Journal of Traditional Chinese Medicine ›› 2023, Vol. 43 ›› Issue (6): 1219-1226.DOI: 10.19852/j.cnki.jtcm.20230915.001
• Research Articles • Previous Articles Next Articles
Efficacy of bioactive compounds of Chaihu (Radix Bupleuri Chinensis) on glaucomatous optic atrophy through interleukin-6/hypoxia inducible factor-1α signal pathway
YANG Xirui1, ZHAO Hui1, SHAN Muhammad2, DONG Feixue3, ZHANG Dandan4, WANG Jixue5( ), YUAN Xingxing6(
), YUAN Xingxing6( )
)
- 1 Ophthalmology Department, the First Affiliated Hospital of Henan University of Traditional Chinese Medicine, Zhengzhou 450000, China
2 Oncology Department, Cancer Hospital of Chinese Academy of Medical Sciences, Beijing 100029, China
3 Ophthalmology Department, the First Affiliated Hospital of Heilongjiang University of Traditional Chinese Medicine, Harbin 150040, China
4 Ophthalmology Department, the First Affiliated Hospital of Heilongjiang University of Traditional Chinese Medicine, Harbin 150040, China
5 Peripheral Vascular Department, the First Affiliated Hospital of Henan University of Traditional Chinese Medicine, Zhengzhou 450000, China
6 Digestive department, Heilongjiang Academy of Traditional Chinese Medicine, Harbin 150006, China, Heilongjiang University of Chinese Medicine, Harbin 150040, China
-
Received:2022-02-22Accepted:2022-05-14Online:2023-10-25Published:2023-09-15 -
Contact:WANG Jixue, Peripheral Vascular Department, the First Affiliated Hospital of Henan University of Traditional Chinese Medicine, Zhengzhou 450000, China. wjxyxr@126.com; YUAN Xingxing, Digestive department, Heilongjiang Academy of Traditional Chinese Medicine, Harbin 150006, China; Heilongjiang University of Chinese Medicine, Harbin 150040, China. yuanxingxing80@163.com. Telephone: +86-15590887703 -
Supported by:Study on the Protective Effect of Tongqiao Mingmu No. 4 on Retinal RGCs in Glaucoma based on Mitochondrial Apoptosis Pathway(QC2018115);Study on the Mechanism of Tongqiao Mingmu Decoction in the Treatment of Glaucomatous Optic Nerve Atrophy Based on p53-SLC7A11 Mediated RGC Ferroptosis to Regulate Microglial Cell Polarization(2022ZDZX127)
Cite this article
YANG Xirui, ZHAO Hui, SHAN Muhammad, DONG Feixue, ZHANG Dandan, WANG Jixue, YUAN Xingxing. Efficacy of bioactive compounds of Chaihu (Radix Bupleuri Chinensis) on glaucomatous optic atrophy through interleukin-6/hypoxia inducible factor-1α signal pathway[J]. Journal of Traditional Chinese Medicine, 2023, 43(6): 1219-1226.
share this article
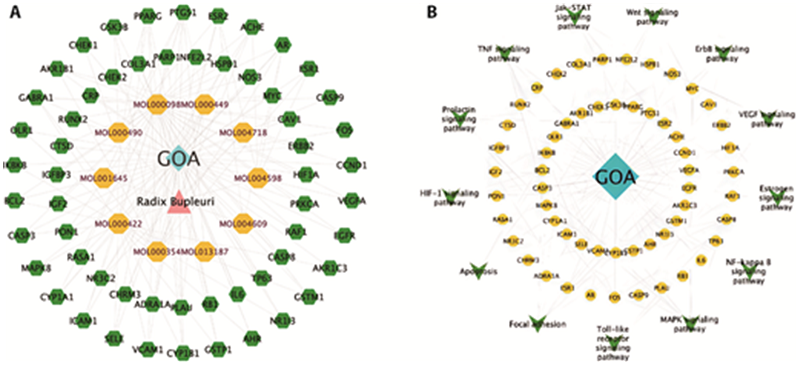
Figure 1 Pharmacological network figures of RB-GOA network A: Compound-target network of potential targets in RB and GOA. The yellow nodes represent the potential active ingredients in RB, the green nodes represent the corresponding targets of the bioactive component of RB-GOA, the red node represents the drug, and the blue node represents the disease. B: Drug-disease-pathway-target network; The yellow nodes represent disease, green nodes represent PPI protein, blue nodes represent pathway, and node size is directly proportional to degree value. RB: Chaihu (Radix Bupleuri Chinensis); GOA: glaucomatous optic atrophy; PPI: protein-protein interaction.
| Term | Number of pathway gene | P value |
|---|---|---|
| TNF signaling pathway | VCAM1, ICAM1, FOS, CASP3, IL-6, CASP8, NFKBIA, MAPK8, IKBKB, SELE | 0.000 |
| P53 signaling pathway | CASP3, CCND1, CASP8, CHEK1, CHEK2, IGFBP3 | 0.000 |
| Prolactin signaling pathway | CCND1, FOS, GSK3B, ESR1, RAF1, MAPK8, ESR2 | 0.000 |
| HIF-1 signaling pathway | EGFR, IL-6, HIF1A, BCL2, VEGFA, HK2, NOS3 | 0.000 |
| Apoptosis | CASP3, CASP9, BCL2, CASP8, NFKBIA, IKBKB | 0.000 |
| Focal adhesion | EGFR, CAV1, CCND1, BCL2, GSK3B, VEGFA, COL3A1, RAF1, MAPK8 | 0.000 |
| Toll-like receptor signaling pathway | FOS, IL-6, CASP8, NFKBIA, MAPK8, IKBKB | 0.000 |
| MAPK signaling pathway | EGFR, FOS, CASP3, RAF1, HSPB1, MAPK8, IKBKB, MYC, RASA1 | 0.000 |
| NF-KappaB signaling pathway | VCAM1, ICAM1, BCL2, NFKBIA, IKBKB, PLAU | 0.000 |
| Estrogen signaling pathway | EGFR, FOS, ESR1, RAF1, NOS3, ESR2 | 0.001 |
| VEGF signaling pathway | CASP9, VEGFA, RAF1, HSPB1, NOS3 | 0.001 |
| ErbB signaling pathway | GSK3B, RAF1, MAPK8, MYC | 0.026 |
| Wnt signaling pathway | CCND1, GSK3B, MAPK8, MYC | 0.648 |
| Jak-STAT signaling pathway | CCND1, IL-6, MYC | 0.815 |
Table 1 Functions of potential target genes based on KEGG pathway analysis
| Term | Number of pathway gene | P value |
|---|---|---|
| TNF signaling pathway | VCAM1, ICAM1, FOS, CASP3, IL-6, CASP8, NFKBIA, MAPK8, IKBKB, SELE | 0.000 |
| P53 signaling pathway | CASP3, CCND1, CASP8, CHEK1, CHEK2, IGFBP3 | 0.000 |
| Prolactin signaling pathway | CCND1, FOS, GSK3B, ESR1, RAF1, MAPK8, ESR2 | 0.000 |
| HIF-1 signaling pathway | EGFR, IL-6, HIF1A, BCL2, VEGFA, HK2, NOS3 | 0.000 |
| Apoptosis | CASP3, CASP9, BCL2, CASP8, NFKBIA, IKBKB | 0.000 |
| Focal adhesion | EGFR, CAV1, CCND1, BCL2, GSK3B, VEGFA, COL3A1, RAF1, MAPK8 | 0.000 |
| Toll-like receptor signaling pathway | FOS, IL-6, CASP8, NFKBIA, MAPK8, IKBKB | 0.000 |
| MAPK signaling pathway | EGFR, FOS, CASP3, RAF1, HSPB1, MAPK8, IKBKB, MYC, RASA1 | 0.000 |
| NF-KappaB signaling pathway | VCAM1, ICAM1, BCL2, NFKBIA, IKBKB, PLAU | 0.000 |
| Estrogen signaling pathway | EGFR, FOS, ESR1, RAF1, NOS3, ESR2 | 0.001 |
| VEGF signaling pathway | CASP9, VEGFA, RAF1, HSPB1, NOS3 | 0.001 |
| ErbB signaling pathway | GSK3B, RAF1, MAPK8, MYC | 0.026 |
| Wnt signaling pathway | CCND1, GSK3B, MAPK8, MYC | 0.648 |
| Jak-STAT signaling pathway | CCND1, IL-6, MYC | 0.815 |
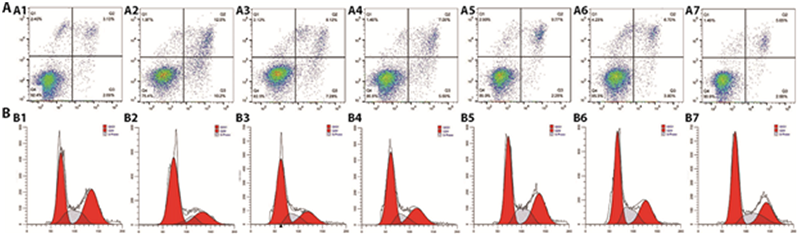
Figure 2 RB reduces cell viability and induces apoptosis in RGCs A: apoptosis rate by flow cytometry assay; A1: control group; A2: model group; A3: RB 0.1 g/L group; A4: RB 1 g/L group; A5: RB 2.5 g/L group; A6: RB 5 g/L group; A7: RB 10 g/L group. B: apoptosis rate by representative flow cytometric data derived from analysis of cell cycle progression in RGCs. B1: control group; B2: model group; B3: RB 0.1 g/L group; B4: RB 1 g/L group; B5: RB 2.5 g/L group; B6: RB 5 g/L group; B7: RB 10 g/L group.The RGCs were divided into control group (n = 3): cells were cultured normally without any drug treatment; Model group (n = 3): cells were cultured for 6 h under normal conditions, and cultured for 6 h in serum free and glucose free medium under anoxic conditions; RB group (n = 3): cells were cultured under normal culture conditions, pre intervened with RB (0.1, 1, 2.5, 5 and 10 g/L for 6 h,, then cultured with serum free and glucose free hypoxia medium for 6 h (maintaining drug concentration), and then cultured with normal reoxygenation for 3 h (maintaining drug concentration). RB: Chaihu (Radix Bupleuri Chinensis); RGCs: retinal ganglion cells.

Figure 3 Effects of RB on expression levels SOD, MDA, GSH-PX, IL-6, and HIF-1α A1: comparison of MDA content in each group; A2: comparison of SOD activity in each group; A3: comparison of GSH-PX activityin each group; B1: relative expression of IL-6 level; B2: relative expression of HIF-1α level; B3: representative Western blot results of the IL-6 and HIF-1α expression levels were shown. RB increased ROS generation and lipid peroxidation in RGCs in a concentration-dependent manner SOD, MDA and GSH-PX levels are presented as percentage mean ± standard deviation relative to control levels, The RGCs were divided into control group (n = 3): cells were cultured normally without any drug treatment; Model group (n = 3): cells were cultured for 6 h under normal conditions, and cultured for 6 h in serum free and glucose free medium under anoxic conditions; RB group (n = 3): cells were cultured under normal culture conditions, pre intervened with RB (0.1, 1, 2.5, 5 and 10 g/L for 6 h,, then cultured with serum free and glucose free hypoxia medium for 6 h (maintaining drug concentration), and then cultured with normal reoxygenation for 3 h (maintaining drug concentration). MDA: malonaldehyde;SOD: superoxidedismutase; GSH-PX: glutathione peroxidase; ROS: reactive oxygen species; RB: Chaihu (Radix Bupleuri Chinensis); IL-6: interleukin- 6; HIF-1α: hypoxia inducible factor 1 alpha. Student t-test and one-way analysis of variance were used for comparison analysis. aP < 0.01 vs the control group, bP < 0.05 vs the Model group and cP < 0.05 vs the RB 0.1 g/L.
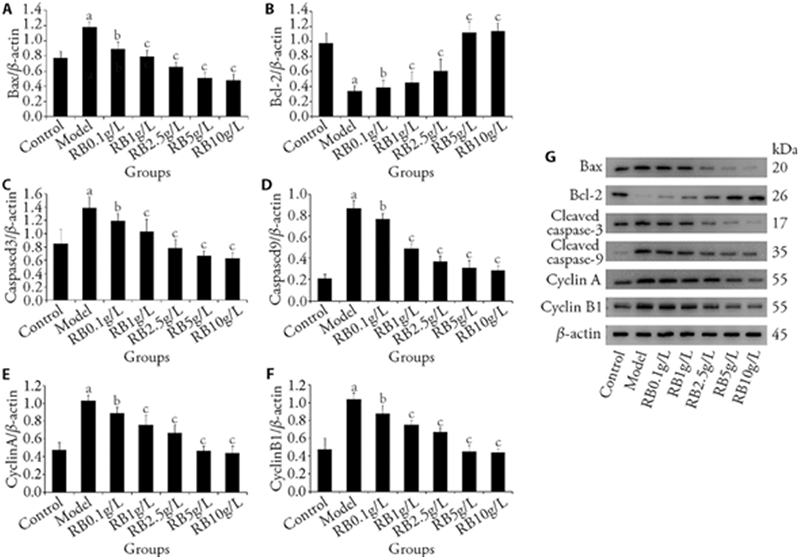
Figure 4 Effects of RB on expression levels Bax, Bcl-2, Caspase-3, Caspase-9, CyclinA and CyclinB1 A: Western blot of Bax expression; B: Western blot of Bcl-2 expression; C: Western blot of Caspase-3 expression; D: Western blot of Caspase-9 expression; E: Western blot of CyclinA expression; F: Western blot of CyclinB1 expression; G: representative Western blot results of the protein expression levels were shown. The RGCs were divided into control group (n = 3): cells were cultured normally without any drug treatment; Model group (n = 3): cells were cultured for 6 h under normal conditions, and cultured for 6 h in serum free and glucose free medium under anoxic conditions; RB group (n = 3): cells were cultured under normal culture conditions, pre intervened with RB (0.1, 1, 2.5, 5 and 10 g/L for 6 h,, then cultured with serum free and glucose free hypoxia medium for 6 h (maintaining drug concentration), and then cultured with normal reoxygenation for 3 h (maintaining drug concentration). RB: Chaihu (Radix Bupleuri Chinensis); Bcl-2: B-cell lymphoma-2; Bax: Bcl-2-associated X protein. Student t-test and one-way analysis of variance were used for comparison analysis; aP < 0.01 vs the control group, bP < 0.05 vs the Model group and cP < 0.05 vs the RB 0.1 g/L.
| 1. |
Weinreb RN, Aung T, Medeiros FA. The pathophysiology and treatment of glaucoma: a review. JAMA 2014; 311: 1901-11.
DOI PMID |
| 2. |
Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol 2006; 90: 262-7.
DOI URL |
| 3. | Wu S, Tian QM, Gao YE, et al. Research progress in the mechanism of apoptosis of optic ganglia cells in glaucoma. Yan Ke Xin Jin Zhan 2019; 39: 882-5. |
| 4. | Yan ML, Yang L, Hou AJ, et al. Research progress on chemical constituents and pharmacological action of Bupleurum. Zhong Yi Yao Xin Xi 2018; 35: 103-9. |
| 5. | Wang P, Chen QL. Effect of processed Bupleurum on cholinesterase activity in whole blood of mice. Zhong Cao Yao 2000; 23: 219. |
| 6. |
Rao PV, Pattabiraman PP, Kopczynski C. Role of the Rho GTPase/Rho kinase signaling pathway in pathogenesis and treatment of glaucoma: Bench to bedside research. Exp Eye Res 2017; 158: 23-32.
DOI PMID |
| 7. | Basavarajappa D, Gupta V, Wall RV, et al. S1PR1 signaling attenuates apoptosis of retinal ganglion cells via modulation of cJun/Bim cascade and Bad phosphorylation in a mouse model of glaucoma. Faseb J 2023; 37: e22710. |
| 8. | Lin CX. Pharmacological research and clinical application of Bupleurum Chinese. Lin Chuang Yi Xue Wen Xian Di Zi Qi Kan 2019; 12: 197. |
| 9. | He XS, Zhu ZF, Lu XL, et al. The effect of saikosaponin d on the expression of VEGF and Ang-2 in hepatoma cells. Di San Jun Yi Da Xue Xue Bao 2011; 33: 1233-5. |
| 10. | Samoilova EB, Horton JL, Hilliard B, Liu TS, Chen Y. IL-6-deficient mice are resistant to experimental autoimmune encephalomyelitis: roles of IL-6 in the activation and differentiation of autoreactive T cells. J Immunol 1998; 161: 6480-6. |
| 11. |
Shihara K, Hirano T. IL-6 in autoimmune disease and chronic inflammatory proliferative disease. Cytokine Growth Factor Rev 2002; 13: 357-68.
DOI URL |
| 12. |
Naldini A, Carraro F, Silvestri S, Bocci V. Hypoxia affects cytokine production and proliferative responses by human peripheral mononuclear cells. J Cell Physiol 1997; 173: 335-42.
DOI PMID |
| 13. |
Lee JW, Bae SH, Jeong JW, Kim SH, Kim KW. Hypoxia-inducible factor (HIF-1)alpha: its protein stability and biological functions. Exp Mol Med 2004; 36: 1-12.
DOI |
| 14. |
Elson DA, Thurston G, Huang LE, et al. Induction of hypervascularity without leakage or inflammation in transgenic mice overexpressing hypoxia-inducible factor-1alpha. Genes Dev 2001; 15: 2520-32.
DOI URL |
| 15. |
Wiener CM, Booth G, Semenza GL. In vivo expression of mRNAs encoding hypoxia-inducible factor 1. Biochem Biophys Res Commun 1996; 225: 485-8.
DOI URL |
| 16. | Ergorul C, Ray A, Huang W, et al. Hypoxia inducible factor-1α (HIF-1α) and some HIF-1 target genes are elevated in experimental glaucoma. J Mol Neurosci 2010; 42: 183-91. |
| 17. | Tezel G, Wax MB. Hypoxia inducible factor-1 alpha in the glaucomatous retina and optic nerve head. Arch Ophthalmol 2004; 12: 1348-56. |
| 18. | Wang PP, Kong FP, Chen XQ. HIF-1 signaling pathway of hypoxic cell stress. Zhejiang Da Xue (Yi Xue Ban) 2011; 12: 559-66. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||

