Journal of Traditional Chinese Medicine ›› 2025, Vol. 45 ›› Issue (3): 586-596.DOI: 10.19852/j.cnki.jtcm.2025.03.013
Previous Articles Next Articles
Effect of electroacupuncture on hypertensive and sympathetic excitability mechanism mediated by the paraventricular nucleus of the hypothalamus in spontaneous hypertensive rats
SUN Jiao1, WANG Yueming3, LYU Jian1, LIU Xin2, YUE Bingnan2, LI Yinyin2, LIU Jipeng2, SUN Yize1, LIU Qingguo2, YAN Liu3( )
)
- 1 Department of Traditional Chinese Medicine, Qilu Hospital (Qingdao), Cheeloo College of Medicine, Shandong University, Qingdao 266035, China
2 School of Acupuncture-Moxibustion and Tuina, Beijing University of Chinese Medicine, Beijing 100029, China
3 Department of Acupuncture, Moxibustion, Massage and Rehabilitation Center, Qingdao Hiser Hospital Affiliated of Qingdao University (Qingdao Traditional Chinese Medicine Hospital), Qingdao 266033, China
-
Received:2024-06-03Accepted:2024-09-27Online:2025-06-15Published:2025-05-21 -
Contact:YAN Liu, Department of Acupuncture, Moxibustion, Massage and Rehabilitation Center, Qingdao Hiser Hospital Affiliated of Qingdao University (Qingdao Traditional Chinese Medicine Hospital), Qingdao 266033, China. 253711794@qq.com,Telephone: +86-18866236350
-
Supported by:Doctoral Research Fund Project of Qilu Hospital of Shandong University (Qingdao): Mechanistic Study on the Improvement of Vertigo Caused by Posterior Circulation Ischemia by Acupuncture through Regulating Cerebral Blood Flow in Rats with Posterior Circulation Ischemia Vertigo(QDKY2023BS19);National Natural Science Foundation of China: From Microrna 9 Regulate P2X7 Receptor of Microglia in Paraventricular Nucleus of Hypothalamus to Explore the Effect of Electroacupuncture on Sympathetic Nerve Excitability in Spontaneously Hypertensive Rats(82074553)
Cite this article
SUN Jiao, WANG Yueming, LYU Jian, LIU Xin, YUE Bingnan, LI Yinyin, LIU Jipeng, SUN Yize, LIU Qingguo, YAN Liu. Effect of electroacupuncture on hypertensive and sympathetic excitability mechanism mediated by the paraventricular nucleus of the hypothalamus in spontaneous hypertensive rats[J]. Journal of Traditional Chinese Medicine, 2025, 45(3): 586-596.
share this article
| Group | n | Day 0 | Day 2 | Day 4 | Day 6 | Day 8 | Day 10 | Day 12 | Day 14 |
|---|---|---|---|---|---|---|---|---|---|
| Control | 16 | 108.6±1.0 | 109.8±0.8 | 111.0±1.4 | 112.0±1.4 | 112.0±1.1 | 113.0±0.9 | 112.8±1.5 | 113.0±0.6 |
| Model | 16 | 169.8±2.1abc | 170.6±1.6abc | 171.2±2.3ab | 172.2±2.4a | 173.0±1.8a | 173.6±2.3a | 173.8±1.8a | 174.4±1.9a |
| Sham | 16 | 174.8±0.8a | 174.2±1.2a | 175.0±0.9a | 175.2±0.8a | 175.8±0.8a | 174.6±1.0a | 174.2±2.1a | 174.4±1.6a |
| EA | 16 | 169.6±1.4abc | 171.2±1.2abc | 170.6±0.5ab | 168.4±1.0abd | 164.8±1.3abd | 160.4±1.0abd | 156.2±1.7abd | 152.4±2.1abd |
| NRA+EA | 16 | 174.8±0.8a | 174.8±0.8a | 174.0±1.1a | 170.4±1.0ab | 166.2±1.2abd | 160.4±1.0abd | 152.8±1.5abd | 144.8±1.7abde |
Table 1 Changes in SBP ($\bar{x}±s$)
| Group | n | Day 0 | Day 2 | Day 4 | Day 6 | Day 8 | Day 10 | Day 12 | Day 14 |
|---|---|---|---|---|---|---|---|---|---|
| Control | 16 | 108.6±1.0 | 109.8±0.8 | 111.0±1.4 | 112.0±1.4 | 112.0±1.1 | 113.0±0.9 | 112.8±1.5 | 113.0±0.6 |
| Model | 16 | 169.8±2.1abc | 170.6±1.6abc | 171.2±2.3ab | 172.2±2.4a | 173.0±1.8a | 173.6±2.3a | 173.8±1.8a | 174.4±1.9a |
| Sham | 16 | 174.8±0.8a | 174.2±1.2a | 175.0±0.9a | 175.2±0.8a | 175.8±0.8a | 174.6±1.0a | 174.2±2.1a | 174.4±1.6a |
| EA | 16 | 169.6±1.4abc | 171.2±1.2abc | 170.6±0.5ab | 168.4±1.0abd | 164.8±1.3abd | 160.4±1.0abd | 156.2±1.7abd | 152.4±2.1abd |
| NRA+EA | 16 | 174.8±0.8a | 174.8±0.8a | 174.0±1.1a | 170.4±1.0ab | 166.2±1.2abd | 160.4±1.0abd | 152.8±1.5abd | 144.8±1.7abde |
| Group | n | Day 0 | Day 2 | Day 4 | Day 6 | Day 8 | Day 10 | Day 12 | Day 14 |
|---|---|---|---|---|---|---|---|---|---|
| Control | 16 | 281.2±4.0 | 288.6±5.0 | 302.8±6.73 | 306.0±5.8 | 300.0±4.2 | 296.0±4.6 | 290.6±4.8 | 288.4±2.9 |
| Model | 16 | 350.2±3.3a | 356.0±4.4a | 368.2±5.5a | 373.8±3.4a | 383.2±5.7a | 397.2±4.0a | 409.2±5.8a | 419.0±6.2a |
| Sham | 16 | 349.6±3.1a | 360.8±1.7a | 375.2±3.7a | 385.6±4.1a | 392.8±3.8a | 398.0±3.5a | 410.4±4.3a | 420.2±2.5a |
| EA | 16 | 351.4±3.0a | 358.8±3.3a | 363.6±3.7a | 357.8±3.9abc | 350.8±3.9abc | 344.6±3.7abc | 334.6±4.8abc | 323.2±5.3abc |
| NRA+EA | 16 | 350.6±3.0a | 363.2±4.3a | 371.0±2.3a | 365.8±2.6ab | 354.8±3.6abc | 343.4±3.0abc | 329.8±4.0abc | 302.4±4.3abcd |
Table 2 Changes in HR ($\bar{x}±s$)
| Group | n | Day 0 | Day 2 | Day 4 | Day 6 | Day 8 | Day 10 | Day 12 | Day 14 |
|---|---|---|---|---|---|---|---|---|---|
| Control | 16 | 281.2±4.0 | 288.6±5.0 | 302.8±6.73 | 306.0±5.8 | 300.0±4.2 | 296.0±4.6 | 290.6±4.8 | 288.4±2.9 |
| Model | 16 | 350.2±3.3a | 356.0±4.4a | 368.2±5.5a | 373.8±3.4a | 383.2±5.7a | 397.2±4.0a | 409.2±5.8a | 419.0±6.2a |
| Sham | 16 | 349.6±3.1a | 360.8±1.7a | 375.2±3.7a | 385.6±4.1a | 392.8±3.8a | 398.0±3.5a | 410.4±4.3a | 420.2±2.5a |
| EA | 16 | 351.4±3.0a | 358.8±3.3a | 363.6±3.7a | 357.8±3.9abc | 350.8±3.9abc | 344.6±3.7abc | 334.6±4.8abc | 323.2±5.3abc |
| NRA+EA | 16 | 350.6±3.0a | 363.2±4.3a | 371.0±2.3a | 365.8±2.6ab | 354.8±3.6abc | 343.4±3.0abc | 329.8±4.0abc | 302.4±4.3abcd |
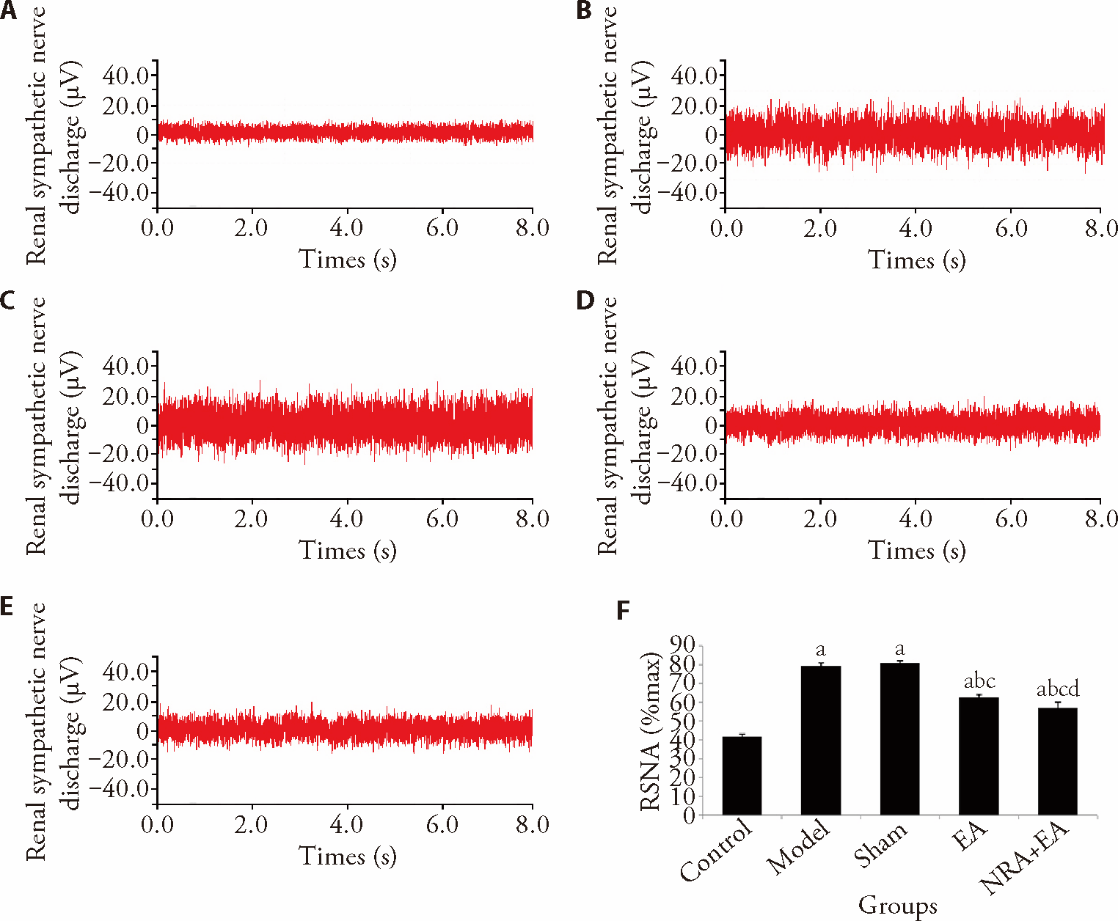
Figure 1 RSNA expression in different groups A: renal sympathetic nerve discharge in the control group; B: renal sympathetic nerve discharge in the model group; C: renal sympathetic nerve discharge in the sham group; D: renal sympathetic nerve discharge in the EA group; E: renal sympathetic nerve discharge in the NRA + EA group; F: renal sympathetic nerve activity (percentage relative to the maximum value). Control and Model groups: fixed with a rat sleeve (as per rats in the EA and NRA + EA groups) without any acupuncture intervention for 14 d; Sham group: inject the Artificial cerebrospinal fluid (10 mmol/L, 100 nL) and fixed with a rat sleeve (as per rats in the EA and NRA + EA groups) without any acupuncture intervention for 14 d; EA group: electro-acupuncture at Taichong (LR3) and Quchi (LI11) acupoints for 14 d; NRA + EA group: inject the N-methyl-D-aspartic acid receptor inhibitor (10 mmol/L, 100 nL) and electro-acupuncture at Taichong (LR3) and Quchi (LI11) acupoints for 14 d. RSNA: renal sympathetic nerve activity; EA: electroacupuncture; NRA + EA: N-methyl-D-aspartate receptor antagonist and electroacupuncture. One-way analysis of variance was adopted, and the least significant difference t-test was used for the comparison among groups. Data were presented as mean ± standard deviation (n = 16). aP < 0.01, vs control group; bP < 0.01, vs model group; cP < 0.01, vs Sham group; dP < 0.05, vs EA group.
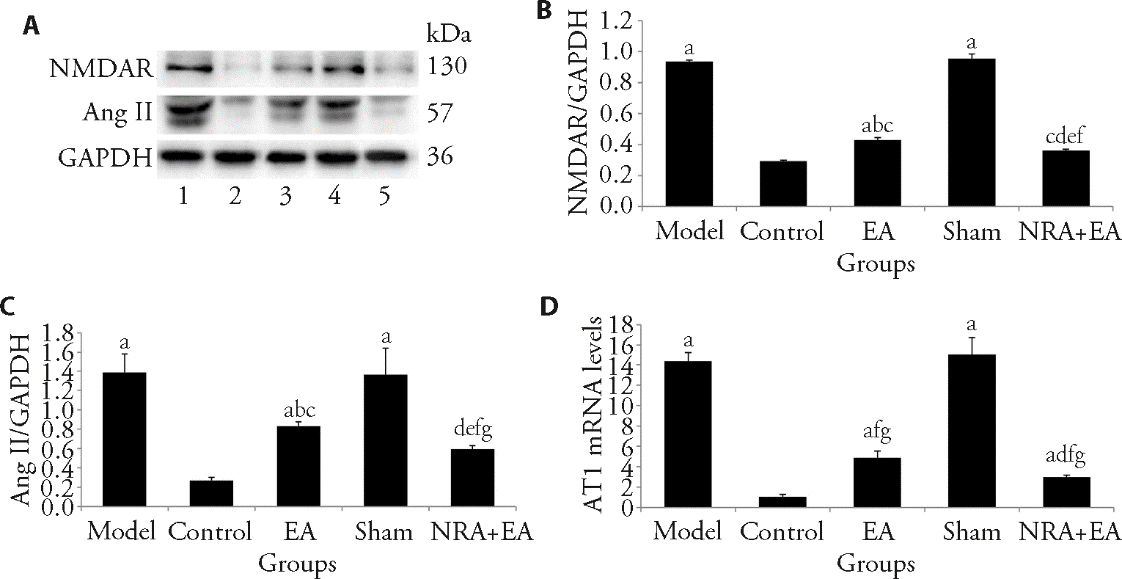
Figure 2 NMDAR, Ang Ⅱ and AT1protein expression in each group of rats A: Western blot analysis of levels of NMDAR and Ang Ⅱ expression in PVN of rats in each group; B: comparison of the protein amount of NMDAR in each group; C: comparison of the protein amount of Ang Ⅱ in each group; D: expression of AT1 mRNA. 1: model group; 2: control group; 3: EA group; 4: sham group; 5: NRA + EA group. Control and model groups: fixed with a rat sleeve (as per rats in the EA and NRA + EA groups) without any acupuncture intervention for 14 d; Sham group: inject the Artificial cerebrospinal fluid (10 mmol/L, 100 nL) and fixed with a rat sleeve (as per rats in the EA and NRA + EA groups) without any acupuncture intervention for 14 d; EA group: electro-acupuncture at Taichong (LR3) and Quchi (LI11) acupoints for 14 d; NRA + EA group: inject the N-methyl-D-aspartic acid receptor inhibitor (10 mmol/L, 100 nL) and electro-acupuncture at Taichong (LR3) and Quchi (LI11) acupoints for 14 d. EA: Electroacupuncture; NRA + EA: N-methyl-D-aspartate receptor antagonist and electroacupuncture; NMDAR: N-methyl-D-aspartate receptor; Ang II: angiotensin II; AT1: angiotensin II type 1. One-way analysis of variance was adopted, and the least significant difference t-test was used for the comparison among groups. Data were presented as mean ± standard deviation (n = 16). aP < 0.01 and eP < 0.05, vs control group; bP < 0.05 and fP < 0.01, vs model group; cP < 0.05 and gP < 0.01, vs Sham group; dP < 0.05, vs EA group.
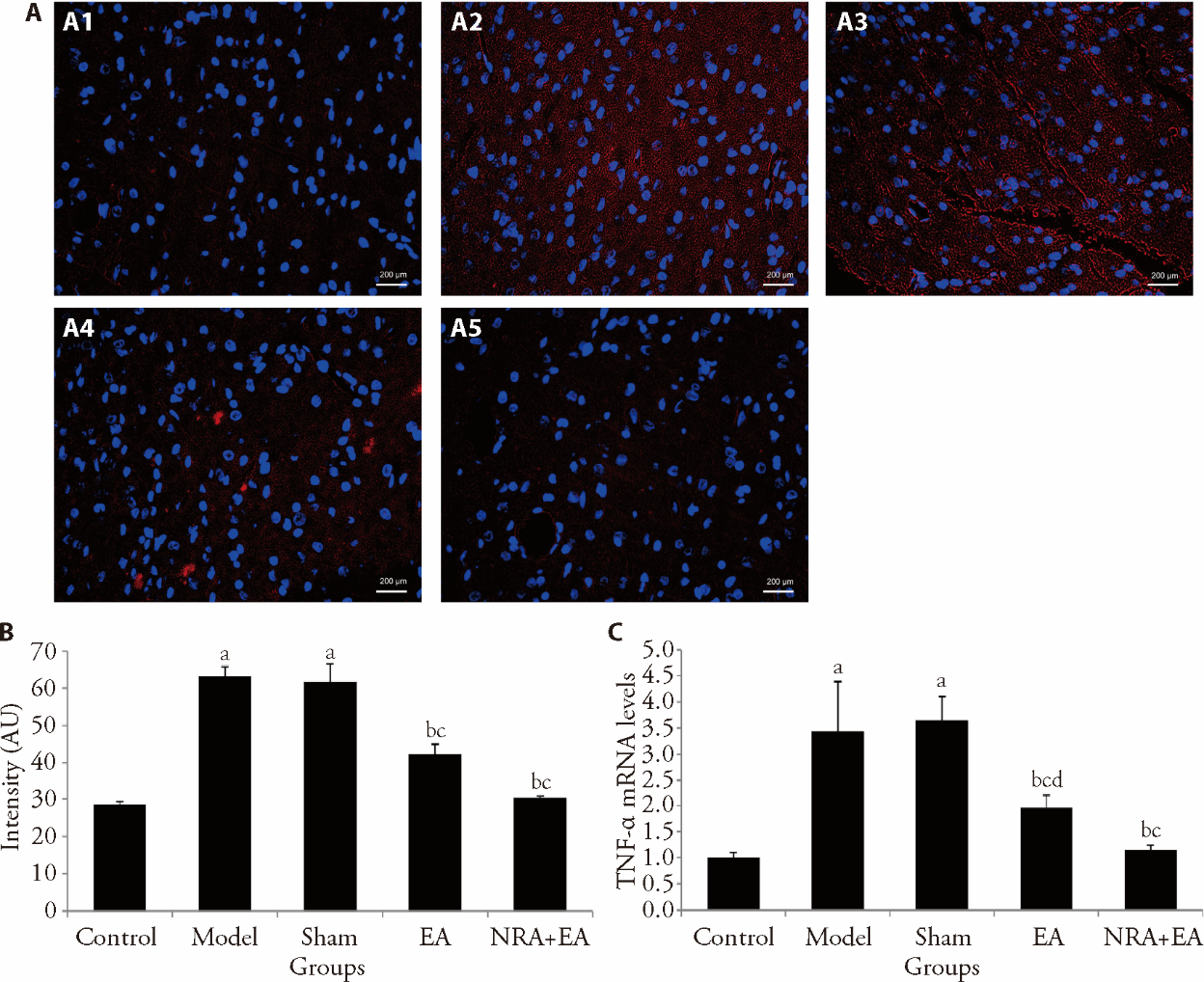
Figure 3 TNF-α levels in the PVN A: immunofluorescence images of TNF-α in different groups. A1: control group; A2: model group; A3: sham group; A4: EA group; A5: NRA+EA group; B: immunofluorescence intensity values of TNF-α in each group; C: expression of TNF-α mRNA. Control and Model groups: fixed with a rat sleeve (as per rats in the EA and NRA + EA groups) without any acupuncture intervention for 14 d; Sham group: inject the Artificial cerebrospinal fluid (10 mmol/L, 100 nL) and fixed with a rat sleeve (as per rats in the EA and NRA + EA groups) without any acupuncture intervention for 14 d; EA group: electro-acupuncture at Taichong (LR3) and Quchi (LI11) acupoints for 14 d; NRA + EA group: inject the N-methyl-D-aspartic acid receptor inhibitor (10 mmol/L, 100 nL) and electro-acupuncture at Taichong (LR3) and Quchi (LI11) acupoints for 14 d. EA: electroacupuncture; NRA + EA:N-methyl-D-aspartate receptor antagonist and electroacupuncture; TNF-α: tumor necrosis factor; PVN: paraventricular nucleus. One-way analysis of variance was adopted, and the least significant difference t-test was used for the comparison among groups. Data were presented as mean ± standard deviation (n = 16). aP < 0.01 and dP < 0.05, vs control group; b P < 0.05, vs model group; cP < 0.05, vs Sham group.
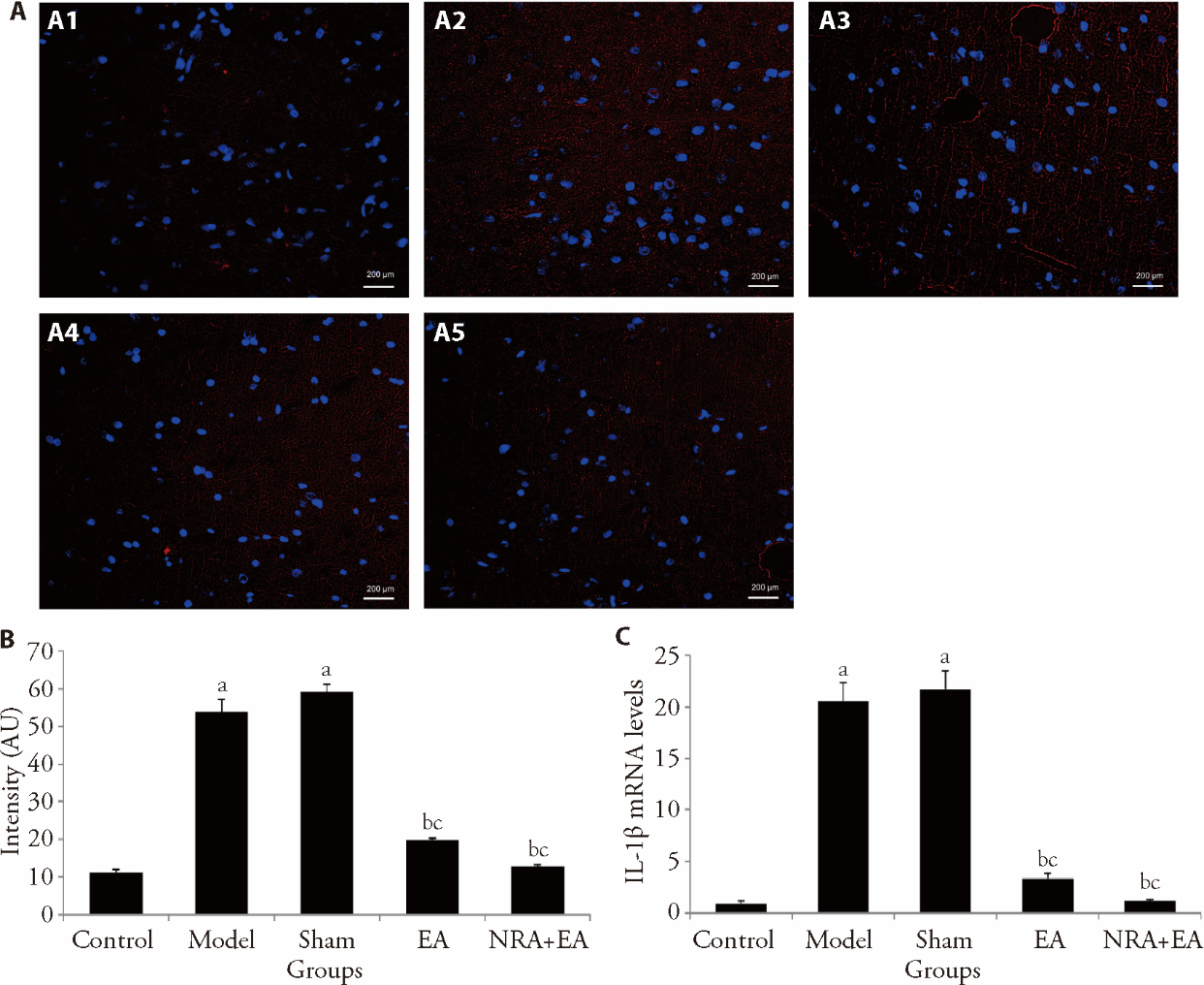
Figure 4 IL-1β levels in the PVN A: immunofluorescence images of IL-1β in different groups. A1: control group; A2: model group; A3: sham group; A4: EA group; A5: NRA + EA group; B: immunofluorescence intensity values of IL-1β in each group; C: expression of IL-1β mRNA. Control and Model groups: fixed with a rat sleeve (as per rats in the EA and NRA + EA groups) without any acupuncture intervention for 14 d; Sham group: inject the Artificial cerebrospinal fluid (10 mmol/L, 100 nL) and fixed with a rat sleeve (as per rats in the EA and NRA + EA groups) without any acupuncture intervention for 14 d; EA group: electro-acupuncture at Taichong (LR3) and Quchi (LI11) acupoints for 14 d; NRA + EA group: inject the N-methyl-D-aspartic acid receptor inhibitor (10 mmol/L, 100 nL) and electro-acupuncture at Taichong (LR3) and Quchi (LI11) acupoints for 14 d. EA: electroacupuncture; NRA+EA: N-methyl-D-aspartate receptor antagonist and electroacupuncture; IL-1β: interleukin-1β; PVN: paraventricular nucleus. One-way analysis of variance was adopted, and the least significant difference t-test was used for the comparison among groups. Data were presented as mean ± standard deviation (n = 16). aP < 0.05, vs control group; bP < 0.05, vs model group; cP < 0.05, vs Sham group.
| Group | n | NE | AVP |
|---|---|---|---|
| Control | 16 | 1.9±0.5 | 1.1±0.3 |
| Model | 16 | 17.9±1.2a | 18.8±1.3a |
| Sham | 16 | 17.5±1.5a | 18.6±1.5a |
| EA | 16 | 7.5±0.7bc | 17.1±0.7bc |
| NRA+EA | 16 | 5.9±0.4bc | 15.4±1.1bc |
Table 3 NE and AVP levels in serum (pg/mL)
| Group | n | NE | AVP |
|---|---|---|---|
| Control | 16 | 1.9±0.5 | 1.1±0.3 |
| Model | 16 | 17.9±1.2a | 18.8±1.3a |
| Sham | 16 | 17.5±1.5a | 18.6±1.5a |
| EA | 16 | 7.5±0.7bc | 17.1±0.7bc |
| NRA+EA | 16 | 5.9±0.4bc | 15.4±1.1bc |
| 1. | Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology/American Heart Association Joint Committee on clinical practice guidelines. Circulation 2022; 145: e876-94. |
| 2. |
Gill D, Georgakis MK, Koskeridis F, et al. Use of genetic variants related to antihypertensive drugs to inform on efficacy and side effects. Circulation 2019; 140: 270-9.
DOI PMID |
| 3. | Zhang M, Zhu Y, Wang J, Li Y, Hua Z. Association between acupuncture and grade 1 hypertension: a systematic review and Meta-analysis. Complement Ther Clin Pract 2022; 49: 101649. |
| 4. | Zhang J, Lyu T, Yang Y, et al. Acupuncture at LR3 and KI3 shows a control effect on essential hypertension and targeted action on cerebral regions related to blood pressure regulation: a resting state functional magnetic resonance imaging study. Acupunct Med 2021; 39: 53-63. |
| 5. |
Guo Q, Liu Q, Sun D, et al. Twirling reinforcing-reducing manipulation-central mechanism underlying antihypertensive effect on spontaneous hypertension in rats. J Tradit Chin Med 2018; 38: 391-8.
PMID |
| 6. |
Bi Q, Wang C, Cheng G, et al. Microglia-derived PDGFB promotes neuronal potassium currents to suppress basal sympathetic tonicity and limit hypertension. Immunity 2022; 55: 1466-82.e9.
DOI PMID |
| 7. | Savić B, Murphy D, Japundžić-Žigon N. The paraventricular nucleus of the hypothalamus in control of blood pressure and blood pressure variability. Front Physiol 2022; 13: 858941. |
| 8. | Sohn R, Jenei-Lanzl Z. Role of the sympathetic nervous system in mild chronic inflammatory diseases: focus on osteoarthritis. Neuroimmunomodulation 2023; 30: 143-66. |
| 9. |
Gao N, Wang H, Xu X, Yang Z, Zhang T. Angiotensin II induces cognitive decline and anxiety-like behavior via disturbing pattern of theta-gamma oscillations. Brain Res Bull 2021; 174: 84-91.
DOI PMID |
| 10. |
Underwood CF, Burke PGR, Kumar NN, et al. Upregulated angiotensin Ia receptors in the hypothalamic paraventricular nucleus sensitize neuroendocrine vasopressin release and blood pressure in a rodent model of polycystic kidney disease. Neuroendocrinology 2022; 112: 1200-13.
DOI PMID |
| 11. | Sun HJ, Chen D, Han Y, et al. Relaxin in paraventricular nucleus contributes to sympathetic overdrive and hypertension via PI3K-Akt pathway. Neuropharmacology 2016; 103: 247-56. |
| 12. | 12. Liu CY, Xie DP, Liu KJ, et al. Oxytocin microinjected into dorsal motor nucleus of the vagus excites gallbladder motility via NMDA receptor-NO-cGMP pathway. Brain Res 2005; 1032: 116-22. |
| 13. | Ji Z, Liang J, Wu J, et al. Effects of electroacupuncture at Taichong (LR3) and Baihui (GV20) on cardiac hypertrophy in rats with spontaneous hypertension. J Tradit Chin Med 2019; 39: 502-8. |
| 14. | Shao R, Wang X, Xu T, Xia Y, Cui D. The balance between AIM2-associated inflammation and autophagy: the role of CHMP2A in brain injury after cardiac arrest. J Neuroinflammation 2021; 18: 257. |
| 15. | Lu P, Liang LW, Xu AL, et al. Pro-inflammatory cytokines in the paraventricular nucleus mediate the adipose afferent reflex in rats. Pflugers Arch 2020; 472: 343-54. |
| 16. |
Kalavi K, Jorjani O, Faghihi MA, Mowla SJ. Cytokine gene expression alterations in human macrophages infected by leishmania major. Cell J 2021; 22: 476-81.
DOI PMID |
| 17. |
Maenosono R, Fukushima T, Kobayashi D, et al. Unplanned hemodialysis initiation and low geriatric nutritional risk index scores are associated with end-stage renal disease outcomes. Sci Rep 2022; 12: 11101.
DOI PMID |
| 18. | Gonzalez JE, Cooke WH. Acute effects of electronic cigarettes on arterial pressure and peripheral sympathetic activity in young nonsmokers. Am J Physiol Heart Circ Physiol 2021; 320: H248-55. |
| 19. | Wang Y, Hu H, Yin J, et al. TLR4 participates in sympathetic hyperactivity Post-MI in the PVN by regulating NF-κB pathway and ROS production. Redox Biol 2019; 24: 101186. |
| 20. | Zhang RM, McNerney KP, Riek AE, Bernal-Mizrachi C. immunity and hypertension. Acta Physiol (Oxf) 2021; 231: e13487. |
| 21. | Ma H, Chen SR, Chen H, et al. α2δ-1 is essential for sympathetic output and NMDA receptor activity potentiated by angiotensin Ⅱ in the hypothalamus. J Neurosci 2018; 38: 6388-98. |
| 22. | Li B, Deng S, Sang B, et al. Revealing the neuroimaging mechanism of acupuncture for poststroke aphasia: a systematic review. Neural Plast 2022; 2022: 5635596. |
| 23. |
Luo X, Huang J, Yu J, et al. Effect of Taichong (LR 3) acupuncture in spontaneously hypertensive rats. J Tradit Chin Med 2019; 39: 74-80.
PMID |
| 24. | Tan YY, Wang YY, Zhang Q. Electroacupuncture of "Quchi" (LI 11) Inhibits the elevation of arterial blood pressure and abnormal sympathetic nerve activity in hypertension rats. Zhen Ci Yan Jiu 2016; 41: 144-9. |
| 25. | Yang JW, Ye Y, Wang XR, et al. Acupuncture attenuates renal sympathetic activity and blood pressure via Beta-Adrenergic receptors in spontaneously hypertensive rats. Neural Plast 2017; 2017: 8696402. |
| 26. | De Matsukawa K, Iwamoto GA, Mitchell JH, et al. Exaggerated renal sympathetic nerve and pressor responses during spontaneously occurring motor activity in hypertensive rats. Am J Physiol Regul Integr Comp Physiol 2023; 324: R497-512. |
| 27. | Collister JP, Taylor-Smith H, Drebes D, et al. Angiotensin Ⅱ-induced hypertension is attenuated by overexpressing Copper/Zinc superoxide dismutase in the brain organum vasculosum of the lamina terminalis. Oxid Med Cell Longev 2016; 2016: 3959087. |
| 28. | Al-Atta A, Spray L, Mohammed A, Shmeleva E, Spyridopoulos I. Arginine vasopressin plays a role in microvascular dysfunction after ST-Elevation myocardial infarction. J Am Heart Assoc 2023; 12: e030473. |
| 29. | Shi Z, Madden CJ, Brooks VL. Arcuate neuropeptide Y inhibits sympathetic nerve activity via multiple neuropathways. J Clin Invest 2017; 127: 2868-80. |
| 30. |
Qiao X, Zhou JJ, Li DP, et al. Src kinases regulate glutamatergic input to hypothalamic presympathetic neurons and sympathetic outflow in hypertension. Hypertension 2017; 69: 154-62.
PMID |
| 31. |
Zhang H, Zhou JJ, Shao JY, et al. Hypothalamic corticotropin-releasing hormone contributes to hypertension in spontaneously hypertensive rats. J Neurosci 2023; 43: 4513-24.
DOI PMID |
| 32. | Stern JE, Son S, Biancardi VC, Zheng H, et al. Astrocytes contribute to angiotensin Ⅱ stimulation of hypothalamic neuronal activity and sympathetic outflow. Hypertension 2016; 68: 1483-93. |
| 33. |
Mohammed M, Berdasco C, Lazartigues E. Brain angiotensin converting enzyme-2 in central cardiovascular regulation. Clin Sci (Lond) 2020; 134: 2535-47.
DOI PMID |
| 34. |
Wang M, Pan W, Xu Y, et al. Microglia-mediated neuroinflammation: a potential target for the treatment of cardiovascular diseases. J Inflamm Res 2022; 15: 3083-94.
DOI PMID |
| 35. | Zhou X, Yang H, Song X, et al. Central blockade of the AT1 receptor attenuates pressor effects via reduction of glutamate release and downregulation of NMDA/AMPA receptors in the rostral ventrolateral medulla of rats with stress-induced hypertension. Hypertens Res 2019; 42: 1142-51. |
| 36. | Pekas EJ, Shin J, Headid RJ, et al. Combined anthocyanins and bromelain supplement improves endothelial function and skeletal muscle oxygenation status in adults: a double-blind placebo-controlled randomised crossover clinical trial. Br J Nutr 2021; 125: 161-71. |
| 37. |
Chen J, Chu Y, Gao M, et al. Cardiac sympathetic afferent ablation to prevent ventricular arrhythmia complicating acute myocardial infarction by inhibiting activated astrocytes. J Cell Mol Med 2022; 26: 4805-13.
DOI PMID |
| 38. |
Gong X, Hu H, Qiao Y, et al. The involvement of renin-angiotensin system in lipopolysaccharide-induced behavioral changes, neuroinflammation, and disturbed insulin signaling. Front Pharmacol 2019; 10: 318.
DOI PMID |
| [1] | LI Yongfeng, CHEN Xinyi, REN Wei, QIAO Haifa. Electroacupuncture stimulation of auricular concha region improves loss of control over stress induced depression-like behavior by modulating 5-hydroxytryptamine 1A receptor [J]. Journal of Traditional Chinese Medicine, 2025, 45(2): 326-334. |
| [2] | HU Junwei, FENG Jiwei, LI Wen, LIU Lumin, LI Xu, XU Ge, LIU Jiandang, CHEN Yuelai. Electroacupuncture improves cyclophosphamide-induced bladder overactivity by reducing mechanotransduction in the rat urothelium [J]. Journal of Traditional Chinese Medicine, 2025, 45(2): 348-358. |
| [3] | LAI Xiaolei, SHANG Juju, LIU Hongxu, HU Jing, LI Xiang, ZHANG Zhenmin, XING Wenlong. Clinical efficacy of Angong Jiangya pill (安宫降压丸) for grade 2 hypertension with liver-fire hyperactivity syndrome: a randomized, double-blind, placebo-controlled, multicenter trial [J]. Journal of Traditional Chinese Medicine, 2025, 45(2): 422-429. |
| [4] | LI Siting, WANG Shaojun, YIN Yehui, DE Gejing, LI Caicai, WANG Ziyan, CAO Wenjie. Electroacupuncture alleviates zymosan-induced colorectal hypersensitivity [J]. Journal of Traditional Chinese Medicine, 2025, 45(1): 32-38. |
| [5] | Emre Bulut, Didem Özkal Eminoğlu, Yasemin Çayır. Effect of electroacupuncture on pain after periodontal flap surgery: a randomized controlled trial [J]. Journal of Traditional Chinese Medicine, 2025, 45(1): 184-191. |
| [6] | ZHANG Boyang, ZHOU Yang, FENG Liyuan, SUI Dan, HE Lei, TONG Dan, WANG Ruoyu, SUI Xue, SONG Jing, WANG Dongyan. A neural regulation mechanism of head electroacupuncture on brain network of patients with stroke related sleep disorders [J]. Journal of Traditional Chinese Medicine, 2024, 44(6): 1268-1276. |
| [7] | XU Yingshan, WU Chunxiao, YU Wei, GUO Hongji, LU Liming, XU Nenggui, TANG Chunzhi. Systematic review and Meta-analysis of brain plasticity associated with electroacupuncture in experimental ischemic stroke [J]. Journal of Traditional Chinese Medicine, 2024, 44(5): 859-870. |
| [8] | ZHANG Fang, YAN Cuina, WENG Zhijun, WU Luyi, QI Li, ZHAO Min, XIN Yuhu, WU Huangan, LIU Huirong. Regulatory role of electroacupuncture on satellite glial cell activity in the colon and dorsal root ganglion of rats with irritable bowel syndrome [J]. Journal of Traditional Chinese Medicine, 2024, 44(5): 981-990. |
| [9] | CHEN Yonglin, OUYANG Ling, MENG Lingling, WU Bufan, PENG Rou, LIU Sitong, HOU Dan, WANG Yaling, JING Xinyue, LU Shengfeng, FU Shuping. Electroacupuncture ameliorates blood-brain barrier disruption after ischemic stroke through histone acetylation regulation at the matrix metalloproteinase 9 and tissue inhibitor of metalloproteinase 2 genes [J]. Journal of Traditional Chinese Medicine, 2024, 44(4): 734-744. |
| [10] | ZHOU Ying, LI Ping, LUAN Jianwei, SHEN Rui, WU Yinglan, XU Qiwen, WANG Xinyue, ZHU Yao, XU Xiangru, LIU Zitian, JIANG Yuning, ZHONG Yong, HE Yun, JIANG Weimin. Study on blood pressure rhythm in hypertensive patients with Yin deficiency syndrome and a random forest model for predicting hypertension with Yin deficiency syndrome [J]. Journal of Traditional Chinese Medicine, 2024, 44(3): 564-571. |
| [11] | WANG Shaosong, SUN Jingqing, FENG Qingyin, LI Bin, WANG Xin, YUAN Fan, CUI Yingxue. Effectivenss of electroacupuncture for skeletal muscle pain in Parkinson's disease: a Clinical randomized controlled trial [J]. Journal of Traditional Chinese Medicine, 2024, 44(2): 388-395. |
| [12] | QIN Xiaoyu, WANG Chunai, XUE Jianjun, ZHANG Jie, LU Xiaoting, DING Shengshuang, GE Long, WANG Minzhen. Efficacy of electroacupuncture on myocardial protection and postoperative rehabilitation in patients undergoing cardiac surgery with cardiopulmonary bypass: a systematic review and Meta-analysis [J]. Journal of Traditional Chinese Medicine, 2024, 44(1): 1-15. |
| [13] | SUN Qianhui, CHENG Kai, DAI Xingye, YANG Zhiwen, WU Xiaoling, XU Chang, QIU Xinghua, GAO Xiaofeng, LIU Daonan, YANG Qirui. Effect of electroacupuncture at Neiguan (PC6) at different time points on myocardial ischemia reperfusion arrhythmia in rats [J]. Journal of Traditional Chinese Medicine, 2024, 44(1): 113-121. |
| [14] | DU Zhongheng, CONG Wenjie, TANG Kejing, ZHENG Qiqi, SONG Zhiwei, CHEN Yong, YANG Su, ZHANG Chunwu, YE Tianshen. Electroacupuncture stimulating Zusanli (ST36), Sanyinjiao (SP6) in mice with collagen-induced arthritis leads to adenosine A2A receptor-mediated alteration of p38α mitogen-activated protein kinase signaling and inhibition of osteoclastogenesis [J]. Journal of Traditional Chinese Medicine, 2023, 43(6): 1103-1109. |
| [15] | JIANG Jianzhen, ZHANG Xin, LUO Zhenguo, SU Chengguo, ZHOU Haiyan, JIANG Yuqing, XIAO Xianjun, CHEN Yunfei, ZHU Jun. Efficacy of electroacupuncture stimulating Zusanli (ST36) and Xuanzhong (GB39) on synovial angiogenesis in rats with adjuvant arthritis [J]. Journal of Traditional Chinese Medicine, 2023, 43(5): 955-962. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||

