Journal of Traditional Chinese Medicine ›› 2025, Vol. 45 ›› Issue (2): 254-265.DOI: 10.19852/j.cnki.jtcm.2025.02.007
• Original articles • Previous Articles Next Articles
Yishen Tongluo formula (益肾通络方) ameliorates kidney injury via modulating inflammation and apoptosis in streptozotocin-induced diabetic kidney disease mice
YUAN Jiayao1, WU Suhui1, MENG Yufan2, LI Hanbing1, LI Genlin1( ), XU Jiangyan3(
), XU Jiangyan3( )
)
- 1 Clinical Pharmacology Teaching and Research Office, School of Medicine, Henan University of Chinese Medicine, Zhengzhou 450000, China
2 School of Pharmacolog, Henan Medical College, Zhengzhou 450000, China
3 Academy of Chinese Medical Sciences, Henan University of Chinese Medicine, Zhengzhou 450000, China
-
Received:2024-03-23Accepted:2024-11-29Online:2025-04-15Published:2025-03-10 -
Contact:Pro. LI Genlin, Clinical Pharmacology Teaching and Research Office, School of Medicine, Henan University of Chinese Medicine, Zhengzhou 450000, China. lgl88@hactcm.edu.cn; Pro. XU Jiangyan, Academy of Chinese Medical Sciences, Henan University of Chinese Medicine, Zhengzhou 450000, China. xujiangyan2008@163.com, Telephone: +86-371-65934070 -
About author:YUAN Jiayao and WU Suhui are co-first authors and contributed equally to this work -
Supported by:National Key Research and Development Plan: Evidence-Based Evaluation and Therapeutic Mechanism Cooperation Study of Yishen Tongluo Formula for Preventing Diabetes Kidney Disease (Phase 3)(2020YFE0201800);Key Science and Technology Projects of Henan Province: Research on Innovative Drug Cooperation of Traditional Chinese Medicine Yishen Tongluo Concentrated Pills (益肾通络浓缩丸)(221111520300);National Natural Science Foundation of China: the Mechanism of Yishen Tongluo Formula Intervention in Diabetic Kidney Disease based on (Yin-Yang-1/Nuclear Factor Erythroid 2-Related Factor 2 Mediated Endothelial Podocyte Interaction Response (No. 82474495) to XU Jiangyan;Key scientific and technological projects of Henan province: Study on the Pharmacodynamic Mechanism of Yishen Tongluo Formula in Treatment of Diabetes Kidney Disease based on the Interaction Regulation of Protein Phosphorylation and Acylation Modification (No. 212102310347) to WU Suhui
Cite this article
YUAN Jiayao, WU Suhui, MENG Yufan, LI Hanbing, LI Genlin, XU Jiangyan. Yishen Tongluo formula (益肾通络方) ameliorates kidney injury via modulating inflammation and apoptosis in streptozotocin-induced diabetic kidney disease mice[J]. Journal of Traditional Chinese Medicine, 2025, 45(2): 254-265.
share this article
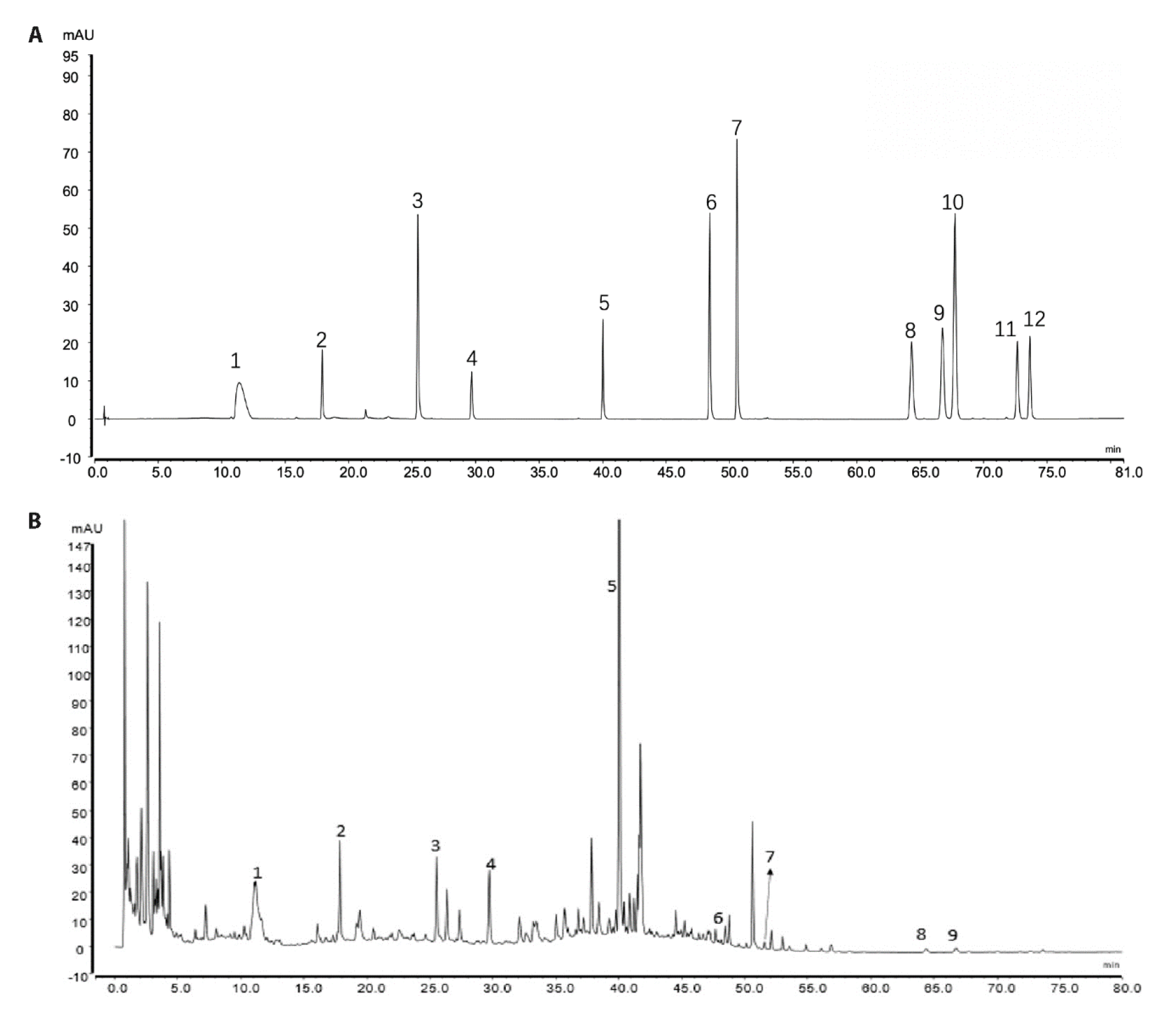
Figure 1 Chromatogram of YSTLF A: mixed standard; B: YSTLF. 1: Monoside; 2: Loganin; 3: Campanulin; 4: Acetoside; 5: Salvianolic acid B; 6: Aloemodin; 7: Rhein; 8: Cryptotanshinone; 9: Chrysophanol; 10: Danshinone I; 11: Emodin Methyl Ether; 12: Danshinone ⅡA. YSTLF: Yishen Tongluo formula.
| Group | n | Body mass (g) | Kidney weight (g) | Kidney index (%) | ||
|---|---|---|---|---|---|---|
| 0 week | 4 weeks | 7 weeks | ||||
| CON | 6 | 46.40±2.47 | 45.52±3.27 | 46.85±3.93 | 0.69±0.09 | 1.78±0.31 |
| MOD | 6 | 33.05±4.50a | 39.57±3.20a | 39.67±4.91a | 0.87±0.06a | 2.61±0.27a |
| YSTLF 4.9 g/kg | 6 | 34.40±6.27 | 37.60±3.88 | 37.02±3.45 | 0.86±0.16 | 2.57±0.06 |
| YSTLF 9.8 g/kg | 6 | 35.25±3.07 | 41.23±5.54 | 39.20±4.20 | 0.85±0.07 | 2.53±0.41 |
| CTP | 6 | 37.55±6.10 | 42.48±6.10 | 41.98±6.81 | 0.85±0.11 | 2.20±0.46 |
Table 1 YSTLF has little effect on the body weight and kidney weight of DKD mice ($\bar{x}±s$)
| Group | n | Body mass (g) | Kidney weight (g) | Kidney index (%) | ||
|---|---|---|---|---|---|---|
| 0 week | 4 weeks | 7 weeks | ||||
| CON | 6 | 46.40±2.47 | 45.52±3.27 | 46.85±3.93 | 0.69±0.09 | 1.78±0.31 |
| MOD | 6 | 33.05±4.50a | 39.57±3.20a | 39.67±4.91a | 0.87±0.06a | 2.61±0.27a |
| YSTLF 4.9 g/kg | 6 | 34.40±6.27 | 37.60±3.88 | 37.02±3.45 | 0.86±0.16 | 2.57±0.06 |
| YSTLF 9.8 g/kg | 6 | 35.25±3.07 | 41.23±5.54 | 39.20±4.20 | 0.85±0.07 | 2.53±0.41 |
| CTP | 6 | 37.55±6.10 | 42.48±6.10 | 41.98±6.81 | 0.85±0.11 | 2.20±0.46 |
| Group | n | FBG (mmol/L) | Urine C-Peptide (ng/mL) | |||
|---|---|---|---|---|---|---|
| 0 week | 2 weeks | 4 weeks | 7 weeks | |||
| CON | 6 | 7.850±1.581 | 8.683±1.566 | 7.717±1.686 | 7.117±1.652 | 0.054±0.009 |
| MOD | 6 | 31.100±2.798a | 31.600±1.920a | 28.467±4.198a | 30.367±3.402a | 0.462±0.127a |
| YSTLF 4.9 g/kg | 6 | 31.150±3.026 | 30.951±4.303 | 30.483±3.731 | 27.750±5.665 | 0.329±0.042b |
| YSTLF 9.8 g/kg | 6 | 31.017±2.573 | 28.183±6.751 | 25.400±5.322 | 23.717±5.338 | 0.235±0.036b |
| CTP | 6 | 31.325±3.950 | 31.202±4.200 | 30.825±4.752 | 25.525±5.055 | 0.244±0.033b |
Table 2 YSTLF has an effect on FBG and urine C-peptide levels of the DKD mice ($\bar{x}±s$)
| Group | n | FBG (mmol/L) | Urine C-Peptide (ng/mL) | |||
|---|---|---|---|---|---|---|
| 0 week | 2 weeks | 4 weeks | 7 weeks | |||
| CON | 6 | 7.850±1.581 | 8.683±1.566 | 7.717±1.686 | 7.117±1.652 | 0.054±0.009 |
| MOD | 6 | 31.100±2.798a | 31.600±1.920a | 28.467±4.198a | 30.367±3.402a | 0.462±0.127a |
| YSTLF 4.9 g/kg | 6 | 31.150±3.026 | 30.951±4.303 | 30.483±3.731 | 27.750±5.665 | 0.329±0.042b |
| YSTLF 9.8 g/kg | 6 | 31.017±2.573 | 28.183±6.751 | 25.400±5.322 | 23.717±5.338 | 0.235±0.036b |
| CTP | 6 | 31.325±3.950 | 31.202±4.200 | 30.825±4.752 | 25.525±5.055 | 0.244±0.033b |
| Group | n | 24-h urine protein (mg/24h) | β2-MG (mg/L) | BUN (mmol/L) | UA (μmol/L) | LDH (U/L) | ||
|---|---|---|---|---|---|---|---|---|
| 0 week | 4 weeks | 7 weeks | ||||||
| CON | 6 | 2.007±0.820 | 1.713±0.735 | 1.688±0.315 | 0.180±0.013 | 2.677±0.577 | 59.798±16.054 | 414.457±42.373 |
| MOD | 6 | 3.978±0.678a | 5.762±1.066a | 7.363±1.466a | 0.232±0.008a | 4.343±0.740a | 106.667±11.778a | 480.322±27.629a |
| YSTLF 4.9 g/kg | 6 | 3.843±0.779 | 5.328±1.523 | 4.518±1.226c | 0.200±0.009b | 2.384±0.588b | 68.364±13.100b | 403.372±62.011b |
| YSTLF 9.8 g/kg | 6 | 4.035±0.987 | 4.967±2.029 | 3.657±1.037b | 0.212±0.008b | 3.363±0.512c | 64.647±16.632b | 418.073±26.474c |
| CTP | 6 | 3.832±1.103 | 5.625±2.825 | 4.665±1.316 | 0.190±0.08b | 2.983±0.609b | 64.243±10.749b | 406.025±23.970b |
Table 3 YSTLF reduced the urine protein content and improved the renal function of DKD mice ($\bar{x}±s$)
| Group | n | 24-h urine protein (mg/24h) | β2-MG (mg/L) | BUN (mmol/L) | UA (μmol/L) | LDH (U/L) | ||
|---|---|---|---|---|---|---|---|---|
| 0 week | 4 weeks | 7 weeks | ||||||
| CON | 6 | 2.007±0.820 | 1.713±0.735 | 1.688±0.315 | 0.180±0.013 | 2.677±0.577 | 59.798±16.054 | 414.457±42.373 |
| MOD | 6 | 3.978±0.678a | 5.762±1.066a | 7.363±1.466a | 0.232±0.008a | 4.343±0.740a | 106.667±11.778a | 480.322±27.629a |
| YSTLF 4.9 g/kg | 6 | 3.843±0.779 | 5.328±1.523 | 4.518±1.226c | 0.200±0.009b | 2.384±0.588b | 68.364±13.100b | 403.372±62.011b |
| YSTLF 9.8 g/kg | 6 | 4.035±0.987 | 4.967±2.029 | 3.657±1.037b | 0.212±0.008b | 3.363±0.512c | 64.647±16.632b | 418.073±26.474c |
| CTP | 6 | 3.832±1.103 | 5.625±2.825 | 4.665±1.316 | 0.190±0.08b | 2.983±0.609b | 64.243±10.749b | 406.025±23.970b |
| Group | n | AST | ALT |
|---|---|---|---|
| CON | 6 | 5.44±1.31 | 6.59±1.36 |
| MOD | 6 | 12.52±1.35a | 19.35±2.79a |
| YSTLF 4.9 g/kg | 6 | 8.11±1.56b | 9.79±1.64b |
| YSTLF 9.8 g/kg | 6 | 9.09±1.50b | 10.79±2.65b |
| CTP | 6 | 9.95±0.74b | 12.85±1.71b |
Table 4 YSTLF promoted liver function of the DKD mice (U/L, $\bar{x}±s$)
| Group | n | AST | ALT |
|---|---|---|---|
| CON | 6 | 5.44±1.31 | 6.59±1.36 |
| MOD | 6 | 12.52±1.35a | 19.35±2.79a |
| YSTLF 4.9 g/kg | 6 | 8.11±1.56b | 9.79±1.64b |
| YSTLF 9.8 g/kg | 6 | 9.09±1.50b | 10.79±2.65b |
| CTP | 6 | 9.95±0.74b | 12.85±1.71b |

Figure 2 YSTLF ameliorated renal injury of the DKD mice A: representative HE staining images (× 400, scale bar = 50 μm) of renal corpuscles and tubules. The black arrow indicates thickened glomerular basement membrane, the yellow arrow indicates mesangial matrix expansion, and the white arrow indicates renal capsule shrinkage and adhesion. A1: CON group, A2: MOD group, A3: YSTLF 4.9 g/kg group, A4: YSTLF 9.8 g/kg group, A5: CTP group. B: glomerular diameters. CON group: drink and eat freely + an equal volume of citrate buffer with model group by intraperitoneal injection + the same volume of pure water was given by gavage; MOD group: with 150 mg/kg STZ dissolved in citrate buffer to induce DKD by intraperitoneal injected. YSTLF 4.9 g/kg group: model group + YSTLF 4.9 g/kg per day; YSTLF 9.8 g/k group: model group + YSTLF 9.8 g/kg per day; CTP group: model group + CTP 12.5 mg/kg per day. DKD: diabetic kidney disease mice; CON: Control group; MOD: Model group; YSTLF: Yishen Tongluo formula; CTP: Captopril group. Statistical analyses were measured using a one-way analysis of variance for multiple comparisons. Data were presented as mean ± standard deviation (n = 6). Compared with the control group, aP < 0.01; compared with the model group, bP < 0.01, cP < 0.05.

Figure 3 YSTLF alleviated glomerulus injury of the DKD mice A: representative electron micrograph images (× 5000, scale bar = 2 μm) of the glomerulus. The black line indicates GBM, the red arrow indicates normal foot processes, and the yellow arrow indicates fused foot processes, A1: CON group, A2: MOD group, A3: YSTLF 4.9 g/kg group, A4: YSTLF 9.8 g/kg group, A5: CTP group. B: TGBM. CON group: drink and eat freely + an equal volume of citrate buffer with model group by intraperitoneal injection + the same volume of pure water was given by gavage; MOD group: with 150 mg/kg STZ dissolved in citrate buffer to induce DKD by intraperitoneal injected. YSTLF 4.9 g/kg group: model group + YSTLF 4.9 g/kg per day; YSTLF 9.8 g/k group: model group + YSTLF 9.8 g/kg per day; CTP group: model group + CTP 12.5 mg/kg per day. DKD: diabetic kidney disease mice; CON: Control group; MOD: Model group; YSTLF: Yishen Tongluo formula; CTP: Captopril group; TGBM: the mean GBM thickness. Statistical analyses were measured using a one-way analysis of variance for multiple comparisons. Data were presented as mean ± standard deviation (n = 6). Compared with the control group, aP < 0.01; compared with the model group, bP < 0.01.
| Group | n | PCX (ng/mL) | MCP-1 (pg/mL) |
|---|---|---|---|
| CON | 6 | 11.58±0.55 | 15.60±1.01 |
| MOD | 6 | 6.94±0.14a | 34.03±1.30a |
| YSTLF 4.9 g/kg | 6 | 7.61±0.60b | 23.16±0.38b |
| YSTLF 9.8 g/kg | 6 | 10.62±0.25b | 21.87±0.96b |
| CTP | 6 | 7.61±0.80b | 21.07±1.91b |
Table 5 YSTLF downregulated inflammation to improve the glomerular and podocyte injury in the DKD mice ($\bar{x}±s$)
| Group | n | PCX (ng/mL) | MCP-1 (pg/mL) |
|---|---|---|---|
| CON | 6 | 11.58±0.55 | 15.60±1.01 |
| MOD | 6 | 6.94±0.14a | 34.03±1.30a |
| YSTLF 4.9 g/kg | 6 | 7.61±0.60b | 23.16±0.38b |
| YSTLF 9.8 g/kg | 6 | 10.62±0.25b | 21.87±0.96b |
| CTP | 6 | 7.61±0.80b | 21.07±1.91b |

Figure 4 YSTLF attenuated kidney apoptosis in DKD mice A: Bax protein expression in renal tissue; B: Bax expression in renal tubules; C: Caspase-3 expression in renal interstitials as determined by immunohistochemistry (×400, scale bar = 20 μm); D: Bax and Caspase-3 protein expression in renal tissue assessed by Western blot. A1, B1, C1: CON group, A2, B2, C2: MOD group, A3, B3, C3: YSTLF 4.9 g/kg group, A4, B4, C4: YSTLF 9.8 g/kg group, A5, B5, C5: CTP group. A6: Average optical density of Bax; B6: Average optical density of Caspase-3 in renal tubules; C6: Average optical density of Caspase-3 in renal interstitial. D1: representative band of Bax and Caspase-3 protein detected by WB; D2: relative protein levels of Bax results; D3: relative protein levels of Caspase-3 results. CON group: drink and eat freely + an equal volume of citrate buffer with model group by intraperitoneal injection + the same volume of pure water was given by gavage; MOD group: with 150 mg/kg STZ dissolved in citrate buffer to induce DKD by intraperitoneal injected. YSTLF 4.9 g/kg group: model group + YSTLF 4.9 g/kg per day; YSTLF 9.8 g/k group: model group + YSTLF 9.8 g/kg per day; CTP group: model group + CTP 12.5 mg/kg per day. DKD: diabetic kidney disease mice; CON: Control group; MOD: Model group; YSTLF: Yishen Tongluo formula; CTP: Captopril group; Bax: BCL-2-associated X. Statistical analyses were measured using a one-way analysis of variance for multiple comparisons. Data were presented as mean ± standard deviation (n = 6). Compared with the control group, aP < 0.01; compared with the model group, bP < 0.01, cP < 0.05.
| 1. | Anders HJ, Huber TB, Isermann B, Schiffer M. CKD in diabetes: diabetic kidney disease versus nondiabetic kidney disease. Nat Rev Nephrol 2018; 14: 361-77. |
| 2. | Magliano DJ, Boyko EJ; IDF Diabetes Atlas 10th edition scientific committee. IDF DIABETES ATLAS [Internet]. 10th edition. Brussels: International Diabetes Federation; Pubmed online, 2021-12-15. cited 2023-10-20; Summary. Available from URL: https://www.ncbi.nlm.nih.gov/books/NBK581934/. |
| 3. |
Kato M, Natarajan R. Epigenetics and epigenomics in diabetic kidney disease and metabolic memory. Nat Rev Nephrol 2019; 15: 327-45.
DOI PMID |
| 4. |
Oshima M, Shimizu M, Yamanouchi M, et al. Trajectories of kidney function in diabetes: a clinicopathological update. Nat Rev Nephrol 2021; 17: 740-50.
DOI PMID |
| 5. | Bellary S, Tahrani AA, Barnett AH. Improving management of diabetic kidney disease: will GLP-1 receptor agonists have a role? Lancet Diabetes Endocrinol 2020; 8: 870-1. |
| 6. | Vartak T, Godson C, Brennan E. Therapeutic potential of pro-resolving mediators in diabetic kidney disease. Adv Drug Deliv Rev 2021; 178: 113965. |
| 7. |
Guo C, Li Y, Zhang R, et al. Protective effect of salidroside against diabetic kidney disease through inhibiting BIM-mediated apoptosis of proximal renal tubular cells in rats. Front Pharmacol 2018; 9: 1433.
DOI PMID |
| 8. |
Lassén E, Bouchareb R, Daehn IS. Podocyte as the link between sterile inflammation and diabetic kidney disease. Kidney Int 2022; 102: 688-90.
DOI PMID |
| 9. | Hao JB, Liu XT, Tang J, et al. The Effect of allograft inflammatory factor-1 on inflammation, oxidative stress, and autophagy via miR-34a/ATG4B pathway in diabetic kidney disease. Oxid Med Cell Longev 2022; 2022: 1668000. |
| 10. |
Niewczas MA, Pavkov ME, Skupien J, et al. A signature of circulating inflammatory proteins and development of end-stage renal disease in diabetes. Nat Med 2019; 25: 805-13.
DOI PMID |
| 11. | Pérez-Morales RE, Del Pino MD, Valdivielso JM, Ortiz A, Mora-Fernández C, Navarro-González JF. Inflammation in diabetic kidney disease. Nephron 2019; 143: 12-6. |
| 12. |
Song S, Shi C, Bian Y, et al. Sestrin2 remedies podocyte injury via orchestrating TSP-1/TGF-β1/Smad3 axis in diabetic kidney disease. Cell Death Dis 2022; 13: 663-77.
DOI PMID |
| 13. | Zhu H, Wang Z, Wang W, Lu Y, He YW, Tian J. Bacterial quorum-sensing signal DSF inhibits LPS-induced inflammations by suppressing toll-like receptor signaling and preventing lysosome-mediated apoptosis in zebrafish. Int J Mol Sci 2022; 23: 7110. |
| 14. | Loretelli C, Rocchio F, D’Addio F, et al. The IL-8-CXCR1/ 2 axis contributes to diabetic kidney disease. Metabolism 2021; 121: 154804. |
| 15. |
Davis TME, Peters KE, Lipscombe R. Apoptosis inhibitor of macrophage and diabetic kidney disease. Cell Mol Immunol 2019; 16: 521-22.
DOI PMID |
| 16. | Maruno S, Tanaka T, Nangaku M. Exploring molecular targets in diabetic kidney disease. Kidney Res Clin Pract 2022; 41: S33-45. |
| 17. | Yuan JY, Li GL, Feng ZH, et al. Une Analyse des caractéristiques du syndrome des patients diabétiques traités par la méthode de tonification du Rein et du drainage des vaisseaux Luo et fondée sur un modèle de forêt aléatoire. Revue Mondiale MC 2022; 1: 28-34. |
| 18. | Ji K, Li G, Wu S, et al. Analysis of Traditional Chinese Medicine syndromes and treatment laws of diabetic kidney disease and the action mechanism of high-frequency Chinese herbs in the treatment of diabetic kidney disease based on real-world study. Chin Med Nat Prod 2022; 2: e200-11. |
| 19. | Zhang X, Zhao L, Xiang S, et al. Yishen Tongluo formula alleviates diabetic kidney disease through regulating Sirt6/TGF-β1/Smad2/3 pathway and promoting degradation of TGF-β1. J Ethnopharmacol 2023; 307: 116243. |
| 20. | Zhao L, Zhang XW, Xie ZS, et al. Pharmacodynamic mechanism of Yishen To ngluo formula in treatment of diabetic kidney disease based on network pharmacology and verification of key regulation pathway. Beijing Zhong Yi Yao Da Xue Xue Bao 2022; 45: 824-34. |
| 21. | Xu Z, Jia K, Wang H, et al. METTL14-regulated PI3K/Akt signaling pathway via PTEN affects HDAC5-mediated epithelial-mesenchymal transition of renal tubular cells in diabetic kidney disease. Cell Death Dis 2021; 12: 32. |
| 22. | Zhang HK, Shi CY, Liu DT, et al. Dynamic changes in cardiac morphology, function, and diffuse myocardial fibrosis duration of diabetes in type 1 and type 2 diabetic mice models using 7.0 T CMR and echocardiography. Front Endocrinol 2023; 14: 1278619. |
| 23. | Zhang H, Shi C, Yang L, et al. Quantification of Early diffuse myocardial fibrosis through 7.0 T cardiac magnetic resonance T 1 mapping in a type 1 diabetic mellitus mouse model. J Magn Reson Imaging JMRI 2023; 57: 167-77. |
| 24. | Ma X, Wang Q. Short-chain fatty acids attenuate renal fibrosis and enhance autophagy of renal tubular cells in diabetic mice through the HDAC2/ULK1 axis. Endocrinol Metab Seoul Korea 2022; 37: 432-43. |
| 25. | Jiang L, Liu X, Hu X, et al. METTL3-mediated m6A modification of TIMP2 mRNA promotes podocyte injury in diabetic nephropathy. Mol Ther J Am Soc Gene Ther 2022; 30: 1721-40. |
| 26. | Xiao X, Yan XM, Li H, et al. Expression and correlation of autophagy-related proteins in kidney tissues of rats with type 1 diabetic nephropathy. Lin Chuang He Shi Yan Yi Xue Za Zhi 2021; 20: 2032-36. |
| 27. |
Shahzad K, Fatima S, Khawaja H, et al. Podocyte-specific Nlrp3 inflammasome activation promotes diabetic kidney disease. Kidney Int 2022; 102: 766-79.
DOI PMID |
| 28. | Abd Allah ESH, Gomaa AMS. Effects of curcumin and captopril on the functions of kidney and nerve in streptozotocin-induced diabetic rats: role of angiotensin converting enzyme 1. Appl Physiol Nutr Metab Physiol Appl Nutr Metab 2015; 40: 1061-7. |
| 29. | Yang Z, Lou X, Zhang J, et al. Association between early markers of renal injury and type 2 diabetic peripheral neuropathy. Diabetes Metab Syndr Obes Targets Ther 2021; 14: 4391. |
| 30. | Lan Q, Zheng L, Zhou X, et al. The value of blood urea nitrogen in the prediction of risks of cardiovascular disease in an older population. Front Cardiovasc Med 2021; 8: 614117. |
| 31. | Chen YT, Hsu HJ, Hsu CK, et al. Correlation between spot and 24 h proteinuria: derivation and validation of equation to estimate daily proteinuria. PLoS One 2019; 14: e0214614. |
| 32. |
Fu H, Liu S, Bastacky SI, Wang X, Tian XJ, Zhou D. Diabetic kidney diseases revisited: a new perspective for a new era. Mol Metab 2019; 30: 250-63.
DOI PMID |
| 33. | Osis G, Traylor AM, Black LM, et al. Expression of lactate dehydrogenase A and B isoforms in the mouse kidney. Am J Physiol - Ren Physiol 2021; 320: F706-18. |
| 34. | Zager RA, Johnson ACM, Becker K. Renal cortical lactate dehydrogenase: a useful, accurate, quantitative marker of in vivo tubular injury and acute renal failure. PLoS One 2013; 8: e66776. |
| 35. | Hanai K, Tauchi E, Nishiwaki Y, et al. Effects of uric acid on kidney function decline differ depending on baseline kidney function in type 2 diabetic patients. Nephrol Dial Transplant 2019; 34:1328-35. |
| 36. |
Barutta F, Bellini S, Canepa S, Durazzo M, Gruden G. Novel biomarkers of diabetic kidney disease: current status and potential clinical application. Acta Diabetol 2021; 58: 819-30.
DOI PMID |
| 37. | Yang M, Luo S, Yang J, et al. Crosstalk between the liver and kidney in diabetic nephropathy. Eur J Pharmacol 2022; 931: 175219. |
| 38. |
Shen Y, Chen W, Han L, et al. VEGF-B antibody and interleukin-22 fusion protein ameliorates diabetic nephropathy through inhibiting lipid accumulation and inflammatory responses. Acta Pharm Sin B 2021; 11: 127-42.
DOI PMID |
| 39. | Kundu A, Dey P, Sarkar P, et al. Protective effects of Croton hookeri on streptozotocin-induced diabetic nephropathy. Food Chem Toxicol Int J Publ Br Ind Biol Res Assoc 2020; 135: 110873. |
| 40. | Dakroub A, Dbouk A, Asfour A, et al. C-peptide in diabetes: a player in a dual hormone disorder? J Cell Physiol 2024; 239:e31212. |
| 41. |
Akhtar M, Taha NM, Nauman A, Mujeeb IB, Al-Nabet ADMH. Diabetic kidney disease: past and present. Adv Anat Pathol 2020; 27: 87-97.
DOI PMID |
| 42. |
Chagnac A, Zingerman B, Rozen-Zvi B, Herman-Edelstein M. Consequences of glomerular hyperfiltration: the role of physical forces in the pathogenesis of chronic kidney disease in diabetes and obesity. Nephron 2019; 143: 38-42.
DOI |
| 43. | Naylor RW, Morais MRPT, Lennon R. Complexities of the glomerular basement membrane. Nat Rev Nephrol 2021; 17: 112-27. |
| 44. | Li C, Guan XM, Wang RY, et al. Berberine mitigates high glucose-induced podocyte apoptosis by modulating autophagy via the mTOR/P70S6K/4EBP 1 pathway. Life Sci 2020; 243: 117277. |
| 45. | Ra D, Wb R, As A. Pathophysiology of diabetic kidney disease: impact of SGLT2 inhibitors. Nat Rev Nephrol 2021; 17: 319-34. |
| 46. |
Tang G, Li S, Zhang C, et al. Clinical efficacies, underlying mechanisms and molecular targets of Chinese medicines for diabetic nephropathy treatment and management. Acta Pharm Sin B 2021; 11: 2749-67.
DOI PMID |
| 47. | Ren J, Xu Y, Lu X, et al. Twist1 in podocytes ameliorates podocyte injury and proteinuria by limiting CCL2-dependent macrophage infiltration. JCI Insight 2021; 6: e148109. |
| 48. | Xing YW, Liu KZ. Azithromycin inhibited oxidative stress and apoptosis of high glucose-induced podocytes by inhibiting STAT1 pathway. Drug Dev Res 2021; 82: 990-8. |
| 49. |
Heng S, Samarajeewa N, Wang Y, Paule SG, Breen J, Nie G. Podocalyxin promotes an impermeable epithelium and inhibits pro-implantation factors to negatively regulate endometrial receptivity. Sci Rep 2021; 11: 24016.
DOI PMID |
| 50. | Takahashi N, Yoshida H, Kimura H, et al. Chronic hypoxia exacerbates diabetic glomerulosclerosis through mesangiolysis and podocyte injury in db/db mice. Nephrol Dial Transplant Off Publ Eur Dial Transpl Assoc - Eur Ren Assoc 2020; 35: 1678-88. |
| 51. | Wang R, Yao C, Liu F. Association between renal podocalyxin expression and renal dysfunction in patients with diabetic nephropathy: a single-center, retrospective case-control study. BioMed Res Int 2020; 2020: 7350781. |
| 52. | Jiang WJ, Xu CT, Du CL, et al. Tubular epithelial cell-to-macrophage communication forms a negative feedback loop via extracellular vesicle transfer to promote renal inflammation and apoptosis in diabetic nephropathy. Theranostics 2022; 12: 324-39. |
| 53. |
Huang F, Wang Q, Guo F, et al. FoxO1-mediated inhibition of STAT1 alleviates tubulointerstitial fibrosis and tubule apoptosis in diabetic kidney disease. EBioMedicine 2019; 48: 491-504.
DOI PMID |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||

