Journal of Traditional Chinese Medicine ›› 2025, Vol. 45 ›› Issue (6): 1228-1237.DOI: 10.19852/j.cnki.jtcm.2025.06.005
• Original Articles • Previous Articles Next Articles
Electroacupuncture alleviates type 2 diabetes mellitus by promoting plasma-derived exosomal circular RNA of enhancer of zeste homolog 1 expression
SHOU Yin1, JIANG Juntao2, HU Jianlin3, JI Wei4, CHEN Chunyan5, HU Li6( ), MA Yuhang7, ZHANG Bimeng1(
), MA Yuhang7, ZHANG Bimeng1( )
)
- 1 Department of Acupuncture-Moxibustion, Shanghai General Hospital, Shanghai Jiaotong University School of Medicine, Shanghai 200000, China
2 Department of Urology, Shanghai General Hospital, Shanghai Jiaotong University School of Medicine, Shanghai 200000, China
3 Department of Reproduction, Shanghai General Hospital, Shanghai Jiaotong University School of Medicine, Shanghai 200000, China
4 Department of Traditional Chinese Orthopedics, Xiangshan TCM Hospital, Shanghai, China
5 Shanghai Research Institute of Qigong, Taiji Health Center, Shanghai University of Traditional Chinese Medicine, Shanghai 200000, China
6 Acumox and Tuina Research Section, College of Acumox and Tuina, Shanghai University of Traditional Chinese Medicine, Shanghai 200000, China
7 Department of Endorcinolgy and Metabolism, Shanghai General Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai 200000, China
-
Received:2024-11-12Accepted:2025-05-07Online:2025-12-15Published:2025-11-24 -
Contact:HU Li, Acumox and Tuina Research Section, College of Acumox and Tuina, Shanghai University of Traditional Chinese Medicine, Shanghai 200000, China. huli6862292006@163.com, Telephone: +86-21-37798133
Prof. ZHANG Bimeng, Department of Acupuncture-Moxibustion, Shanghai General Hospital, Shanghai Jiaotong University School of Medicine, Shanghai 200000, China. acusci2007@126.com -
About author:SHOU Yin and HU Jianlin are co-first authors and contributed equally to this work -
Supported by:Science and Technology Committee of Songjiang District, Shanghai(2024SJKJGG001);project: Mechanistic Study on the Therapeutic Effect of Electroacupuncture in Diabetic Osteoporosis via Exosomal circular RNA of Enhancer of Zeste Homolog 1-Regulated miR-128/ Transient Receptor Potential Vanilloid 1 Axis-Mediated Ferroptosis in Osteoclasts; the Shanghai Municipal Health Bureau [grant number: 2020LP010];Project: a Randomized Controlled Study of Traditional Chinese Acupuncture Combined with Rehabilitation Training for the Treatment of Intensive Care Unit-Acquired Weakness; the Science and Technology Commission of Shanghai Municipality (grant number: 20511101204);Project: Traditional Chinese Medicine Data Collection and Governance for Early Screening and Stratified Diagnosis and Treatment of Pancreatic Cancer; the Shanghai University of Traditional Chinese Medicine (grant number: 2021LK100);Project: the Role of Sestrin 2/mechanistic Target of Rapamycin-Mediated Autophagy in Age-Related Skeletal Muscle Atrophy and the Effects of Acupuncture;Shanghai General Hospital(grant number: 202220);Project: Research on the Construction of Talent Evaluation System by the Southern Outpatient Party Branch; and Shanghai Municipal Commission of Health and Family Planning [grant number: ZY(2021-2023)-0208];Project: Special Program of the Integrated Chinese and Western Medicine Innovation Research Institute
Cite this article
SHOU Yin, JIANG Juntao, HU Jianlin, JI Wei, CHEN Chunyan, HU Li, MA Yuhang, ZHANG Bimeng. Electroacupuncture alleviates type 2 diabetes mellitus by promoting plasma-derived exosomal circular RNA of enhancer of zeste homolog 1 expression[J]. Journal of Traditional Chinese Medicine, 2025, 45(6): 1228-1237.
share this article
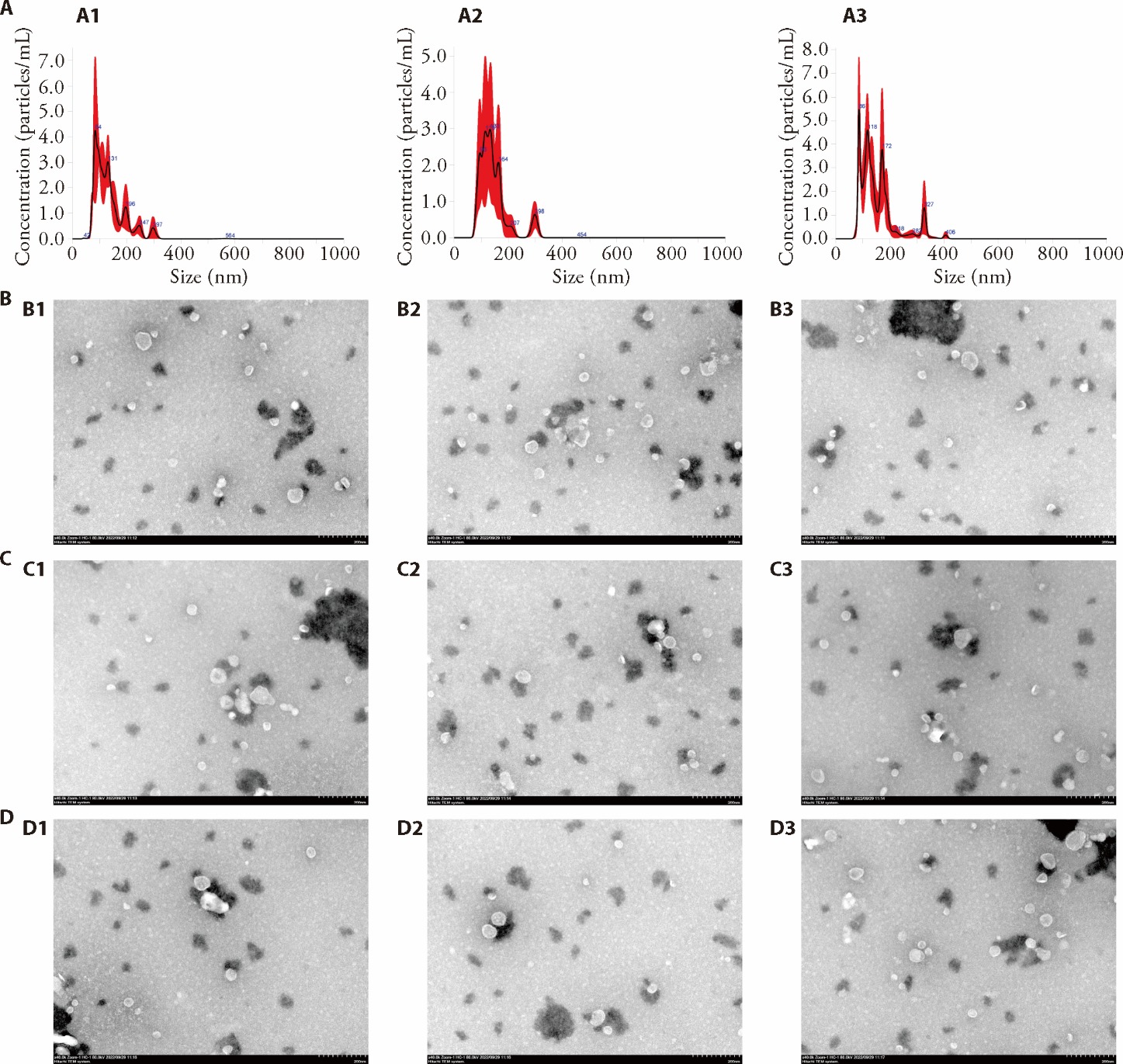
Figure 1 Evaluation of the quality of exosomes A: size distribution of plasma exosomes assessed using NTA; A1: Control; A2: T2DM; A3: T2DM + EA; B: morphological appearance of plasma exosomes under a transmission electron microscope (Control); B1: Control-1; B2: Control-2; B3: Control-3; C: morphological appearance of plasma exosomes under a transmission electron microscope (T2DM); C1:T2DM-1; C2:T2DM-2; C3:T2DM-3; D: morphological appearance of plasma exosomes under a transmission electron microscope (T2DM + EA); D1: T2DM + EA-1; D1: T2DM + EA-1; D1: T2DM + EA-1. NTA: nanoparticle tracking analysis; T2DM: type 2 diabetes; EA: electroacupuncture. Control (low-fat diet), T2DM (HFD + STZ 50 mg/kg), and T2DM + EA (T2DM mice treated with electroacupuncture at ST36 and BL20 for 4 weeks). Briefly, for the mice in the T2DM model + EA group, the acupuncture Zusanli (ST36) and Pishu (BL20) points (frequency 2 Hz, intensity 1-3 mA, intermittent waveform, serial length 30 s, increasing once every 5 min, lasting for 15 min) were stimulated once every 2 d, 3 times a week, for 4 weeks (12 times in total). Before EA treatment, T2DM mice were under 2% isoflurane anesthesia.
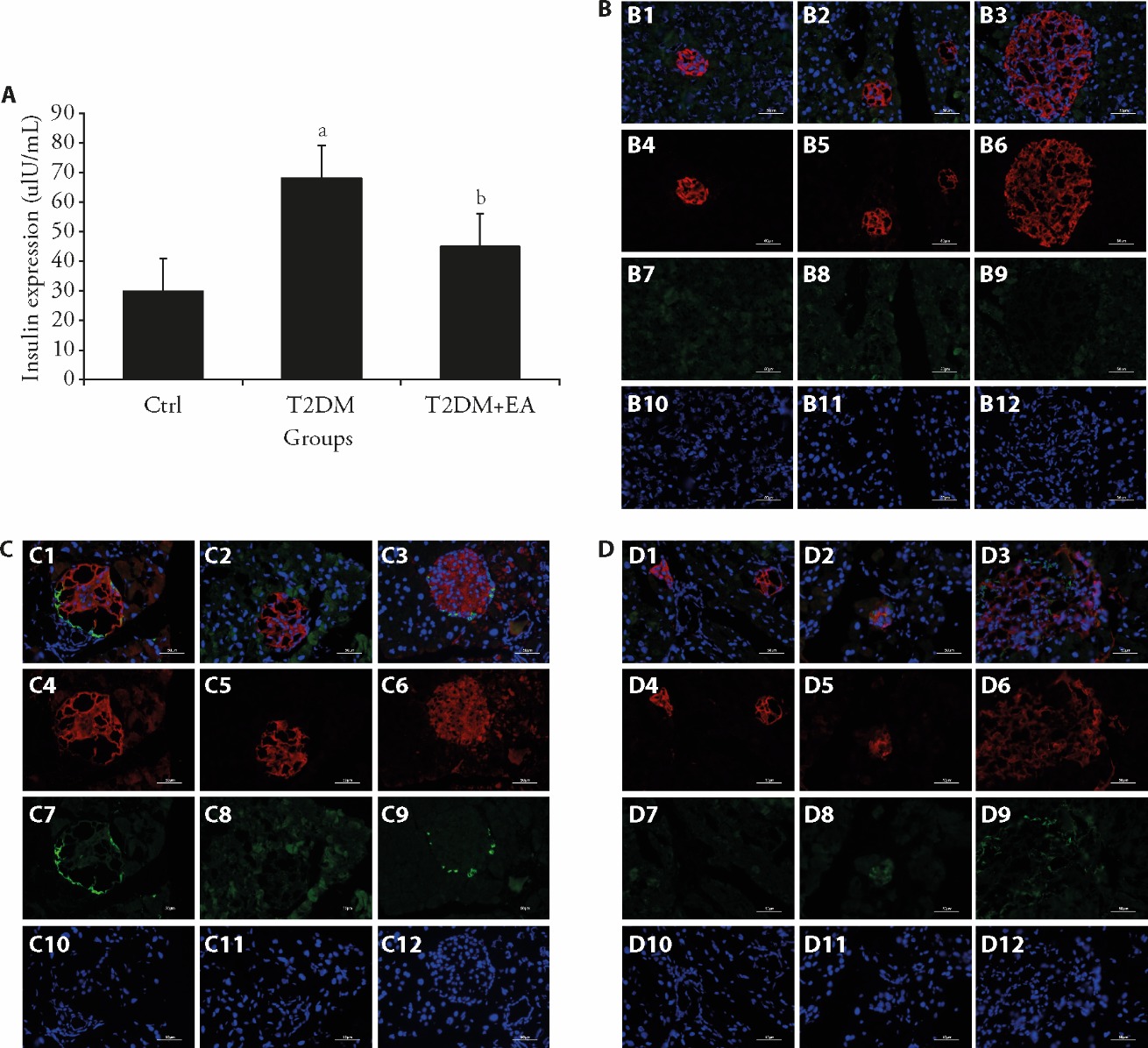
Figure 2 EA treatment reduced the insulin level and cell apoptosis in pancreatic tissue of T2DM A: serum insulin level detected using ELISA; B: immunofluorescence analysis of insulin and c-caspase 3 expression in pancreatic tissue; C: immunofluorescence analysis of insulin and c-caspase 3 expression in pancreatic tissue; D: immunofluorescence analysis of insulin and c-caspase 3 expression in pancreatic tissue; B1, C1, D1: merge-1; B2, C2, D2: merge-2; B3, C3, D3: merge-3; B4, C4, D4: insulin-1; B5, C5, D5: insulin-2; B6, C6, D6: insulin-3; B7, C7, D7: c-caspase3-1; B8, C8, D8: c-caspase3-2; B9, C9, D9: c-caspase3-3; B10, C10, D10: DAPI-1; B11, C11, D11: DAPI-2; B12, C12, D12: DAPI-3; D1: merge-1; D2: merge-2; D3: merge-3; D4: insulin-1; D5: insulin-2; D6: insulin-3; D7: c-caspase3-1; D8: c-caspase3-2; D9: c-caspase3-3; D10: DAPI-1; D11: DAPI-2; D12: DAPI-3. Control (low-fat diet), T2DM (HFD + STZ), and T2DM + CircEZH1-siRNA (T2DM mice administered CircEZH1-siRNA, 20 nmol/20 g). Briefly, for the mice in the T2DM model + EA group, the acupuncture Zusanli (ST36) and Pishu (BL20) points (frequency 2 Hz, intensity 1-3 mA, intermittent waveform, serial length 30 s, increasing once every 5 min, lasting for 15 min) were stimulated once every 2 d, 3 times a week, for 4 weeks (12 times in total). Before EA treatment, T2DM mice were under 2% isoflurane anesthesia. T2DM: type 2 diabetes, EA: electroacupuncture; ELISA: enzyme-linked immunosorbent assay; DAPI: 4′,6-diamidino-2-phenylindole; ANOVA: analysis of variance. The statistical analyses included one-way ANOVA and t-tests to compare differences among treatment groups. Error bars represent the mean?± standard deviation (n = 3). Compared with the ctrl group, aP < 0.001; compared with T2DM group, bP < 0.05.
| Group | n | circEZH1/GAPDH relative expression | circEZH2/GAPDH relative expression |
|---|---|---|---|
| Ctrl-exo | 3 | 1.00±0.43 | 1.00±0.21 |
| T2DM-exo | 3 | 0.06±0.01a | 0.79±0.14 |
| T2DM+EA-exo | 3 | 0.42±0.17b | 1.02±0.15 |
Table 1 CircEZH1 and CircEZH2 expression in plasma exosomes detected using qPCR ($\bar{x}$± s)
| Group | n | circEZH1/GAPDH relative expression | circEZH2/GAPDH relative expression |
|---|---|---|---|
| Ctrl-exo | 3 | 1.00±0.43 | 1.00±0.21 |
| T2DM-exo | 3 | 0.06±0.01a | 0.79±0.14 |
| T2DM+EA-exo | 3 | 0.42±0.17b | 1.02±0.15 |
| Group | n | Cell viability of Min6 (%) | Apoptosis (%) | ||||
|---|---|---|---|---|---|---|---|
| 0 h | 24 h | 48 h | 72 h | 96 h | |||
| OE-NC | 5 | 100.00±3.44 | 225.81±16.15 | 410.09±2.04 | 627.22±25.83 | 793.71±35.24 | 6.51±0.36 |
| CircEZH1-OE | 5 | 100.00±1.43 | 253.32±4.71 | 523.34±25.23a | 765.49±49.01c | 1012.72±28.5a | 1.73±0.04a |
| siRNA-NC | 5 | 100.00±7.33 | 210.23±9.41 | 397.33±15.98 | 613.46±46.85 | 765.41±53.34 | 6.57±0.14 |
| CircEZH1-siRNA | 5 | 100.00±6.61 | 226.26±8.45 | 267.43±7.94b | 491.17±62.66d | 658.6±55.82e | 9.81±0.28c |
Table 2 CircEZH1 expression affected Min6 cell viability and apoptosis ($\bar{x}$ ± s)
| Group | n | Cell viability of Min6 (%) | Apoptosis (%) | ||||
|---|---|---|---|---|---|---|---|
| 0 h | 24 h | 48 h | 72 h | 96 h | |||
| OE-NC | 5 | 100.00±3.44 | 225.81±16.15 | 410.09±2.04 | 627.22±25.83 | 793.71±35.24 | 6.51±0.36 |
| CircEZH1-OE | 5 | 100.00±1.43 | 253.32±4.71 | 523.34±25.23a | 765.49±49.01c | 1012.72±28.5a | 1.73±0.04a |
| siRNA-NC | 5 | 100.00±7.33 | 210.23±9.41 | 397.33±15.98 | 613.46±46.85 | 765.41±53.34 | 6.57±0.14 |
| CircEZH1-siRNA | 5 | 100.00±6.61 | 226.26±8.45 | 267.43±7.94b | 491.17±62.66d | 658.6±55.82e | 9.81±0.28c |
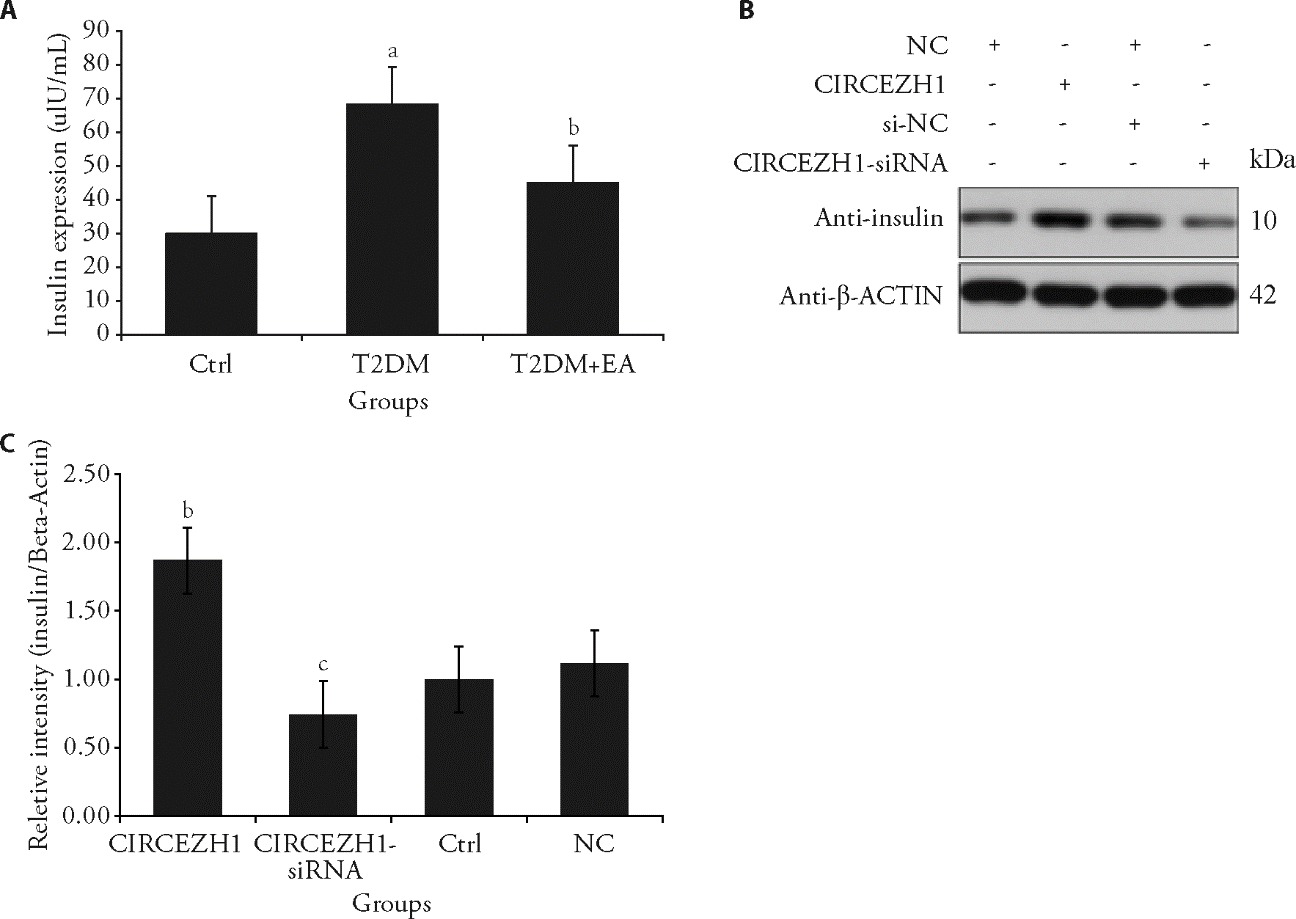
Figure 3 Effect of CircEZH1 expression on the insulin level in Min6 cells A: insulin level in Min6 cell supernatant detected using ELISA. Control (low-fat diet), T2DM (HFD + STZ 50 mg/kg), and T2DM + EA (T2DM mice treated with electroacupuncture at ST36 and BL20 for 4 weeks); B: representative band of insulin protein in in Min6 cells detected using Western blotting; C: relative protein levels of insulin results. Control (scramble siRNA or empty vector), CircEZH1 overexpression, CircEZH1 knockdown (CircEZH1 siRNA) and NC (Min6 cell without CircEZH1overexpression). HFD: high-fat diet; STZ: streptozotocin; T2DM: type 2 diabetes mellitus; EA: electroacupuncture; ST36: Zusanli acupoint; BL20: Pishu acupoint; siRNA: Small interfering RNA; CircEZH1: Circular RNA enhancer of zeste homolog 1. Briefly, for the mice in the T2DM model + EA group, the acupuncture Zusanli (ST36) and Pishu (BL20) points (frequency 2 Hz, intensity 1-3 mA, intermittent waveform, serial length 30 s, increasing once every 5 min, lasting for 15 min) were stimulated once every 2 d, 3 times a week, for 4 weeks (12 times in total). Before EA treatment, T2DM mice were under 2% isoflurane anesthesia. OE-NC: Min6 cell without CircEZH1overexpression; CircEZH1: circular RNA of Enhancer of Zeste Homolog 1; ELISA: enzyme-linked immunosorbent assay; ANOVA: analysis of variance. Error bars represent the mean?±?standard deviation (n = 3). Compared with OE-NC group, aP < 0.05, bP < 0.001; compared with siRNA-NC group, cP < 0.0.05. The statistical analyses included one-way ANOVA and t-tests to compare differences among treatment groups.
| Group | n | Blood Glucose (mg/dL) | Fasting glucose (mg/dL) | Summed GTT (AUC) | ||||
|---|---|---|---|---|---|---|---|---|
| 0 min | 30 min | 60 min | 90 min | 120 min | ||||
| Ctrl | 5 | 107±11 | 107±11 | 21681±1252 | 104±9 | 93±11 | 107±11 | 21681±1252 |
| T2DM | 5 | 222±22a | 222±22a | 39166±2498a | 284±50a | 196±14a | 222±22a | 39166±2498a |
| T2DM+CircEZH1-siRNA | 5 | 251±5a | 251±5ab | 47590±3093ab | 319±22a | 234±16a | 251±5ac | 47590±3093ab |
Table 3 CircEZH1 deletion alters glucose tolerance ($\bar{x}$ ± s)
| Group | n | Blood Glucose (mg/dL) | Fasting glucose (mg/dL) | Summed GTT (AUC) | ||||
|---|---|---|---|---|---|---|---|---|
| 0 min | 30 min | 60 min | 90 min | 120 min | ||||
| Ctrl | 5 | 107±11 | 107±11 | 21681±1252 | 104±9 | 93±11 | 107±11 | 21681±1252 |
| T2DM | 5 | 222±22a | 222±22a | 39166±2498a | 284±50a | 196±14a | 222±22a | 39166±2498a |
| T2DM+CircEZH1-siRNA | 5 | 251±5a | 251±5ab | 47590±3093ab | 319±22a | 234±16a | 251±5ac | 47590±3093ab |
| Group | n | Blood Glucose (mg/dL) | Fasting glucose (mg/dL) | Summed GTT (AUC) | ||||
|---|---|---|---|---|---|---|---|---|
| 0 min | 30 min | 60 min | 90 min | 120 min | ||||
| Ctrl | 5 | 147±18 | 96±21 | 78±23 | 81±22 | 87±18 | 147±18 | 11161±2352 |
| T2DM | 5 | 219±18a | 125±14c | 107±15c | 111±16c | 126±25f | 219±18a | 15466±1500f |
| T2DM+CircEZH1-siRNA | 5 | 276±12ab | 169±9ab | 140±17ad | 138±4ae | 156±14ae | 276±12ab | 19910±655ad |
Table 4 CircEZH1 deletion promotes insulin resistance ($\bar{x}$ ± s)
| Group | n | Blood Glucose (mg/dL) | Fasting glucose (mg/dL) | Summed GTT (AUC) | ||||
|---|---|---|---|---|---|---|---|---|
| 0 min | 30 min | 60 min | 90 min | 120 min | ||||
| Ctrl | 5 | 147±18 | 96±21 | 78±23 | 81±22 | 87±18 | 147±18 | 11161±2352 |
| T2DM | 5 | 219±18a | 125±14c | 107±15c | 111±16c | 126±25f | 219±18a | 15466±1500f |
| T2DM+CircEZH1-siRNA | 5 | 276±12ab | 169±9ab | 140±17ad | 138±4ae | 156±14ae | 276±12ab | 19910±655ad |
| 1. |
Singh DD, Shati AA, Alfaifi MY, et al. Development of dementia in type 2 diabetes patients: mechanisms of insulin resistance and antidiabetic drug development. Cells 2022; 11: 3767.
DOI URL |
| 2. |
Ogurtsova K, da Rocha Fernandes JD, Huang Y, et al. IDF diabetes atlas: global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract 2017; 128: 40-50.
DOI URL |
| 3. |
Chu N, Chan JCN, Chow E. Pharmacomicrobiomics in Western Medicine and Traditional Chinese Medicine in type 2 diabetes. Front Endocrinol (Lausanne) 2022; 13: 857090.
DOI URL |
| 4. | Holt RIG, DeVries JH, Hess-Fischl A, et al. The management of type 1 diabetes in adults. A consensus report by the american diabetes association (ADA) and the european association for the study of diabetes (EASD). Diabetes Care 2021; 44: 2589-625. |
| 5. | Sheng J, Jin X, Zhu J, Chen Y, Liu X. The effectiveness of acupoint catgut embedding therapy for abdominal obesity: a systematic review and Meta-analysis. Evid Based Complement Alternat Med 2019; 2019: 9714313. |
| 6. |
Hsu CH, Hwang KC, Chao CL, Lin JG, Kao ST, Chou P. Effects of electroacupuncture in reducing weight and waist circumference in obese women: a randomized crossover trial. Int J Obes (Lond) 2005; 29: 1379-84.
DOI |
| 7. |
Lan D, Xu N, Sun J, et al. Electroacupuncture mitigates endothelial dysfunction via effects on the PI3K/Akt signalling pathway in high fat diet-induced insulin-resistant rats. Acupunct Med 2018; 36: 162-9.
DOI URL |
| 8. | Shou Y, Hu L, Zhang W, Gao Y, Xu P, Zhang B. Determination of electroacupuncture effects on circRNAs in plasma exosomes in diabetic mice: an RNA-sequencing approach. Evid Based Complement Alternat Med 2019; 2019: 7543049. |
| 9. |
Biondi B, Kahaly GJ, Robertson RP. Thyroid dysfunction and diabetes mellitus: two closely associated disorders. Endocr Rev 2019; 40: 789-824.
DOI PMID |
| 10. |
Kalra S, Aggarwal S, Khandelwal D. Thyroid dysfunction and type 2 diabetes mellitus: screening strategies and implications for management. Diabetes Ther 2019; 10: 2035-44.
DOI PMID |
| 11. |
Aguayo-Mazzucato C, Zavacki AM, Marinelarena A, et al. Thyroid hormone promotes postnatal rat pancreatic β-cell development and glucose-responsive insulin secretion through MAFA. Diabetes 2013; 62: 1569-80.
DOI PMID |
| 12. |
Cheng FK. An overview of the contribution of acupuncture to thyroid disorders. J Integr Med 2018; 16: 375-83.
DOI URL |
| 13. |
Sun Y, Wang W, Tang Y, et al. Microarray profiling and functional analysis of differentially expressed plasma exosomal circular RNAs in Graves' disease. Biol Res 2020; 53: 32.
DOI PMID |
| 14. | Ling C, Bacos K, Rönn T. Epigenetics of type 2 diabetes mellitus and weight change-a tool for precision medicine? Nat Rev Endocrinol 2022; 18: 433-48. |
| 15. | Guo CF, Li R, Song SS, et al. Effects of electroacupuncture on the glucose-lipid metabolism and the expression of ZAG and GLUT4 in the femoral quadriceps and adipose tissue in the rats with type 2 diabetes mellitus. Zhong Guo Zhen Jiu 2023; 43: 1425-30. |
| 16. |
Liu M, Liu Z, Xu B, Zhang W, Cai J. Review of systematic reviews and Meta-analyses investigating Traditional Chinese Medicine treatment for type 2 diabetes mellitus. J Tradit Chin Med 2016; 36: 555-63.
PMID |
| 17. | Zheng R, Qing P, Han M, et al. The Effect of acupuncture on glucose metabolism and lipid profiles in patients with PCOS: a systematic review and Meta-analysis of randomized controlled trials. Evid Based Complement Alternat Med 2021; 2021: 5555028. |
| 18. |
Martinez B, Peplow PV. Treatment of insulin resistance by acupuncture: a review of human and animal studies. Acupunct Med 2016; 34: 310-9.
DOI PMID |
| 19. |
Chung YC, Chen YI, Lin CM, et al. Electroacupuncture combined with acarbose improves insulin sensitivity via peroxisome proliferator-activated receptor γ activation and produces a stronger glucose-lowering effect than acarbose alone in a rat model of steroid-induced insulin resistance. Acupunct Med 2020; 38: 335-42.
DOI URL |
| 20. |
Kalwat MA, Cobb MH. Mechanisms of the amplifying pathway of insulin secretion in the β cell. Pharmacol Ther 2017; 179: 17-30.
DOI URL |
| 21. |
Boland BB, Rhodes CJ, Grimsby JS. The dynamic plasticity of insulin production in β-cells. Mol Metab 2017; 6: 958-73.
DOI PMID |
| 22. | Cao BY, Li R, Tian HH, et al. Effect of electroacupuncture at "Weiwanxiashu" (EX-B 3) on islet morphology and the expression of pancreatic glucagon-like peptide-1 receptor in type 2 diabetes rats. Zhen Ci Yan Jiu 2017; 42: 107-13. |
| 23. | Wei WJ, Liao MH, Tan YH, et al. Effect of electroacupuncture on renal vascular microcirculation in diabetic mice based on in vivo two-photon microscopy imaging. Zhen Ci Yan Jiu 2022; 47: 497-503. |
| 24. | Song S, Li R, Cao B, et al. Mechanism of electroacupuncture regulating IRS-1 phosphorylation in skeletal muscle to improve insulin sensitivity. Evid Based Complement Alternat Med 2021; 2021: 8631475. |
| 25. | Fradin D, Bougnères P. T2DM: why epigenetics? J Nutr Metab 2011; 2011: 647514. |
| 26. | Piunti A, Shilatifard A. The roles of Polycomb repressive complexes in mammalian development and cancer. Nat Rev Mol Cell Biol 2021; 22: 326-45. |
| 27. |
Dhawan S, Tschen SI, Bhushan A. Bmi-1 regulates the Ink4a/Arf locus to control pancreatic beta-cell proliferation. Genes Dev 2009; 23: 906-11.
DOI URL |
| 28. |
Lu TT, Heyne S, Dror E, et al. The polycomb-dependent epigenome controls β cell dysfunction, dedifferentiation, and diabetes. Cell Metab 2018; 27: 1294-308.e7.
DOI URL |
| 29. |
Chen H, Gu X, Su IH, et al. Polycomb protein Ezh2 regulates pancreatic beta-cell Ink4a/Arf expression and regeneration in diabetes mellitus. Genes Dev 2009; 23: 975-985.
DOI URL |
| 30. |
Dahlby T, Simon C, Backe MB, et al. Enhancer of zeste homolog 2 (EZH2) mediates glucolipotoxicity-Induced apoptosis in β-cells. Int J Mol Sci 2020; 21: 8016.
DOI URL |
| [1] | ZHAO Ping, HE Xingbo, HAN Xudong, CHEN Xinyue, LI Zhanglong, SONG Jike, XING Wenjia, WU Jiangfeng, GUO Bin, BI Hongsheng. Mechanism of electroacupuncture involve in lens-induced myopia guinea pigs by inhibiting wnt/β-catenin signaling pathway [J]. Journal of Traditional Chinese Medicine, 2025, 45(4): 796-805. |
| [2] | ZHENG Ruwen, DONG Xu, WANG Tianyi, FENG Liyuan, ZHANG Hongyan, HUO Hong, ZHANG Ying, ZHANG Qianshi, ZHU Xingyan, WANG Dongyan. Electroacupuncture versus conventional acupuncture of scalp motor area for post-stroke wrist dyskinesia and its effect on muscle function: a randomized, controlled clinical trial [J]. Journal of Traditional Chinese Medicine, 2025, 45(4): 852-859. |
| [3] | SUN Jiao, WANG Yueming, LYU Jian, LIU Xin, YUE Bingnan, LI Yinyin, LIU Jipeng, SUN Yize, LIU Qingguo, YAN Liu. Effect of electroacupuncture on hypertensive and sympathetic excitability mechanism mediated by the paraventricular nucleus of the hypothalamus in spontaneous hypertensive rats [J]. Journal of Traditional Chinese Medicine, 2025, 45(3): 586-596. |
| [4] | LI Yongfeng, CHEN Xinyi, REN Wei, QIAO Haifa. Electroacupuncture stimulation of auricular concha region improves loss of control over stress induced depression-like behavior by modulating 5-hydroxytryptamine 1A receptor [J]. Journal of Traditional Chinese Medicine, 2025, 45(2): 326-334. |
| [5] | HU Junwei, FENG Jiwei, LI Wen, LIU Lumin, LI Xu, XU Ge, LIU Jiandang, CHEN Yuelai. Electroacupuncture improves cyclophosphamide-induced bladder overactivity by reducing mechanotransduction in the rat urothelium [J]. Journal of Traditional Chinese Medicine, 2025, 45(2): 348-358. |
| [6] | LI Siting, WANG Shaojun, YIN Yehui, DE Gejing, LI Caicai, WANG Ziyan, CAO Wenjie. Electroacupuncture alleviates zymosan-induced colorectal hypersensitivity [J]. Journal of Traditional Chinese Medicine, 2025, 45(1): 32-38. |
| [7] | Emre Bulut, Didem Özkal Eminoğlu, Yasemin Çayır. Effect of electroacupuncture on pain after periodontal flap surgery: a randomized controlled trial [J]. Journal of Traditional Chinese Medicine, 2025, 45(1): 184-191. |
| [8] | ZHANG Boyang, ZHOU Yang, FENG Liyuan, SUI Dan, HE Lei, TONG Dan, WANG Ruoyu, SUI Xue, SONG Jing, WANG Dongyan. A neural regulation mechanism of head electroacupuncture on brain network of patients with stroke related sleep disorders [J]. Journal of Traditional Chinese Medicine, 2024, 44(6): 1268-1276. |
| [9] | XU Yingshan, WU Chunxiao, YU Wei, GUO Hongji, LU Liming, XU Nenggui, TANG Chunzhi. Systematic review and Meta-analysis of brain plasticity associated with electroacupuncture in experimental ischemic stroke [J]. Journal of Traditional Chinese Medicine, 2024, 44(5): 859-870. |
| [10] | ZHANG Fang, YAN Cuina, WENG Zhijun, WU Luyi, QI Li, ZHAO Min, XIN Yuhu, WU Huangan, LIU Huirong. Regulatory role of electroacupuncture on satellite glial cell activity in the colon and dorsal root ganglion of rats with irritable bowel syndrome [J]. Journal of Traditional Chinese Medicine, 2024, 44(5): 981-990. |
| [11] | CHEN Yonglin, OUYANG Ling, MENG Lingling, WU Bufan, PENG Rou, LIU Sitong, HOU Dan, WANG Yaling, JING Xinyue, LU Shengfeng, FU Shuping. Electroacupuncture ameliorates blood-brain barrier disruption after ischemic stroke through histone acetylation regulation at the matrix metalloproteinase 9 and tissue inhibitor of metalloproteinase 2 genes [J]. Journal of Traditional Chinese Medicine, 2024, 44(4): 734-744. |
| [12] | WANG Shaosong, SUN Jingqing, FENG Qingyin, LI Bin, WANG Xin, YUAN Fan, CUI Yingxue. Effectivenss of electroacupuncture for skeletal muscle pain in Parkinson's disease: a Clinical randomized controlled trial [J]. Journal of Traditional Chinese Medicine, 2024, 44(2): 388-395. |
| [13] | QIN Xiaoyu, WANG Chunai, XUE Jianjun, ZHANG Jie, LU Xiaoting, DING Shengshuang, GE Long, WANG Minzhen. Efficacy of electroacupuncture on myocardial protection and postoperative rehabilitation in patients undergoing cardiac surgery with cardiopulmonary bypass: a systematic review and Meta-analysis [J]. Journal of Traditional Chinese Medicine, 2024, 44(1): 1-15. |
| [14] | SUN Qianhui, CHENG Kai, DAI Xingye, YANG Zhiwen, WU Xiaoling, XU Chang, QIU Xinghua, GAO Xiaofeng, LIU Daonan, YANG Qirui. Effect of electroacupuncture at Neiguan (PC6) at different time points on myocardial ischemia reperfusion arrhythmia in rats [J]. Journal of Traditional Chinese Medicine, 2024, 44(1): 113-121. |
| [15] | DU Zhongheng, CONG Wenjie, TANG Kejing, ZHENG Qiqi, SONG Zhiwei, CHEN Yong, YANG Su, ZHANG Chunwu, YE Tianshen. Electroacupuncture stimulating Zusanli (ST36), Sanyinjiao (SP6) in mice with collagen-induced arthritis leads to adenosine A2A receptor-mediated alteration of p38α mitogen-activated protein kinase signaling and inhibition of osteoclastogenesis [J]. Journal of Traditional Chinese Medicine, 2023, 43(6): 1103-1109. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||

