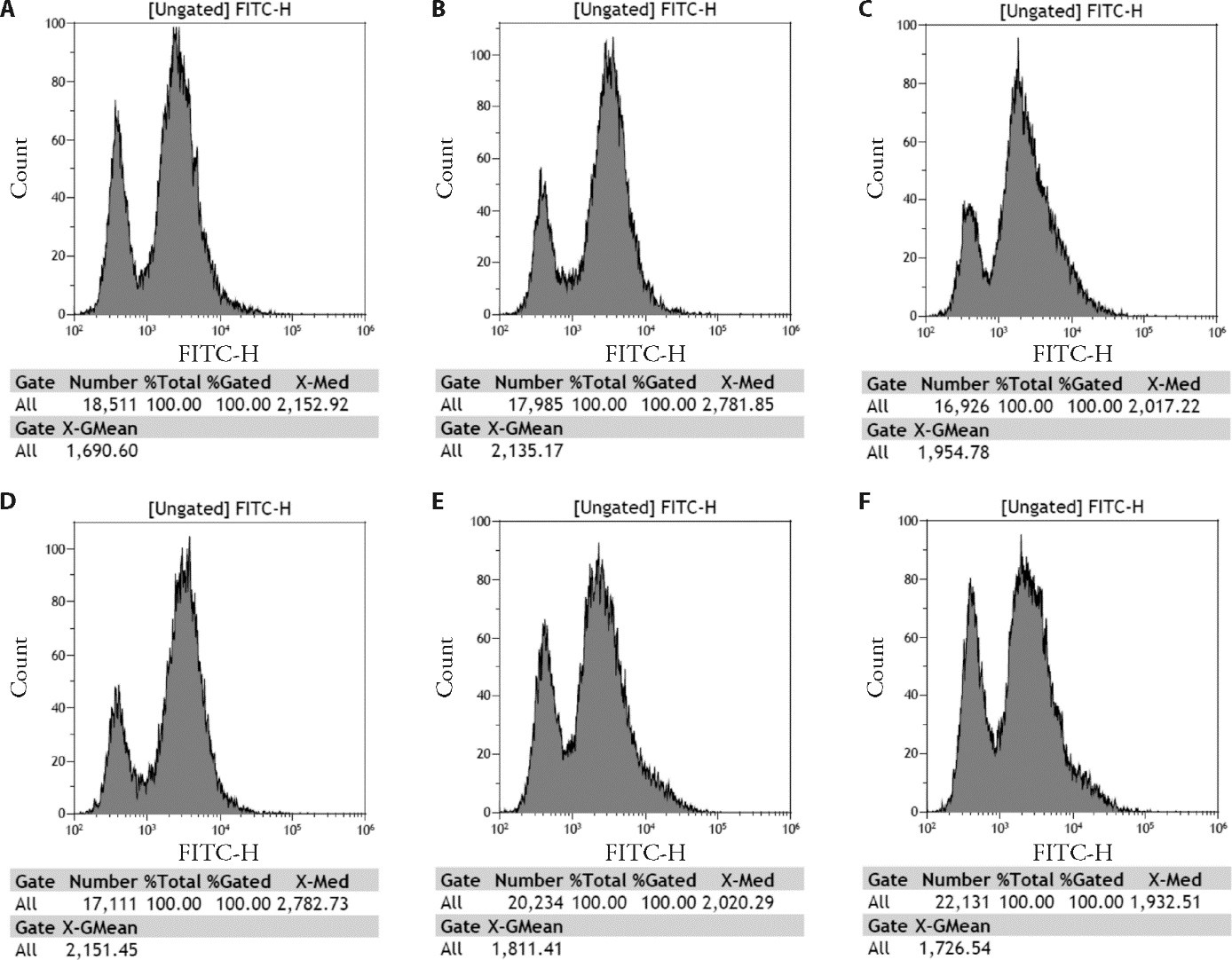
Figure 4 Intracellular Ca2+ concentration detection A: the control group; B the HG group; C: the Sch A + HG group; D: the negative control + HG group; E: the si-DNase I + HG group; F: the si-DNase I + HG + Sch A group. HG: treated with 30 mmol/L high glucose; SchA + HG: treated with 9 μmol/L Sch A and 30 mmol/L high glucose for 48h; NC: treated with negative control siRNA and 30 mmol/L high glucose; si-DNase I + HG: treated with DNase I siRNA and 30 mmol/L high glucose; si-DNase I + HG + SchA: co-treated with 9 μmol/L Sch A and DNase I siRNA under 30 mmol/L high glucose condition for 48 h; Control group: treated with equivalent volume of normal saline. The Ca2+ concentration was evaluated by the average fluorescence intensity. DNase I: deoxyribonuclease I; Sch A: schizandrin A; HG: high glucose; NC: negative control.
| 1. |
Sun H, Saeedi P, Karuranga S, et al. IDF diabetes atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract 2022; 183: 109119.
DOI URL |
| 2. |
Cheng F, Li Y, Zheng H, et al. Mediating effect of body mass index and dyslipidemia on the relation of uric acid and type 2 diabetes: results from China health and retirement longitudinal study. Front Public Health 2021; 9: 823739.
DOI URL |
| 3. |
So WY, Cheng Q, Chen L, et al. High glucose represses beta-klotho expression and impairs fibroblast growth factor 21 action in mouse pancreatic islets: involvement of peroxisome proliferator-activated receptor gamma signaling. Diabetes 2013; 62: 3751-59.
DOI URL |
| 4. | Rojas J, Bermudez V, Palmar J, et al. Pancreatic beta cell death: novel potential mechanisms in diabetes therapy. J Diabetes Res 2018; 2018: 9601801. |
| 5. |
Zhou Z, Zhu C, Ren J, et al. A graphene-based real-time fluorescent assay of deoxyribonuclease I activity and inhibition. Anal Chim Acta 2012; 740: 88-92.
DOI PMID |
| 6. |
Zhu B, Gong Y, Chen P, et al. Increased DNase I activity in diabetes might be associated with injury of pancreas. Mol Cell Biochem 2014; 393: 23-32.
DOI PMID |
| 7. | Zhu B, Zhang L, Zhang YY, et al. DNase I aggravates islet beta-cell apoptosis in type 2 diabetes. Mol Med Rep 2016; 3: 4577-84. |
| 8. |
Bai L, Li X, He L, et al. Antidiabetic potential of flavonoids from Traditional Chinese Medicine: a review. Am J Chin Med 2019; 47: 933-57.
DOI URL |
| 9. |
Qiao Z, Du X, Zhuang W, et al. Schisandra Chinensis acidic polysaccharide improves the insulin resistance in type 2 diabetic rats by inhibiting inflammation. J Med Food 2020; 23: 358-66.
DOI PMID |
| 10. |
Choi HJ, Yeon MH, Jun HS. Schisandrae chinensis Fructus extract ameliorates muscle atrophy in streptozotocin-induced diabetic mice by downregulation of the CREB-KLF15 and autophagy-lysosomal pathways. Cells 2021; 10: 2283.
DOI URL |
| 11. | Chen BC, Tu SL, Zheng BA, et al. Schizandrin A exhibits potent anticancer activity in colorectal cancer cells by inhibiting heat shock factor 1. Biosci Rep 2020; 40: BSR20200203. |
| 12. |
Zhou F, Wang M, Ju J, et al. Schizandrin A protects against cerebral ischemia-reperfusion injury by suppressing inflammation and oxidative stress and regulating the AMPK/Nrf2 pathway regulation. Am J Transl Res 2019; 11: 199-209.
PMID |
| 13. |
Li S, Xie R, Jiang C, et al. Schizandrin A alleviates LPS-induced injury in human keratinocyte cell hacat through a microRNA-127-dependent regulation. Cell Physiol Biochem 2018; 49: 2229-39.
DOI PMID |
| 14. | Dong Y, Qian C, Wan G, et al. Schizandrin A protects human retinal pigment epithelial cell line ARPE-19 against HG-induced cell injury by regulation of miR-145. Mol Ther Nucleic Acids 2020; 19: 42-9. |
| 15. |
Men X, Peng L, Wang H, et al. Involvement of the Ca2+-responsive transactivator in high glucose-induced beta-cell apoptosis. J Endocrinol 2013; 216: 231-43.
DOI URL |
| 16. |
Snyder J, Lackey AI, Brown GS, et al. GRK2 contributes to glucose mediated calcium responses and insulin secretion in pancreatic islet cells. Sci Rep 2021; 11: 11129.
DOI PMID |
| 17. |
Xiao E, Luo L. Alternative therapies for diabetes: a comparison of western and Traditional Chinese Medicine approaches. Curr Diabetes Rev 2018; 14: 487-96.
DOI URL |
| 18. | Wang H, Shi S, Wang S. Can highly cited herbs in ancient Traditional Chinese Medicine formulas and modern publications predict therapeutic targets for diabetes mellitus? J Ethnopharmacol 2018; 213: 101-10. |
| 19. |
Zhang TT, Jiang JG. Active ingredients of Traditional Chinese Medicine in the treatment of diabetes and diabetic complications. Expert Opin Investig Drugs 2012; 21: 1625-42.
DOI URL |
| 20. |
Zhang IX, Raghavan M, Satin LS. The endoplasmic reticulum and calcium homeostasis in pancreatic beta cells. Endocrinology 2020; 161: bqz028.
DOI URL |
| 21. |
Alevriadou BR, Patel A, Noble M, et al. Molecular nature and physiological role of the mitochondrial calcium uniporter channel. Am J Physiol Cell Physiol 2021; 320: C465-82.
DOI URL |
| 22. |
Hughes JW, Ustione A, Lavagnino Z, et al. Regulation of islet glucagon secretion: beyond calcium. Diabetes Obes Metab 2018; 20 Suppl 2: 127-36.
DOI URL |
| 23. |
Gil-Rivera M, Medina-Gali RM, Martinez-Pinna J, et al. Physiology of pancreatic beta-cells: ion channels and molecular mechanisms implicated in stimulus-secretion coupling. Int Rev Cell Mol Biol 2021; 359: 287-323.
DOI PMID |
| [1] | JIN Shenyi, LIU Yahua, HAN Xu, CAI Mengjie, XU Jiatuo, LU Hao, CHEN Qingguang. Dark red tongue color formation caused by hyperglycemia is attributed to decreased blood flow of tongue tissue partially due to nuclear factor-kappa B pathway activation [J]. Journal of Traditional Chinese Medicine, 2023, 43(6): 1118-1125. |
| [2] | HENG Xianpei, WANG Zhita, YANG Liuqing, LI Liang, HUANG Suping. Dangua Fang (丹瓜方) regulating tricarboxylic acid cycle and respiratory chain and its mechanism in diabetic rats [J]. Journal of Traditional Chinese Medicine, 2023, 43(6): 1150-1159. |
| [3] | QIN Xihui, PANG Jianli, XIONG Guan, FENG Jie. Bo′s abdominal acupuncture improves disordered metabolism in obese type 2 diabetic rats through regulating fibroblast growth factor 21 and its related adipokines [J]. Journal of Traditional Chinese Medicine, 2023, 43(6): 1200-1208. |
| [4] | JIANG Li, FU Qiang, WANG Shidong, ZHAO Jinxi, CHEN Yu, LI Jiayue, XIAO Yonghua, HUANG Weijun, SUN Ruixi, XIAO Yao, SHEN Aijia, WANG Junheng, LIU Jiangteng, FU Xiaozhe, LI Yuanyuan, ZHAO Yu, XUE Taiqi. Effects of Shenlian formula (参连方) on microbiota and inflammatory cytokines in adults with type 2 diabetes: a double-blind randomized clinical trial [J]. Journal of Traditional Chinese Medicine, 2023, 43(4): 760-769. |
| [5] | JIA Lihong, TIE Defu, FAN Zhaohui, CHEN Dan, CHEN Qizhu, CHEN Jun, BO Huaben. Mechanism underlying Fanmugua (Fructus Caricae) leaf multicomponent synergistic therapy for anemia: data mining based on hematopoietic network [J]. Journal of Traditional Chinese Medicine, 2023, 43(3): 542-551. |
| [6] | QU Yilun, CHENG Haimei, WANG Qian, LI Shuang, DUAN Shuwei, FENG Zhe, LI Weizhen, JIANG Shuangshuang, YANG Hongtao, MAO Yonghui, GENG Yanqiu, LI Jijun, LIU Yuning, TIAN Jinzhou, LIU Hongfang, DONG Zheyi, CHEN Xiangmei. Noninvasive identificational diagnosis of diabetic nephropathy and non-diabetic renal disease based on clinical characteristics of Traditional Chinese Medicine symptom pattern and conventional medicine [J]. Journal of Traditional Chinese Medicine, 2023, 43(3): 588-593. |
| [7] | ZHAO Jun, LI Xia, ZHENG Hui, YE Kun, WANG Xin, WANG Xuefei, SUN Runquan, LI Zhigang. Effects of acupuncture on functional gastrointestinal disorders: special effects, coeffects, synergistic effects in terms of single or compatible acupoints [J]. Journal of Traditional Chinese Medicine, 2023, 43(2): 397-408. |
| [8] | You WU, Yuli HU, Wei LIU, Boju SUN, Chengfei ZHANG, Lili WU, Tonghua LIU. Flavonoids from traditional Chinese herbs for diabetes in rats: a network Meta-analysis [J]. Journal of Traditional Chinese Medicine, 2022, 42(1): 1-8. |
| [9] | XIA Xichao, LI Bin, QIU Ju, TIAN Gang, CHEN Changdong, LA Ming, ZHANG Ke, QI Jinxu, LI Yanyan, GAO Huashan, SHAO Xiangyang, SU Congying, WANG Mengqi, OUYANG Jingfeng. Antioxidative and immunological effects of Cyclocarya paliurus polysaccharides on the spleen injury of diabetic rats [J]. Journal of Traditional Chinese Medicine, 2021, 41(5): 739-746. |
| [10] | YIN Yundong, FANG Zhaohui, WU Yuanyuan, YOU Liangzhen. Effect of Shenzhu Tiaopi granule(参术调脾颗粒) on hepatic insulin resistance in diabetic Goto-Kakizakirats via liver kinase B1/adenosine 5'-monophosphate/mammalian target of rapamycin signaling pathway [J]. Journal of Traditional Chinese Medicine, 2021, 41(1): 107-116. |
| [11] | Wang Jianxing, Yu Xiaohan, Jiang Yan, Wang Yan, Li Ying, Han Shuying. Effects of a fermented buckwheat flower and leaf extract on the blood glucose and lipid profile of type 2 diabetic db/db mice [J]. Journal of Traditional Chinese Medicine, 2020, 40(2): 197-203. |
| [12] | Rangachari Balamurugan, Jeong Hwa Kim, Mi-na Jo, Chenglian Xue, Jin kyu Park, Jae Kwon Lee. Bee wax coated water-soluble fraction of bee venom improved altered glucose homeostasis in streptozotocin-induced diabetic rats [J]. Journal of Traditional Chinese Medicine, 2019, 39(06): 842-852. |
| [13] | Zhang Zexi, Zhang Lei, Xu Hansong. Effect of Astragalus polysaccharide in treatment of diabetes mellitus: a narrative review [J]. Journal of Traditional Chinese Medicine, 2019, 39(01): 133-138. |
| [14] | Yan Bin, Wang Jingbo, Zhang Hong, Tian Guoqing, Liu Yuqin. Effect of icariin on apoptosis in hippocampal neurons cultured in high glucose [J]. Journal of Traditional Chinese Medicine, 2018, 38(04): 556-561. |
| [15] | Zhang Yi, Mo Fangfang, Zhang Dongwei, Gao Sihua, Zhao Dandan, Yu Na, Mu Qianqian, Zuo Jiacheng, Ma Yue. Jiangtang Xiaoke granule attenuates glucose metabolism disorder via regulating endoplasmic reticulum stress in the liver of type 2 diabetes mellitus mice [J]. Journal of Traditional Chinese Medicine, 2018, 38(04): 570-578. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||

