Journal of Traditional Chinese Medicine ›› 2025, Vol. 45 ›› Issue (2): 326-334.DOI: 10.19852/j.cnki.jtcm.2025.02.014
• Original articles • Previous Articles Next Articles
Electroacupuncture stimulation of auricular concha region improves loss of control over stress induced depression-like behavior by modulating 5-hydroxytryptamine 1A receptor
LI Yongfeng1, CHEN Xinyi1, REN Wei2, QIAO Haifa1( )
)
- 1 Acupuncture and Moxibustion College, Shaanxi University of Traditional Chinese Medicine, Xianyang 712046, China
2 Faculty of Education, Shaanxi Normal University, Xi’an 710062, China
-
Received:2023-10-24Accepted:2024-03-08Online:2025-04-15Published:2025-03-10 -
Contact:QIAO Haifa, Acupuncture and Moxibustion College, Shaanxi University of Traditional Chinese Medicine, Xianyang 712046, China. 1511006@sntcm.edu.cn, Telephone: +86-29-38180212 -
Supported by:Natural Science Foundation of Shaanxi Province: Acupuncture’s Anxiolytic Effects in Uncontrollable Stress: Modulation of the Median Raphe Nucleus to Ventral Hippocampus Serotonergic Pathway(2022JQ-949);National Natural Science Foundation of China: Study on the Mechanisms of Learning and Memory Impairment induced by Chronic Pain and the Intervention Effects of Acupuncture(82004489)
Cite this article
LI Yongfeng, CHEN Xinyi, REN Wei, QIAO Haifa. Electroacupuncture stimulation of auricular concha region improves loss of control over stress induced depression-like behavior by modulating 5-hydroxytryptamine 1A receptor[J]. Journal of Traditional Chinese Medicine, 2025, 45(2): 326-334.
share this article
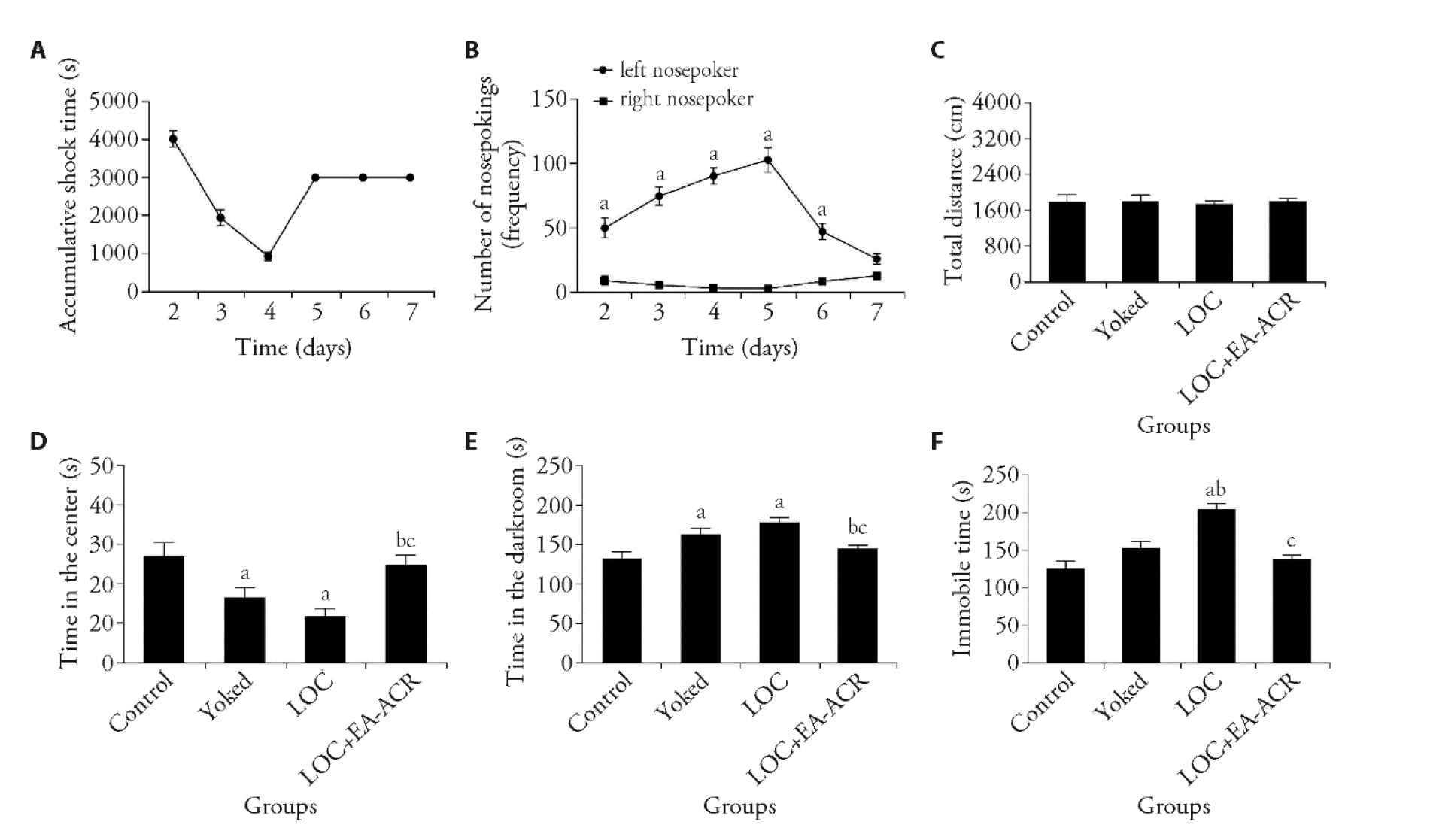
Figure 1 Effects of EA-ACR stimulation on depression-like behavior in LOC mice A: the accumulative shock time during Days 2-7 from the mice of LOC group; B: total number of nosepoker touches during Days 2-7, there was a significant difference in the number of times the nosepoker was touched between the left and right sides; C: there were no observable differences of total distance in the four groups; D: time in the center of the open field; E: time in the darkroom of the Light-dark transition test; F: immobile time of the forced swimming. Control: days 2-4: Ms (0 mA, ≤ 1 min), Enp-off, 30-60s Ri, 5B × 10 s, 10 min Rb; days 5-7: 5b × 10 min us, Enp-invalid; days 8-12 EA-ACR (0 mA/0 Hz) × 20 min × 5 d.; LOC: days 2-4: Ms (0.15 mA, ≤ 1 min), Enp-off, 30-60 s Ri, 5B × 10 s, 10 min Rb; days 5-7: 5b × 10 min us, Enp-invalid; days 8-12 EA-ACR (0 mA/0 Hz) × 20 min × 5 d; Yoked: days 2-7 received the same foot shock intensity and pattern as the LOC, but without control over the shock; days 8-12 EA-ACR (0 mA/0 Hz) × 20 min × 5 d; LOC + EA-ACR: days 2-4: Ms (0.15 mA, ≤ 1 min), Enp-off, 30-60 s Ri, 5B × 10 s, 10 min Rb. days 5-7: 5b × 10 min us, Enp-invalid. days 8-12 EA-ACR (1 mA/2 Hz) × 20 min × 5 d. EA: electroacupuncture; ACR: auricular concha region; LOC: loss of control over stress model; Ms: (0.15 mA, ≤ 1 min) - mild shock (0.15 ma, up to 1 min); Enp-off: effective nose-poke turns off shock 30-60 s; Ri: rest interval (30-60 s), 5B × 10 s: 5 blocks of 10 shocks; 10 min Rb: 10-minute rest between blocks. Statistical analyses were measured using one-way analysis of variance followed by post hoc Bonferroni correction for multiple comparisons. Data are presented as mean ± standard error of mean (n = 11). Compared with control group, aP < 0.01; compared with yoked group, bP < 0.01; compared with LOC group, cP < 0.01.
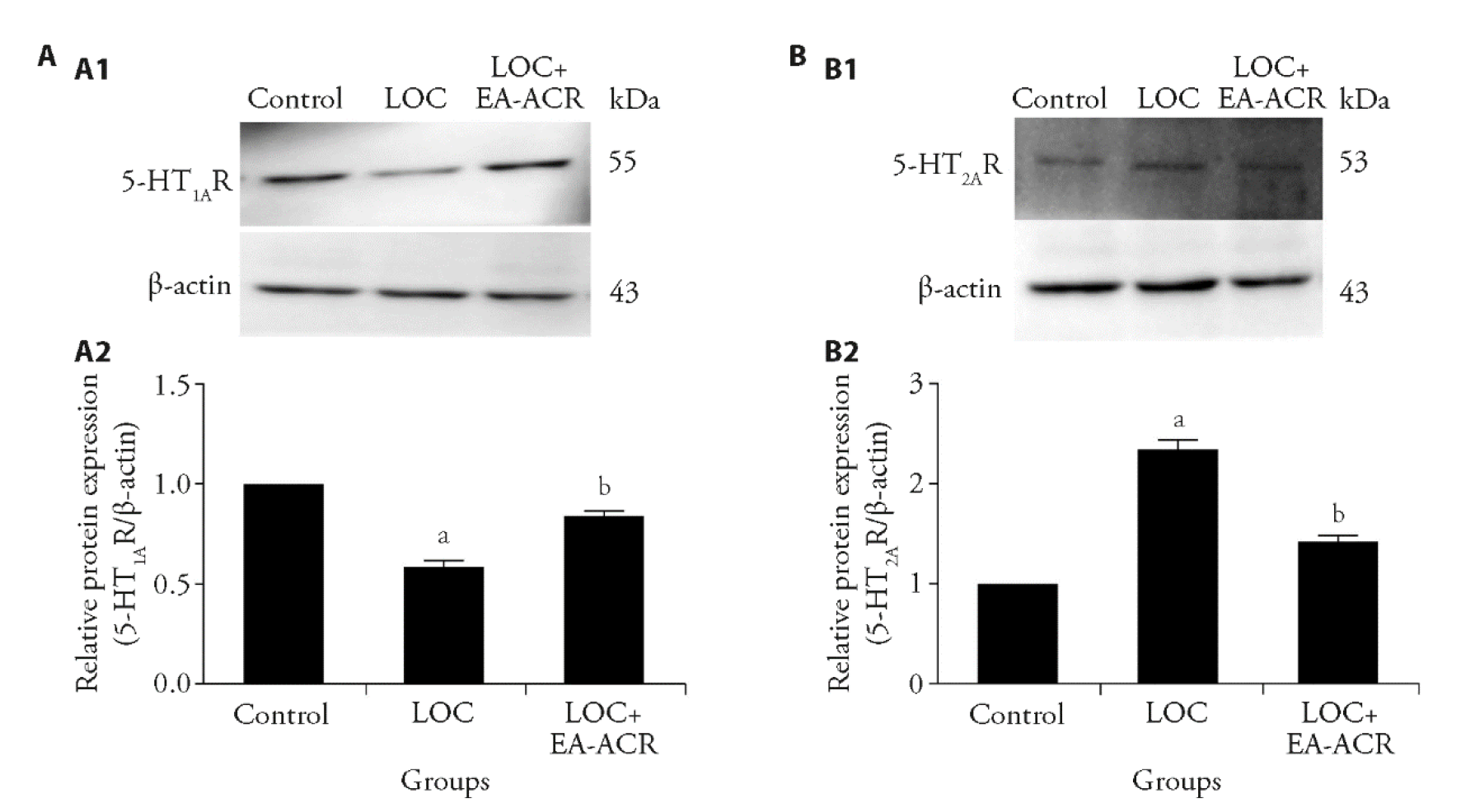
Figure 2 Effect of EA-ACR on 5-HT1AR and 5-HT2AR protein expression in Hip of LOC mice A1: representative Western blot of 5-HT1AR protein expression in the hippocampus; A2: Western blot analysis of the EA-ACR effect 5-HT1AR protein expression with β-actin as loading control among different groups; B1: representative Western blot of 5-HT2AR protein expression in the hippocampus; B2: western blot analysis of the EA-ACR effect 5-HT2AR protein expression with β-actin as loading control among different groups. Control: days 2-4: Ms (0 mA, ≤ 1 min), Enp-off, 30-60s Ri, 5B × 10 s, 10 min Rb. days 5-7: 5b × 10 min us; days 8-12 EA-ACR (0 mA/0 Hz) × 20 min × 5 d. Enp-invalid; LOC: days 2-4: Ms (0.15 mA, ≤ 1 min), Enp-off, 30-60 s Ri, 5B × 10 s, 10 min Rb; days 5-7: 5b × 10 min us; days 8-12 EA-ACR (0 mA/0 Hz) × 20 min × 5 d; LOC+ EA-ACR: days 2-4: Ms (0.15 mA, ≤ 1 min), Enp-off, 30-60s Ri, 5B × 10s, 10 min Rb; days 5-7: 5b × 10 min us; days 8-12 EA-ACR (1 mA/2 Hz) × 20 min × 5 d. 5-HT1AR: hydroxytryptamine 1A receptor; LOC: loss of control over stress model; EA: electroacupuncture; ACR: stimulation of auricular concha region; Ms: (0.15 mA, ≤ 1 min) - mild shock (0.15 ma, up to 1 min); Enp-off: effective nose-poke turns off shock 30-60s; Ri: rest interval (30-60 s), 5B ×10 s: 5 blocks of 10 shocks; 10 min Rb: 10-minute rest between blocks. Statistical analyses were measured using one-way analysis of variance followed by post hoc Bonferroni correction for multiple comparisons. Data are presented as mean ± standard error of mean (n = 4). Compared with control group, aP < 0.01; compared with LOC group, bP < 0.01.
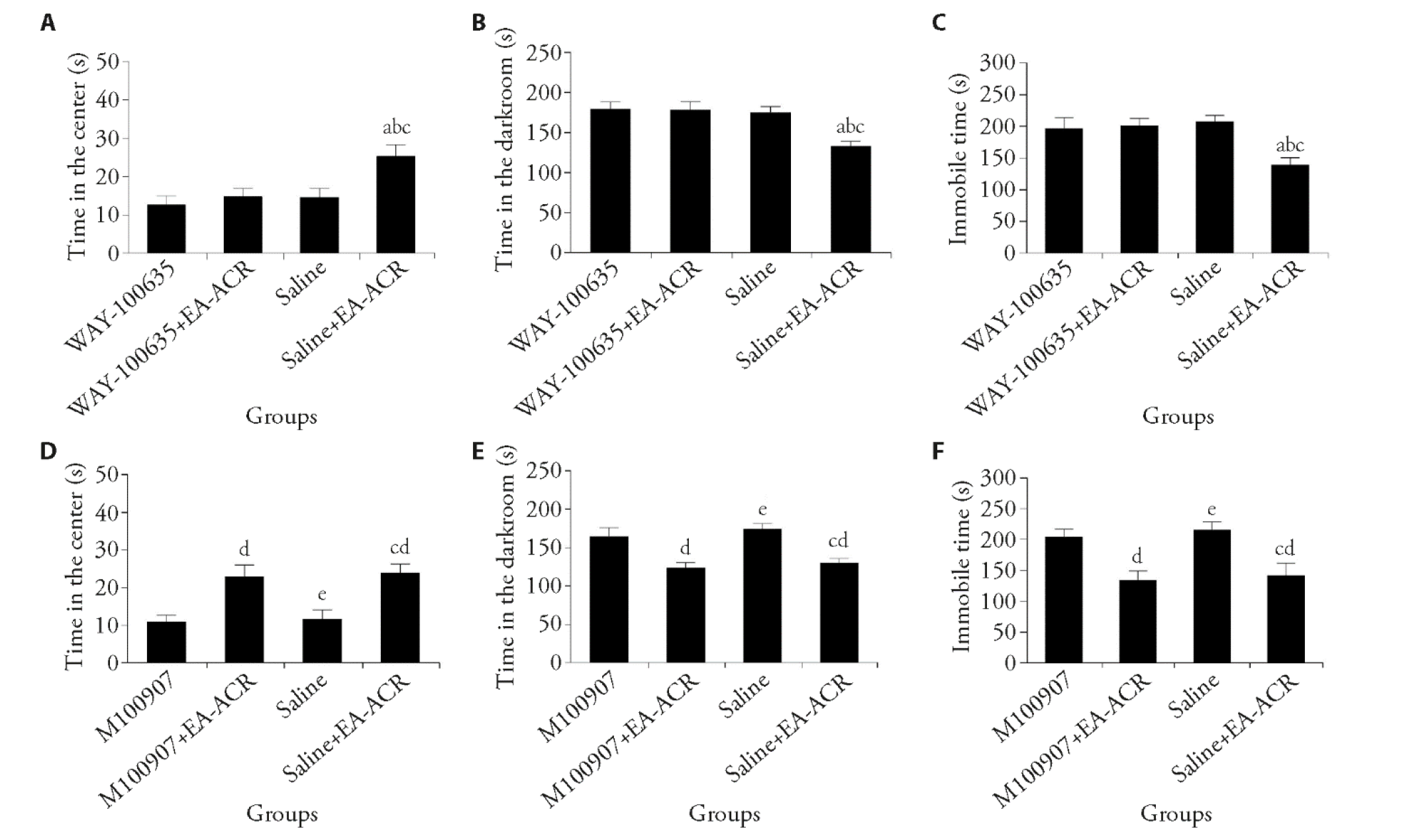
Figure 3 WAY-100635 antagonizes 5-HT1AR and EA-ACR does not improve LOC-induced depression-like behaviors A: time in the center of the open field; B: time in the darkroom of the Light-dark transition test; C: immobile time of the Forced swimming; D: time in the center of the open field; E: time in the darkroom of the Light-dark transition test; F: immobile time of the forced swimming. WAY-100635: LOC + 0.5 ng/nL 150 ng per side N-[2-[4-(2-methoxyphenyl)piperazin-1-yl] ethyl]-N- pyridin-2-ylcyclo hexane carboxamide (150 ng/side); M100907: LOC + 1 ng/nL 300ng per side (R-(+)-a- (2,3-dimethoxyphenil)-1-[4- fluorophenylethyl)]-4-piperidinemethanol (300 ng/side); WAY-100635 + EA-ACR: LOC + 0.5 ng/nL 150 ng per side WAY-100635 + EA-ACR; M100907+EA-ACR: LOC + 1 ng/nL 300 ng per side M100907 + EA-ACR; Saline: 300 nL 0.9%Nacl; Saline + EA-ACR: 300 nL 0.9%Nacl + EA-ACR. Control: control of non-foot-shock; LOC: loss of control over stress model; EA: electroacupuncture; ACR: stimulation of auricular concha region. Statistical analyses were measured using one-way analysis of variance followed by post hoc Bonferroni correction for multiple comparisons; Data are presented as mean ± standard error of mean (n = 8). Compared with WAY-100635 group, aP < 0.01; compared with WAY-100635 + EA-ACR group, bP < 0.01; compared with saline group, cP < 0.01; Compared with M100907 group, dP < 0.01; compared with M100907 + EA-ACR group, eP < 0.01.
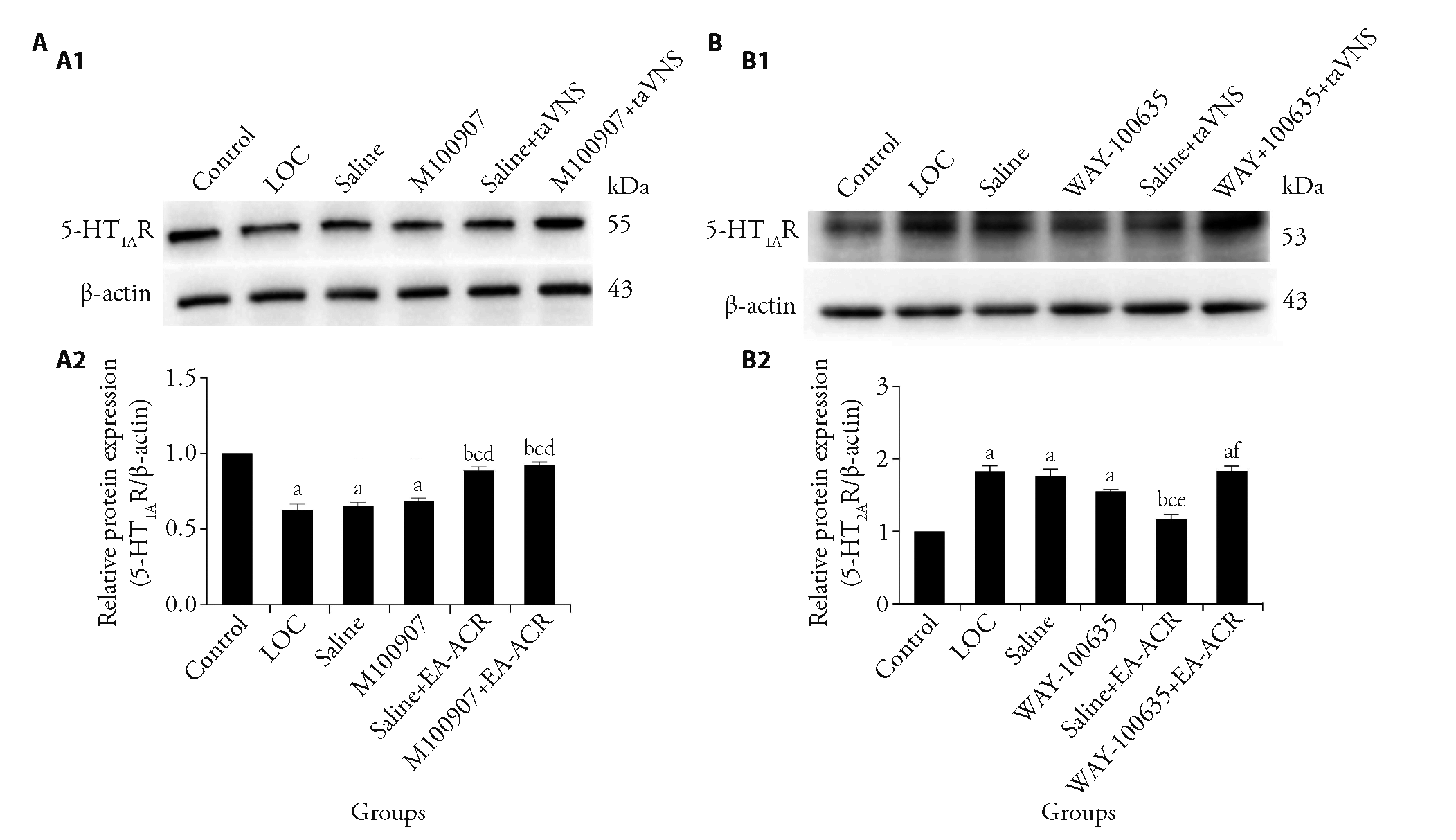
Figure 4 EA-ACR regulates LOC-induced depression-like behavior through 5-HT1AR A1: representative Western blot of 5-HT1AR protein expression in the hippocampus; A2: effect of electrodes on 5-HT1AR protein expression in Hip of LOC mice after antagonism of 5-HT2AR; B1: representative Western blot of 5-HT2AR protein expression in the hippocampus; B2: Effect of electrodes on 5-HT2AR protein expression in Hip of LOC mice after antagonism of 5-HT1AR. WAY-100635: LOC + 0.5 ng/nL 150 ng per side N-[2-[4-(2-methoxyphenyl)piperazin-1-yl] ethyl]-N-pyridin-2-ylcyclo hexane carboxamide (150 ng/side); M100907: LOC + 1 ng/nL 300 ng per side (R-(+)-a- (2,3-dimethoxyphenil)-1-[4- fluorophenylethyl)]-4- piperidinemethanol (300 ng/side); WAY-100635 + EA-ACR: LOC + 0.5 ng/nL 150 ng per side WAY-100635 + EA-ACR; M100907+EA-ACR: LOC + 1 ng/nL 300 ng per side M100907 + EA-ACR; Saline: 300 nL 0.9%Nacl; Saline + EA-ACR: 300 nL 0.9%Nacl + EA-ACR. Control: control of non-foot-shock; LOC: loss of control over stress model; EA: electroacupuncture; ACR: stimulation of auricular concha region. Statistical analyses were measured using one-way analysis of variance followed by post hoc Bonferroni correction for multiple comparisons; Data are presented as mean ± standard error of mean (n = 4). Compared with control group, aP < 0.01; Compared with LOC group, bP < 0.01; compared with saline group, cP < 0.01; compared with M100907 group, dP < 0.01; compared with WAY-100635 group, eP < 0.01; compared with Saline + EA-ACR group, fP < 0.01.
| 1. |
Hannibal KE, Bishop MD. Chronic stress, cortisol dysfunction, and pain: a psychoneuroendocrine rationale for stress management in pain rehabilitation. Phys Ther 2014; 94: 1816-25.
DOI PMID |
| 2. | Marin MF, Lord C, Andrews J, et al. Chronic stress, cognitive functioning and mental health. Neurobiol Learn Mem 2011; 96: 583-95. |
| 3. |
Güdül ÖZ H, Nazik E. The relationship between fear of COVID-19 and depression, anxiety and stress in persons with disabilities: A cross-sectional study. Arch Psychiat Nurs 2023; 43: 15-21.
DOI PMID |
| 4. |
Yao L, Li Y, Qian Z, et al. Loss of control over mild aversive events produces significant helplessness in mice. Behav Brain Res 2019; 376: 112173.
DOI |
| 5. | McEwen BS, Nasca C, Gray JD. Stress effects on neuronal structure: hippocampus, amygdala, and prefrontal cortex. Neuropsychopharmacol 2016; 41: 3-23. |
| 6. | van Tol MJ, van der Wee N JA, van den Heuvel OA, et al. Regional brain volume in depression and anxiety disorders. Arch Gen Psychiat 2010; 67: 1002-11. |
| 7. |
McEwen BS, Eiland L, Hunter RG, et al. Stress and anxiety: structural plasticity and epigenetic regulation as a consequence of stress. Neuropharmacology 2012; 62: 3-12.
DOI PMID |
| 8. |
Revest JM, Dupret D, Koehl M, et al. Adult hippocampal neurogenesis is involved in anxiety-related behaviors. Mol Psychiatr 2009; 14: 959-67.
DOI PMID |
| 9. |
Desrosiers A, Vine V, Klemanski DH, et al. Mindfulness and emotion regulation in depression and anxiety: common and distinct mechanisms of action. Depress Anxiety 2013; 30: 654-61.
DOI PMID |
| 10. |
Hein E, Nowak M, Kiess O, et al. Auricular transcutaneous electrical nerve stimulation in depressed patients: a randomized controlled pilot study. J Neural Transm 2013; 120: 821-27.
DOI PMID |
| 11. | Zhang Y, Liu J, Li H, et al. Transcutaneous auricular vagus nerve stimulation at 1 Hz modulates locus coeruleus activity and resting state functional connectivity in patients with migraine: an fMRI study. Neuroimage Clin 2019; 24: 101971. |
| 12. | Yuan TF, Li A, Sun X, et al. Vagus nerve stimulation in treating depression: a tale of two stories. CURR Curr Mol Med 2016; 16: 33-9. |
| 13. |
Miller BR, Hen R. The current state of the neurogenic theory of depression and anxiety. Curr Opin Neurobiol 2015; 30: 51-8.
DOI PMID |
| 14. | Feng L, Xing H, Zhang K. The therapeutic potential of Traditional Chinese Medicine in depression: targeting adult hippocampal neurogenesis. Phytomedicine 2022; 98: 1-21. |
| 15. |
Dorr AE, Debonnel G. Effect of vagus nerve stimulation on serotonergic and noradrenergic transmission. J Pharmacol Exp Ther 2006; 318: 890-98.
PMID |
| 16. | Morrissette DA, Stahl SM. Modulating the serotonin system in the treatment of major depressive disorder. Cns Spectrums 2014; 19: 54-68. |
| 17. | Samuels BA, Mendez-David I, Faye C, et al. Serotonin 1A and serotonin 4 receptors: essential mediators of the neurogenic and behavioral actions of antidepressants. J. Neurosci 2016; 22: 26-45. |
| 18. |
Xiang M, Jiang Y, Hu Z, et al. Serotonin receptors 2A and 1A modulate anxiety-like behavior in post-traumatic stress disordered mice. Am J Transl Res 2019; 11: 2288.
PMID |
| 19. |
Taciak PP, Lysenko N, Mazurek AP. Drugs which influence serotonin transporter and serotonergic receptors: pharmacological and clinical properties in the treatment of depression. Pharmacol Rep 2018; 70: 37-46.
DOI PMID |
| 20. |
Boldrini M, Underwood MD, Mann JJ, et al. Serotonin-1A autoreceptor binding in the dorsal raphe nucleus of depressed suicides. J Psychiatr Res 2008; 42: 433-42.
DOI PMID |
| 21. | Dasiel BE, Narváez Manuel, Patrizia A, et al. Receptor-receptor interactions in multiple 5-HT1A heteroreceptor complexes in raphe-hippocampal 5-HT transmission and their relevance for depression and its treatment. Molecules 2018; 23: 1341. |
| 22. |
Kattalai Kailasam V, Anand P, et al. Establishing an animal model for National Acupuncture Detoxification Association (NADA) Auricular Acupuncture Protocol. Neurosci Lett 2016; 624: 29-33.
DOI PMID |
| 23. |
Light GA, Naatanen R. Mismatch negativity is a breakthrough biomarker for understanding and treating psychotic disorders. Proc Natl Acad Sci USA 2013; 110: 15175-76.
DOI PMID |
| 24. |
Chang Y, Xu J, Pang X, et al. Mismatch negativity indices of enhanced preattentive automatic processing in panic disorder as measured by a multi-feature paradigm. Biol Psychol 2015; 105: 77-82.
DOI PMID |
| 25. |
Petit-Demouliere B, Chenu F, Bourin M. Forced swimming test in mice: a review of antidepressant activity. Psychopharmacology 2005; 177: 245-55.
DOI PMID |
| 26. | Chang Y, Xu J, Shi N, et al. Dysfunction of preattentive visual information processing among patients with major depressive disorder. Biol Psychiatry 2011; 69: 742-7. |
| 27. | Amodeo DA, Rivera E, Cook Jr EH, et al. 5HT2A receptor blockade in dorsomedial striatum reduces repetitive behaviors in BTBR mice J. Genes Brain Behav 2017; 16: 342-51. |
| 28. | Jiang YF, Liu J, Yang J, et al. Involvement of the dorsal hippocampus 5-HT1A receptors in the regulation of depressive-like behaviors in hemiparkinsonian rats. Neuropsychobiology 2020; 79: 198-207. |
| 29. | Beck AT. Cognitive models of depression. Adv Cogn Psychol 2002; 14: 29-61. |
| 30. |
Dobson KS. A Meta-analysis of the efficacy of cognitive therapy for depression. J Consult Clin Psychol 1989; 57: 414.
PMID |
| 31. | Rong PJ, Fang JL, Wang LP, et al. Transcutaneous vagus nerve stimulation for the treatment of depression: a study protocol for a double blinded randomized clinical trial. BMC Complement Med Ther 2012; 12: 255. |
| 32. | Rong P, Liu J, Wang L, et al. Effect of transcutaneous auricular vagus nerve stimulation on major depressive disorder: a nonrandomized controlled pilot study. J Affect Disord 2016; 195: 172-9. |
| 33. | Zobel A, Joe A, Freymann N, et al. Changes in regional cerebral blood flow by therapeutic vagus nerve stimulation in depression: an exploratory approach. Psychiatry Res 2005; 139: 165-79. |
| 34. | Yang Y, Wang ZH, Jin S, et al. Opposite monosynaptic scaling of BLP-vCA 1 inputs governs hopefulness- and helplessness-modulated spatial learning and memory. Nat. Commun 2016; 7: 11935. |
| 35. | Yu J, Xu W, Luo Y, et al. Dynamic monitoring of depressive behavior induced by nonylphenol and its effect on synaptic plasticity in rats. Sci Total Environ 2019; 689: 1012-22. |
| 36. | Li S, Wang Y, Gao G, et al. Transcutaneous auricular vagus nerve stimulation at 20 Hz improves depression-like behaviors and down -regulates the hyperactivity of HPA axis in chronic unpredictable mild stress model rats. Front Neurosci-Switz 2020; 14: 680. |
| 37. |
Bowles S, Hickman J, Peng X, et al. Vagus nerve stimulation drives selective circuit modulation through cholinergic reinforcement. Neuron 2022; 110: 2867-85.
DOI PMID |
| 38. |
McDevitt RA, Neumaier JF. Regulation of dorsal raphe nucleus function by serotonin autoreceptors: a behavioral perspective. J Chem Neuroanat 2011; 41: 234-46.
DOI PMID |
| 39. | Lei S. Serotonergic modulation of neural activities in the entorhinal cortex. J Physiol Pharmacol 2012; 4: 201-10. |
| 40. | Quesseveur G, Nguyen HT, Gardier AM, et al. 5-HT2 ligands in the treatment of anxiety and depression. Expert Opin Investig Drugs 2012; 21: 1701-25. |
| 41. |
Boothman LJ, Sharp T. A role for midbrain raphe gamma aminobutyric acid neurons in 5-hydroxytryptamine feedback control. Neuroreport 2005; 16: 891-6.
PMID |
| 42. |
Martín-Ruiz R, Puig MV, Celada P, et al. Control of serotonergic function in medial prefrontal cortex by serotonin-2A receptors through a glutamate-dependent mechanism. J Neurosci 2001; 21: 9856-66.
PMID |
| 43. | Diaz SL, Maroteaux L. Implication of 5-HT (2B) receptors in the serotonin syndrome. Neuropharmacology 2011; 61: 495-502. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||

