Journal of Traditional Chinese Medicine ›› 2025, Vol. 45 ›› Issue (3): 561-570.DOI: 10.19852/j.cnki.jtcm.2025.03.011
Previous Articles Next Articles
Bushen Tongluo recipe (补肾通络方) improves oxidative stress homeostasis, inhibits transforming growth factor/Notch signaling pathway, and regulates the lncRNA maternally expressed gene 3/miR-145 axis to delay diabetic kidney disease
XU Bojun, TAO Tian, ZHAO Liangbin, ZHENG Hui, ZHAN huakui( ), GUO Julan(
), GUO Julan( )
)
- Department of Nephrology, Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu 610072, China
-
Received:2024-04-22Accepted:2024-09-30Online:2025-06-15Published:2025-05-21 -
Contact:ZHAN huakui, Department of Nephrology, Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu 610072, China. zhanhuak@cdutcm.edu.cn;GUO Julan, Department of Nephrology, Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu 610072, China. 530349862@qq.com,Telephone: +86-18980880223, +86-18981916045 -
About author:XU Bojun and TAO Tian are co-first authors and contributed equally to this work
Cite this article
XU Bojun, TAO Tian, ZHAO Liangbin, ZHENG Hui, ZHAN huakui, GUO Julan. Bushen Tongluo recipe (补肾通络方) improves oxidative stress homeostasis, inhibits transforming growth factor/Notch signaling pathway, and regulates the lncRNA maternally expressed gene 3/miR-145 axis to delay diabetic kidney disease[J]. Journal of Traditional Chinese Medicine, 2025, 45(3): 561-570.
share this article
| Group | n | IBW (g) | STZ 3 d body weight (g) | High lipid/treatment body weight (g) | KC (%) | ||
|---|---|---|---|---|---|---|---|
| 1W | 5W | 10W | |||||
| Control | 5 | 279.924±1.708 | 349.502±11.552 | 379.500±9.649 | 499.500±11.032 | 569.500±4.155 | 0.568±0.013 |
| Model | 5 | 279.588±2.859 | 272.386±5.211a | 279.166±4.383a | 288.212±4.359a | 317.516±4.161a | 1.432±0.058a |
| Losartan | 5 | 279.733±3.515 | 324.003±3.574 | 324.201±3.749b | 379.613±4.409b | 430.276±4.948b | 0.868±0.024b |
| BSTLR-L | 5 | 280.300±3.827 | 279.638±4.604 | 299.086±4.416b | 356.498±3.511b | 392.542±3.750b | 1.025±0.013b |
| BSTLR-M | 5 | 279.690±1.801 | 290.316±5.448b | 309.722±5.132b | 375.180±4.152b | 425.740±5.422b | 0.885±0.015b |
| BSTLR-H | 5 | 280.020±2.505 | 308.908±4.851b | 329.892±4.663b | 394.546±4.077b | 444.170±5.454b | 0.761±0.025b |
Table 1 Body weight and kidney coefficient ($\bar{x}±s$)
| Group | n | IBW (g) | STZ 3 d body weight (g) | High lipid/treatment body weight (g) | KC (%) | ||
|---|---|---|---|---|---|---|---|
| 1W | 5W | 10W | |||||
| Control | 5 | 279.924±1.708 | 349.502±11.552 | 379.500±9.649 | 499.500±11.032 | 569.500±4.155 | 0.568±0.013 |
| Model | 5 | 279.588±2.859 | 272.386±5.211a | 279.166±4.383a | 288.212±4.359a | 317.516±4.161a | 1.432±0.058a |
| Losartan | 5 | 279.733±3.515 | 324.003±3.574 | 324.201±3.749b | 379.613±4.409b | 430.276±4.948b | 0.868±0.024b |
| BSTLR-L | 5 | 280.300±3.827 | 279.638±4.604 | 299.086±4.416b | 356.498±3.511b | 392.542±3.750b | 1.025±0.013b |
| BSTLR-M | 5 | 279.690±1.801 | 290.316±5.448b | 309.722±5.132b | 375.180±4.152b | 425.740±5.422b | 0.885±0.015b |
| BSTLR-H | 5 | 280.020±2.505 | 308.908±4.851b | 329.892±4.663b | 394.546±4.077b | 444.170±5.454b | 0.761±0.025b |

Figure 1 Effect of BSTLR on renal function in DKD rats A: concentrations of UP, α1-MG, GLU, BUN, CREA, and CysC in rats of 1 week; B: concentrations of UP, α1-MG, GLU, BUN, CREA, and CysC in rats of 5 week; C: concentrations of UP, α1-MG, GLU, BUN, CREA, and CysC in rats of 10 week; A1, B1, C1: UP; A2, B2, C2: α1-MG; A3, B3, C3: GLU; A4, B4, C4: BUN; A5, B5, C5: CREA; A6, B6, C6: CysC. Control and Model groups were gavaged with equal amounts of distilled water, the Losartan group was gavaged with Losartan (50 mg/kg), and the BSTLR-L, BSTLR-M, and BSTLR-H groups were gavaged with BSTLR at concentrations of 1.2, 2.4, and 4.8 g/mL, respectively, once a day. BSTLR: Bushen Tongluo recipe; DKD: diabetic kidney disease; UP: urinary protein; α1-MG: α1-microglobulin; GLU: glucose; BUN: blood urea nitrogen; CREA: creatinine; CysC: cystatin C. Data were represented as mean ± standard deviation (n = 5). Statistical differences between groups were determined by one-way analysis of variance. Compared with control group, aP < 0.05; compared with model group, bP < 0.05.

Figure 2 Effect of BSTLR on renal injury in DKD rats A: HE staining of kidney tissues; A1: Control (× 100, scale bar, 200 μm) ; A2: Model (× 100, scale bar, 200 μm); A3: Losartan (× 100, scale bar, 200 μm); A4: BSTLR-L (× 100, scale bar, 200 μm); A5: BSTLR-M (× 100, scale bar, 200 μm); A6: BSTLR-H (× 100, scale bar, 200 μm); A7: Control (× 400, scale bar, 50 μm); A8: Model (× 400, scale bar, 50 μm); A9: Losartan (× 400, scale bar, 50 μm); A10: BSTLR-L (× 400, scale bar, 50 μm); A11: BSTLR-M (× 400, scale bar, 50 μm); A12: BSTLR-H (× 400, scale bar, 50 μm). B: activities of MDA, SOD, and CAT in kidney tissues; B1: SOD; B2: MDA; B3: CAT; C: expression levels of Nephrin, Desmin, and Podocin in kidney tissues; C1: protein band; C2: Nephrin; C3: Desmin; C4: Podocin. Control and Model groups were gavaged with equal amounts of distilled water, the Losartan group was gavaged with Losartan (50 mg/kg), and the BSTLR-L, BSTLR-M, and BSTLR-H groups were gavaged with BSTLR at concentrations of 1.2, 2.4, and 4.8 g/mL, respectively, once a day. BSTLR: Bushen Tongluo recipe; DKD: diabetic kidney disease; SOD: superoxide dismutase; MDA: malondialdehyde; CAT: catalase. Data were represented as mean ± standard deviation (n = 5). Statistical differences between groups were determined by one-way analysis of variance. Compared with control group, aP < 0.05; compared with model group, bP < 0.05.

Figure 3 Effect of BSTLR on renal injury in DKD rats A: immunohistochemical staining of FN (× 400, scale bar, 50 μm) in kidney tissues. B: immunohistochemical staining of E-cad (× 400, scale bar, 50 μm) in kidney tissues. C: immunohistochemical staining of α-SMA (×400, scale bar, 50 μm) in kidney tissues. D: immunohistochemical staining of LN (× 400, scale bar, 50 μm) in kidney tissues. E: immunohistochemical staining of Vim (× 400, scale bar, 50 μm) in kidney tissues. F: immunohistochemical staining of Col Ⅳ (× 400, scale bar, 50 μm) in kidney tissues. A1, B1, C1, D1, E1, F1: Control; A2, B2, C2, D2, E2, F2: Model; A3, B3, C3, D3, E3, F3: Losartan; A4, B4, C4, D4, E4, F4: BSTLR-L; A5, B5, C5, D5, E5, F5: BSTLR-M; A6, B6, C6, D6, E6, F6: BSTLR-H; G: positive expression levels of FN; H: positive expression levels of E-cad; I: positive expression levels of α-SMA; J: positive expression levels of LN; K: positive expression levels of Vim; L: positive expression levels of Col IV. Control and Model groups were gavaged with equal amounts of distilled water, the Losartan group was gavaged with Losartan (50 mg/kg), and the BSTLR-L, BSTLR-M, and BSTLR-H groups were gavaged with BSTLR at concentrations of 1.2, 2.4, and 4.8 g/mL, respectively, once a day. BSTLR: Bushen Tongluo recipe; DKD: diabetic kidney disease; FN: fibronectin; E-cad: E-cadherin; α-SMA: α-smooth muscle actin; LN: laminin; Vim: vimentin; Col IV: collagen type Ⅳ. Data were represented as mean ± standard deviation (n = 5). Statistical differences between groups were determined by one-way analysis of variance. Compared with the control group, aP < 0.05; compared with the model group, bP < 0.05.
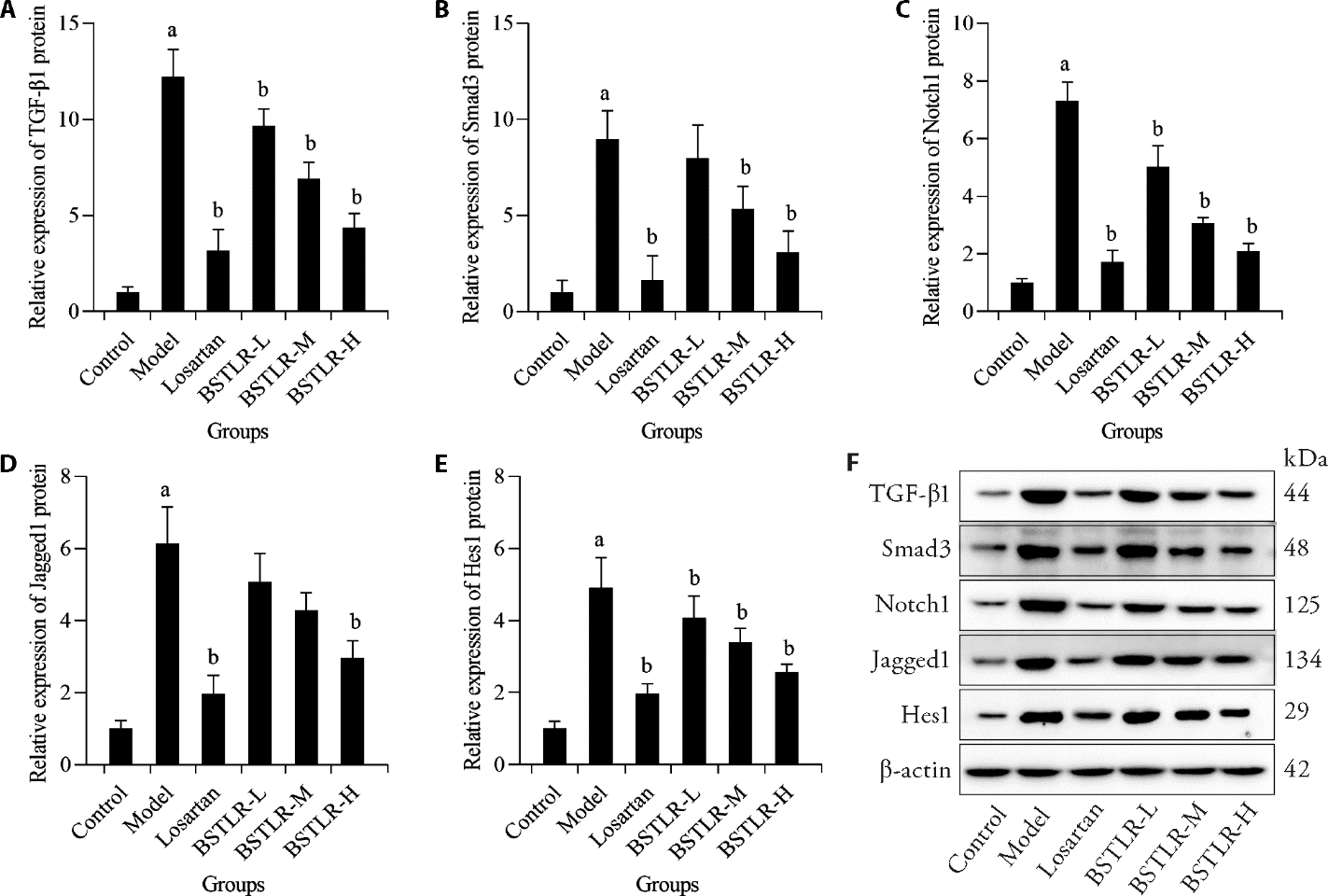
Figure 4 Effect of BSTLR on renal TGF/Notch pathway in DKD rats A: expression levels of TGF-β1 in kidney tissues; B: expression levels of Smad3 in kidney tissues; C: expression levels of Notch1 in kidney tissues; D: expression levels of Jagged1 in kidney tissues; E: expression levels of Hes1 in kidney tissues; F: protein band. Control and Model groups were gavaged with equal amounts of distilled water, the Losartan group was gavaged with Losartan (50 mg/kg), and the BSTLR-L, BSTLR-M, and BSTLR-H groups were gavaged with BSTLR at concentrations of 1.2, 2.4, and 4.8 g/mL, respectively, once a day. BSTLR: Bushen Tongluo recipe; DKD: diabetic kidney disease; TGF-β1: transforming growth factor-β1; Smad3: mothers against decapentaplegic homolog 3; Hes1: hairy and enhancer of split 1. Data were represented as mean ± standard deviation (n = 5). Statistical differences between groups were determined by one-way analysis of variance. Compared with the control group, aP < 0.05; compared with the model group, bP < 0.05.
| 1. | Tinajero MG, Malik VS. An update on the epidemiology of type 2 diabetes: a global perspective. Endocrinol Metab Clin North Am 2021; 50: 337-55. |
| 2. | Guo W, Song Y, Sun Y, et al. Systemic immune-inflammation index is associated with diabetic kidney disease in type 2 diabetes mellitus patients: evidence from NHANES 2011-2018. Front Endocrinol (Lausanne) 2022; 13: 1071465. |
| 3. | Rayego-Mateos S, Rodrigues-Diez RR, Fernandez-Fernandez B, et al. Targeting inflammation to treat diabetic kidney disease: the road to 2030. Kidney Int 2023; 103: 282-96. |
| 4. | Barrera-Chimal J, Lima-Posada I, Bakris GL, et al. Mineralocorticoid receptor antagonists in diabetic kidney disease -mechanistic and therapeutic effects. Nat Rev Nephrol 2022; 18: 56-70. |
| 5. | Park EG, Pyo SJ, Cui Y, et al. Tumor immune microenvironment lncRNAs. Brief Bioinform 2022; 23: bbab504. |
| 6. | Chen T, Shi Z, Zhao Y, et al. LncRNA Airn maintains LSEC differentiation to alleviate liver fibrosis via the KLF2-eNOS-sGC pathway. BMC Med 2022; 20: 335. |
| 7. | Wu YY, Wu S, Li XF, et al. LncRNA MEG3 reverses CCl(4)-induced liver fibrosis by targeting NLRC5. Eur J Pharmacol 2021; 911: 174462. |
| 8. |
He Y, Dan Y, Gao X, et al. DNMT1-mediated lncRNA MEG3 methylation accelerates endothelial-mesenchymal transition in diabetic retinopathy through the PI3K/Akt/mTOR signaling pathway. Am J Physiol Endocrinol Metab 2021; 320: E598-e608.
DOI PMID |
| 9. | Xu BW, Rao Y, Wang L, et al. LncRNA MEG3 inhibits renal fibrinoid necrosis of diabetic nephropathy via the MEG3/miR-21/ORAI1 axis. Mol Biol Rep 2023; 50: 3283-295. |
| 10. | Zha F, Qu X, Tang B, et al. Long non-coding RNA MEG3 promotes fibrosis and inflammatory response in diabetic nephropathy via miR-181a/Egr-1/TLR4 axis. Mol Biol Rep 2019; 11: 3716-730. |
| 11. |
Mahtal N, Lenoir O. MicroRNAs in kidney injury and disease. Nat Rev Nephrol 2022; 18: 643-62.
DOI PMID |
| 12. | Qin R, Huang W, Huang Y, et al. lncRNA MEG3 modulates hepatic stellate cell activation by sponging miR-145 to regulate PPARγ. Mol Med Rep 2022; 25: 3. |
| 13. | Wang T, Cui S, Liu X, et al. LncTUG1 ameliorates renal tubular fibrosis in experimental diabetic nephropathy through the miR-145-5p/dual-specificity phosphatase 6 axis. Ren Fail 2023; 45: 2173950. |
| 14. | Liu XJ, Hu XK, Yang H, et al. A review of Traditional Chinese Medicine on treatment of diabetic nephropathy and the involved mechanisms. Am J Chin Med 2022; 50: 1739-79. |
| 15. | Liu H, Wang G, Wang J, et al. Bushentongluo recipe (BSTL) attenuates bone destruction by inhibiting NF-κB/RANK/RANKL pathway in collagen-induced arthritis (CIA) rats. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 2021; 37: 205-11. |
| 16. |
Yuan H, Xiao L, Min W, et al. Bu-Shen-Tong-Luo decoction prevents bone loss via inhibition of bone resorption and enhancement of angiogenesis in ovariectomy-induced osteoporosis of rats. J Ethnopharmacol 2018; 220: 228-38.
DOI PMID |
| 17. |
Xiang E, Han B, Zhang Q, et al. Human umbilical cord-derived mesenchymal stem cells prevent the progression of early diabetic nephropathy through inhibiting inflammation and fibrosis. Stem Cell Res Ther 2020; 11: 336.
DOI PMID |
| 18. | Mohamed RH, Sedky AA. Sitagliptin's renoprotective effect in a diabetic nephropathy model in rats: the potential role of PI3K/AKT pathway. Fundam Clin Pharmacol 2022; 36: 324-37. |
| 19. |
Wang Z, Fu W, Huo M, et al. Spatial-resolved metabolomics reveals tissue-specific metabolic reprogramming in diabetic nephropathy by using mass spectrometry imaging. Acta Pharm Sin B 2021; 11: 3665-77.
DOI PMID |
| 20. | An X, Zhang Y, Cao Y, et al. Punicalagin protects diabetic nephropathy by inhibiting pyroptosis based on TXNIP/NLRP3 pathway. Nutrients 22 2020; 12: 1516. |
| 21. |
Liu J, Zhang J, Hou MH, et al. Clinical efficacy of linagliptin combined with irbesartan in patients with diabetic nephropathy. Pak J Med Sci 2022; 38: 52-6.
DOI PMID |
| 22. | Teuma L, Eshwaran R, Tawokam Fongang U, et al. Glucosamine inhibits extracellular matrix accumulation in experimental diabetic nephropathy. Front Nutr 2022; 9: 1048305. |
| 23. | Tang C, Wang M, Liu J, et al. A cyclopentanone compound attenuates the over-accumulation of extracellular matrix and fibrosis in diabetic nephropathy via downregulating the TGF-β/p38MAPK axis. Biomedicines 2022; 10: 3270. |
| 24. | Liu J, Sun M, Xia Y, et al. Phloretin ameliorates diabetic nephropathy by inhibiting nephrin and podocin reduction through a non-hypoglycemic effect. Food Funct 2022; 13: 6613-22. |
| 25. | Ashraf A, Akhtar T. Sitagliptin ameliorates diabetic nephropathy by upregulating renal nephrin and podocin expression through modulation of adipokines levels. Fundam Clin Pharmacol 2023; 37: 549-55. |
| 26. | Ma T, Li X, Zhu Y, et al. Excessive activation of Notch signaling in macrophages promote kidney inflammation, fibrosis, and necroptosis. Front Immunol 2022; 13: 835879. |
| 27. | Chen J, Ou Z, Gao T, et al. Ginkgolide B alleviates oxidative stress and ferroptosis by inhibiting GPX4 ubiquitination to improve diabetic nephropathy. Biomed Pharmacother 2022; 156: 113953. |
| 28. | Ma L, Wu F, Shao Q, et al. Baicalin alleviates oxidative stress and inflammation in diabetic nephropathy via Nrf2 and MAPK signaling pathway. Drug Des Devel Ther 2021; 15: 3207-21. |
| 29. | Sapian S, Budin SB, Taib IS, et al. Role of polyphenol in regulating oxidative stress, inflammation, fibrosis, and apoptosis in diabetic nephropathy. Drug Des Devel Ther 2022; 22: 453-70. |
| 30. | Wu H, Xu F, Huang X, et al. Lupenone improves type 2 diabetic nephropathy by regulating NF-κB pathway-mediated inflammation and TGF-β1/Smad/CTGF -associated fibrosis. Phytomedicine 2023; 118: 154959. |
| 31. | Luo J, Jiang J, Huang H, et al. C-peptide ameliorates high glucose-induced podocyte dysfunction through the regulation of the Notch and TGF-β signaling pathways. Drug Dev Res 2021; 142: 170557. |
| 32. | Liu B, Deng C, Tan P. Ombuin ameliorates diabetic nephropathy in rats by anti-inflammation and antifibrosis involving Notch 1 and PPAR γ signaling pathways. Drug Dev Res 2022; 83: 1270-80. |
| 33. |
Li J, Jiang X, Duan L, et al. Long non-coding RNA MEG3 impacts diabetic nephropathy progression through sponging miR-145. Am J Transl Res 2019; 11: 6691-8.
PMID |
| 34. | Guo K, Qian K, Shi Y, et al. LncRNA-MIAT promotes thyroid cancer progression and function as ceRNA to target EZH 2 by sponging miR-150-5p. Cell Death Dis 2021; 12: 1097. |
| 35. |
Karagkouni D, Karavangeli A, Paraskevopoulou MD, et al. Characterizing miRNA-lncRNA interplay. Methods Mol Biol 2021; 2372: 243-62.
DOI PMID |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||

