Journal of Traditional Chinese Medicine ›› 2024, Vol. 44 ›› Issue (5): 906-915.DOI: 10.19852/j.cnki.jtcm.2024.05.002
Previous Articles Next Articles
Weitiao No. 3 (微调3号方) enhances the efficacy of anti-programmed cell death protein-1 immunotherapy by modulating the intestinal microbiota in an orthotopic model of gastric cancer mice
HUANG Xiaona, LI Yuzhen, ZHU Chenyang, ZHU Hengzhou, JIANG Chenyu, ZHU Xiaodan, ZHANG Chencen, JIN Chunhui( )
)
- Department of Oncology, Wuxi Hospital Affiliated to Nanjing University of Chinese Medicine (Wuxi Hospital of Traditional Chinese Medicine), Wuxi 214071, China
-
Received:2023-07-23Accepted:2023-12-05Online:2024-10-15Published:2024-09-11 -
Contact:Prof. JIN Chunhui, Department of Oncology, Wuxi Hospital Affiliated to Nanjing University of Chinese Medicine (Wuxi Hospital of Traditional Chinese Medicine), Wuxi 214071, China. wxzy013@njucm.edu.cn Telephone: +86-13861868628 -
Supported by:National Natural Science Foundation of China: to Explore the Role and Mechanism of Weitiao No. 3 in Promoting Immunotherapy of Gastric Cancer by Activating Peroxisome Proliferator-activated Receptor-γ/AMP-activated Protein Kinase Signaling Pathway through Short Chain Fatty Acid based on Intestinal Flora(82274269);Nature Foundation of Nanjing University of Chinese Medicine: to Investigate the Effect and Mechanism of Weitiao No. 3 on Immunotherapy of Gastric Cancer based on Peroxisome Proliferator-activated Receptor-γ/AMP-activated Protein Kinase Signaling Pathway(XZR2020079);Wuxi Administration of Traditional Chinese Medicine Scientific Research Project: Clinical and Basic Study on the Effect of Weitiao No. 3 Mixture on Immunotherapy of Gastric Cancer by Regulating Intestinal Microecology(ZYKJ201906)
Cite this article
HUANG Xiaona, LI Yuzhen, ZHU Chenyang, ZHU Hengzhou, JIANG Chenyu, ZHU Xiaodan, ZHANG Chencen, JIN Chunhui. Weitiao No. 3 (微调3号方) enhances the efficacy of anti-programmed cell death protein-1 immunotherapy by modulating the intestinal microbiota in an orthotopic model of gastric cancer mice[J]. Journal of Traditional Chinese Medicine, 2024, 44(5): 906-915.
share this article
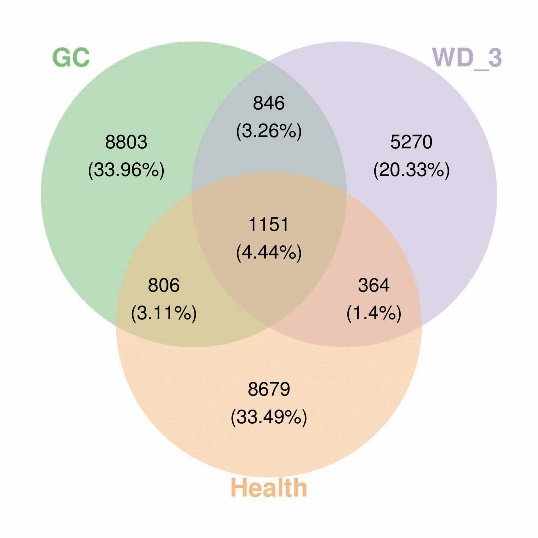
Figure 1 Effects of WD-3 on the composition of the intestinal microbiota Venn diagram of OTUs in the three groups (Health, GC, and WD-3). Health group: healthy participants; GC group: GC patients. WD-3 group: GC patients treated with WD-3. GC: gastric cancer; WD-3: Weitiao No. 3; OTUs: operational taxonomic units.

Figure 2 Cluster heat map of the top 50 bacteria in terms of average abundance at the genus level Health group: healthy participants; GC group: GC patients. WD-3 group: GC patients treated with WD-3. GC: gastric cancer; WD-3: Weitiao No. 3.
| Group | n | Bifidobacterium | Coprococcus | Saccharopolyspora |
|---|---|---|---|---|
| Health | 10 | 20.266±11.387 | 4.553±3.543 | 0.000±0.000 |
| GC | 17 | 2.099±2.821a | 0.249±0.271a | 0.000±0.000 |
| WD-3 | 12 | 24.541±20.546b | 1.214±0.379b | 0.044±0.017b |
Table 1 Relative abundances of Bifidobacterium, Coprococcus, and Saccharopolyspora in the three groups (%, $\bar{x}±s$)
| Group | n | Bifidobacterium | Coprococcus | Saccharopolyspora |
|---|---|---|---|---|
| Health | 10 | 20.266±11.387 | 4.553±3.543 | 0.000±0.000 |
| GC | 17 | 2.099±2.821a | 0.249±0.271a | 0.000±0.000 |
| WD-3 | 12 | 24.541±20.546b | 1.214±0.379b | 0.044±0.017b |

Figure 3 Effects of WD-3 on the efficacy of immunotherapy in GC mice A: IHC staining. Magnification × 200; Scar bar = 20 μm. A1: CD3 expression in the control group; A2: CD3 expression in the anti-PD-1 group; A3: CD3 expression in the WD-3 group; A4: CD3 expression in the anti-PD-1 + WD-3 group; A5: CD4 expression in the control group; A6: CD4 expression in the anti-PD-1 group; A7: CD4 expression in the WD-3 group; A8: CD4 expression in the anti-PD-1+WD-3 group; A9: CD8 expression in the control group; A10: CD8 expression in the anti-PD-1 group; A11: CD8 expression in the WD-3 group; A12: CD8 expression in the anti-PD-1+WD-3 group; A13: FOXP3 expression in the control group; A14: FOXP3 expression in the anti-PD-1 group; A15: FOXP3 expression in the WD-3 group; A16: FOXP3 expression in the anti-PD-1 + WD-3 group; A17: Ki67 expression in the control group; A18: Ki67 expression in the anti-PD-1 group; A19: Ki67 expression in the WD-3 group; A20: Ki67 expression in the anti-PD-1 + WD-3 group; B: TUNEL staining was used to detect cell apoptosis of GC tissues. Magnification × 200; Scar bar = 20 μm. B1: cell apoptosis in the control group; B2: cell apoptosis in the anti-PD-1 group; B3: cell apoptosis in the WD-3 group; B4: cell apoptosis in the anti-PD-1 + WD-3 group. Control group: GC mice with normal saline; anti-PD-1 group: GC mice treated with PD-1 inhibitor; WD-3 group: GC mice treated with WD-3; anti-PD-1+WD-3 group: GC mice treated with PD-1 inhibitor and WD-3. GC: gastric cancer; anti-PD-1: anti-programmed cell death protein-1; WD-3: Weitiao No. 3; IHC: immunohistochemistry; TUNEL: terminal-deoxynucleotidyl-transferase -mediated deoxyuridine triphosphate nick end labeling; FOXP3: forkhead box protein P3.
| Group | n | CD3 | CD4 | CD8 | FOXP3 | Ki67 |
|---|---|---|---|---|---|---|
| Control | 6 | 0.106±0.016 | 0.084±0.02 | 0.089±0.020 | 0.300±0.040 | 0.309±0.036 |
| Anti-PD-1 | 6 | 0.244±0.0279a | 0.145±0.025a | 0.156±0.028a | 0.161±0.029a | 0.162±0.025a |
| WD-3 | 6 | 0.201±0.026a | 0.128±0.021a | 0.131±0.016a | 0.247±0.029a | 0.244±0.020a |
| Anti-PD-1+WD-3 | 6 | 0.414±0.034abc | 0.221±0.031abc | 0.210±0.035abc | 0.078±0.015abc | 0.102±0.023abc |
Table 2 Mean optical density for Immunohistochemistry ($\bar{x}±s$)
| Group | n | CD3 | CD4 | CD8 | FOXP3 | Ki67 |
|---|---|---|---|---|---|---|
| Control | 6 | 0.106±0.016 | 0.084±0.02 | 0.089±0.020 | 0.300±0.040 | 0.309±0.036 |
| Anti-PD-1 | 6 | 0.244±0.0279a | 0.145±0.025a | 0.156±0.028a | 0.161±0.029a | 0.162±0.025a |
| WD-3 | 6 | 0.201±0.026a | 0.128±0.021a | 0.131±0.016a | 0.247±0.029a | 0.244±0.020a |
| Anti-PD-1+WD-3 | 6 | 0.414±0.034abc | 0.221±0.031abc | 0.210±0.035abc | 0.078±0.015abc | 0.102±0.023abc |
| Group | n | TNF-α | IL-2 | IL-6 | IL-10 | IFN-γ | TGF-β |
|---|---|---|---|---|---|---|---|
| Control | 6 | 33.6±2.9 | 28.8±3.1 | 203.8±10.1 | 65.2±7.5 | 186.4±5.7 | 226.0±6.2 |
| Anti-PD-1 | 6 | 59.5±5.1a | 45.8±3.9a | 116.9±11.1a | 40.8±3.1a | 314.1±12.7a | 166.0±4.8a |
| WD-3 | 6 | 54.7±4.4a | 42.5±2.7a | 129.7±16.7a | 43.7±5.9a | 298.6±17.2a | 175.6±11.7a |
| Anti-PD-1+WD-3 | 6 | 83.9±8.7abc | 64.2±5.2abc | 80.8±11.9abc | 32.8±6.1ade | 353.0±6.4abc | 124.4±11.8abc |
Table 3 Inflammatory factor concentration (pg/mL, $\bar{x}±s$)
| Group | n | TNF-α | IL-2 | IL-6 | IL-10 | IFN-γ | TGF-β |
|---|---|---|---|---|---|---|---|
| Control | 6 | 33.6±2.9 | 28.8±3.1 | 203.8±10.1 | 65.2±7.5 | 186.4±5.7 | 226.0±6.2 |
| Anti-PD-1 | 6 | 59.5±5.1a | 45.8±3.9a | 116.9±11.1a | 40.8±3.1a | 314.1±12.7a | 166.0±4.8a |
| WD-3 | 6 | 54.7±4.4a | 42.5±2.7a | 129.7±16.7a | 43.7±5.9a | 298.6±17.2a | 175.6±11.7a |
| Anti-PD-1+WD-3 | 6 | 83.9±8.7abc | 64.2±5.2abc | 80.8±11.9abc | 32.8±6.1ade | 353.0±6.4abc | 124.4±11.8abc |

Figure 4 Effects of intestinal flora on the efficacy of immunotherapy in GC mice A: IHC staining. Magnification × 100; Scar bar = 50 μm. A1: CD3 expression in the control group; A2: CD3 expression in the anti-PD-1 group; A3: CD3 expression in the intestinal flora group; A4: CD3 expression in the anti-PD-1 + intestinal flora group; A5: CD4 expression in the control group; A6: CD4 expression in the anti-PD-1 group; A7: CD4 expression in the intestinal flora group; A8: CD4 expression in the anti-PD-1 + intestinal flora group; A9: CD8 expression in the control group; A10: CD8 expression in the anti-PD-1 group; A11: CD8 expression in the intestinal flora group; A12: CD8 expression in the anti-PD-1 + intestinal flora group; A13: FOXP3 expression in the control group; A14: FOXP3 expression in the anti-PD-1 group; A15: FOXP3 expression in the intestinal flora group; A16: FOXP3 expression in the anti-PD-1+ intestinal flora group; A17: Ki67 expression in the control group; A18: Ki67 expression in the anti-PD-1 group; A19: Ki67 expression in the intestinal flora group; A20: Ki67 expression in the anti-PD-1 + intestinal flora group.; B: TUNEL staining was used to detect the cell apoptosis of GC tumors. Magnification × 100; Scar bar = 50 μm. B1: cell apoptosis in the control group; B2: cell apoptosis in the anti-PD-1 group; B3: cell apoptosis in the intestinal flora group; B4: cell apoptosis in the anti-PD-1+ intestinal flora group. Control group: GC mice with normal saline; anti-PD-1 group: GC mice treated with PD-1 inhibitor; intestinal flora group: GC mice treated with intestinal flora; anti-PD-1 + intestinal flora group: GC mice treated with PD-1 inhibitor and intestinal flora. GC: gastric cancer; anti-PD-1: anti-programmed cell death protein-1; IHC: immunohistochemistry; TUNEL: terminal-deoxynucleotidyl-transferase-mediated deoxyuridine triphosphate nick end labeling; FOXP3: forkhead box protein P3.
| Group | n | CD3 | CD4 | CD8 | FOXP3 | Ki67 |
|---|---|---|---|---|---|---|
| Control | 6 | 0.101±0.014 | 0.086±0.013 | 0.045±0.013 | 0.306±0.017 | 0.299±0.016 |
| Anti-PD-1 | 6 | 0.304±0.030a | 0.262±0.034a | 0.155±0.016a | 0.165±0.018a | 0.152±0.021a |
| Intestinal flora | 6 | 0.219±0.022a | 0.153±0.011a | 0.103±0.011a | 0.213±0.012a | 0.218±0.037a |
| Anti-PD-1+ intestinal flora | 6 | 0.528±0.018abc | 0.404±0.019abc | 0.221±0.016abc | 0.057±0.011abc | 0.056±0.013abc |
Table 4 Mean optical density for Immunohistochemistry ($\bar{x}±s$)
| Group | n | CD3 | CD4 | CD8 | FOXP3 | Ki67 |
|---|---|---|---|---|---|---|
| Control | 6 | 0.101±0.014 | 0.086±0.013 | 0.045±0.013 | 0.306±0.017 | 0.299±0.016 |
| Anti-PD-1 | 6 | 0.304±0.030a | 0.262±0.034a | 0.155±0.016a | 0.165±0.018a | 0.152±0.021a |
| Intestinal flora | 6 | 0.219±0.022a | 0.153±0.011a | 0.103±0.011a | 0.213±0.012a | 0.218±0.037a |
| Anti-PD-1+ intestinal flora | 6 | 0.528±0.018abc | 0.404±0.019abc | 0.221±0.016abc | 0.057±0.011abc | 0.056±0.013abc |
| Group | n | TNF-α | IL-2 | IL-6 | IL-10 | IFN-γ | TGF-β |
|---|---|---|---|---|---|---|---|
| Control | 6 | 36.0±3.4 | 31.8±4.4 | 231.0±7.4 | 75.3±2.7 | 190.8±7.3 | 234.9±3.8 |
| Anti-PD-1 | 6 | 66.8±2.6a | 59.8±4.3a | 114.8±11.2a | 40.4±3.7a | 340.0±4.0a | 164.6±7.7a |
| Intestinal flora | 6 | 51.7±3.2a | 47.5±3.0a | 174.1±13.5a | 56.9±5.4a | 293.0±5.9a | 197.7±3.8a |
| Anti-PD-1+ intestinal flora | 6 | 99.4±1.9abc | 85.2±2.7abc | 52.5±11.6abc | 23.7±6.0adc | 446.1±12.5abc | 115.8±6.4abc |
Table 5 Inflammatory factor concentration (pg/mL, $\bar{x}±s$)
| Group | n | TNF-α | IL-2 | IL-6 | IL-10 | IFN-γ | TGF-β |
|---|---|---|---|---|---|---|---|
| Control | 6 | 36.0±3.4 | 31.8±4.4 | 231.0±7.4 | 75.3±2.7 | 190.8±7.3 | 234.9±3.8 |
| Anti-PD-1 | 6 | 66.8±2.6a | 59.8±4.3a | 114.8±11.2a | 40.4±3.7a | 340.0±4.0a | 164.6±7.7a |
| Intestinal flora | 6 | 51.7±3.2a | 47.5±3.0a | 174.1±13.5a | 56.9±5.4a | 293.0±5.9a | 197.7±3.8a |
| Anti-PD-1+ intestinal flora | 6 | 99.4±1.9abc | 85.2±2.7abc | 52.5±11.6abc | 23.7±6.0adc | 446.1±12.5abc | 115.8±6.4abc |
| 1. | Sung H, Ferlay J, Siegel RL. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021; 71: 209-49. |
| 2. | Song Z, Wu Y, Yang J, et al. Progress in the treatment of advanced gastric cancer. Tumour Biol 2017; 39: 1010428317714626. |
| 3. | Tan Z. Recent advances in the surgical treatment of advanced gastric cancer: a review. Med Sci Monit 2019; 25: 3537-41. |
| 4. |
Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade Science 2018; 359: 1350-5.
DOI PMID |
| 5. |
Janjigian YY, Shitara K, Moehler M, et al. Cancer immunotherapy using checkpoint blockadeFirst-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet 2021; 398: 27-40.
DOI PMID |
| 6. | Gopalakrishnan V, Helmink BA, Spencer CN, et al. The influence of the gut microbiome on cancer, immunity, and cancer immunotherapy. Cancer Cell 2018; 33: 570-80. |
| 7. |
Li W, Deng Y, Chu Q, et al. Gut microbiome and cancer immunotherapy. Cancer Lett 2019; 447: 41-7.
DOI PMID |
| 8. |
Helmink BA, Khan MAW, Hermann A, et al. The microbiome, cancer, and cancer therapy. Nat Med 2019; 25: 377-88.
DOI PMID |
| 9. |
Panebianco C, Andriulli A, Pazienza V. Pharmacomicrobiomics: exploiting the drug-microbiota interactions in anticancer therapies. Microbiome 2018; 6: 92.
DOI PMID |
| 10. |
Derosa L, Routy B, Fidelle M, et al. Gut bacteria composition drives primary resistance to cancer immunotherapy in renal cell carcinoma patients. Eur Urol 2020; 78: 195-206.
DOI PMID |
| 11. | Sui H, Zhang L, Gu K, et al. YYFZBJS ameliorates colorectal cancer progression in Apc (Min/+) mice by remodeling gut microbiota and inhibiting regulatory T-cell generation. Cell Commun Signal 2020; 18: 113. |
| 12. | Shao S, Jia R, Zhao L, et al. Xiao-Chai-Hu-Tang ameliorates tumor growth in cancer comorbid depressive symptoms via modulating gut microbiota-mediated TLR4/MyD88/NF-κB signaling pathway. Phytomedicine 2021; 88: 153606. |
| 13. | Zhu X, Zhao L, You J, et al. WD-3 inhibits the proliferation of breast cancer cells by regulating the glycolytic pathway. Bosn J Basic Med Sci 2020; 20: 226-35. |
| 14. | Xue Q, You JL, Wang WS, Gong SX. Clinical analysis of Chinese medicine WD-3 treat colorectal cancer liver metastasis. Neimenggu Zhong Yi Yao 2015; 12: 1-2. |
| 15. | Xue Q, You JL, Gong SX, Zhao JF. Clinical analysis of Chinese medicine WD-3 combined with Xiaozhenggao to treat advanced pancreatic cancer. Liaoning Zhong Yi Yao Da Xue Xue Bao 2016; 18: 184-6. |
| 16. | Jin C, Zhang BN, Wei Z, Ma B, Pan Q, Hu P. Effects of WD-3 on tumor growth and the expression of integrin αvβ3 and ERK1/2 in mice bearing human gastric cancer using the 18F-RGD PET/CT imaging system. Mol Med Rep 2017; 16: 9295-300. |
| 17. |
Dermani FK, Samadi P, Rahmani G, Kohlan AK, Najafi R. PD-1/PD-L1 immune checkpoint: potential target for cancer therapy. J Cell Physiol 2019; 234: 1313-25.
DOI PMID |
| 18. | Fuchs CS, Doi T, Jang RW, et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 clinical KEYNOTE-059 trial. JAMA Oncol 2018; 4: e180013. |
| 19. | Guo J, Shen Y, Hu S, Rui T, Liu J, Yuan Y. Neobavaisoflavone inhibits antitumor immunosuppression via myeloid-derived suppressor cells. Int Immunopharmacol 2022; 111: 109103. |
| 20. | Chen S, Li R, Chen Y, et al. Scutellarin enhances anti-tumor immune responses by reducing TNFR2-expressing CD4(+) Foxp3(+) regulatory T cells. Biomed Pharmacother 2022; 151: 113187. |
| 21. | Bailly C. Anticancer properties of lobetyolin, an essential component of radix codonopsis (Dangshen). Nat Prod Bioprospect 2021; 11: 143-53. |
| 22. |
Li X, He Y, Zeng P, et al. Molecular basis for Poria cocos mushroom polysaccharide used as an antitumour drug in China. J Cell Mol Med 2019; 23: 4-20.
DOI PMID |
| 23. | Bailly C. Atractylenolides, essential components of Atractylodes-based traditional herbal medicines: antioxidant, anti-inflammatory and anticancer properties. Eur J Pharmacol 2021; 891: 173735. |
| 24. | Badgeley A, Anwar H, Modi K, Murphy P, Lakshmikuttyamma A. Effect of probiotics and gut microbiota on anti-cancer drugs: mechanistic perspectives. Biochim Biophys Acta Rev Cancer 2021; 1875: 188494. |
| 25. | Ying HZ, Xie W, Wang MC, He JQ, Zhang HH, Yu CH. Gut microbiota: an emerging therapeutic approach of herbal medicine for prevention of colorectal cancer. Front Cell Infect Microbiol 2022; 12: 969526. |
| 26. | Chen YZ, Yuan MY, Chen YL, et al. The gut microbiota and Traditional Chinese Medicine: a new clinical frontier on cancer. Curr Drug Targets 2021; 22: 1222-31. |
| 27. | Meng C, Bai C, Brown TD, Hood LE, Tian Q. Human gut microbiota and gastrointestinal cancer. Genomics Proteomics Bioinformatics 2018; 16: 33-49. |
| 28. |
Matson V, Fessler J, Bao R. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science 2018; 359: 104-8.
DOI PMID |
| 29. |
Sivan A, Corrales L, Hubert N, et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science 2015; 350: 1084-9.
DOI PMID |
| 30. | Xia W, Khan I, Li XA, et al. Adaptogenic flower buds exert cancer preventive effects by enhancing the SCFA-producers, strengthening the epithelial tight junction complex and immune responses. Pharmacol Res 2020; 159: 104809. |
| 31. | He Y, Fu L, Li Y, et al. Gut microbial metabolites facilitate anticancer therapy efficacy by modulating cytotoxic CD8(+) T cell immunity. Cell Metab 2021; 33: 988-1000.e7. |
| 32. |
Sayed AM, Abdel-Wahab NM, Hassan HM, Abdelmohsen UR. Saccharopolyspora: an underexplored source for bioactive natural products. J Appl Microbiol 2020; 128: 314-29.
DOI PMID |
| 33. |
Rooks MG, Garrett WS. Gut microbiota, metabolites and host immunity. Nat Rev Immunol 2016; 16: 341-52.
DOI PMID |
| 34. | Luu M, Weigand K, Wedi F, et al. Regulation of the effector function of CD8(+) T cells by gut microbiota-derived metabolite butyrate. Sci Rep 2018; 8: 14430. |
| 35. | Zhang SL, Mao YQ, Zhang ZY, et al. Pectin supplement significantly enhanced the anti-PD-1 efficacy in tumor-bearing mice humanized with gut microbiota from patients with colorectal cancer. Theranostics 2021; 11: 4155-70. |
| 36. | Nomura M, Nagatomo R, Doi K, et al. Association of short-chain fatty acids in the gut microbiome with clinical response to treatment with nivolumab or pembrolizumab in patients with solid cancer tumors. JAMA Netw Open 2020; 3: e202895. |
| 37. | Naraoka Y, Yamaguchi T, Hu A, Akimoto K, Kobayashi H. Short chain fatty acids upregulate adipokine production in type 2 diabetes-derived human adipocytes. Acta Endocrinol 2018; 14: 287-93. |
| 38. | Lu Y, Yao J, Gong C, et al. Gentiopicroside ameliorates diabetic peripheral neuropathy by modulating PPAR-γ/AMPK/ACC signaling pathway. Cell Physiol Biochem 2018; 50: 585-96. |
| 39. | Li W, Wong CC, Zhang X, et al. CAB39L elicited an anti-Warburg effect via a LKB1-AMPK-PGC1α axis to inhibit gastric tumorigenesis. Oncogene 2018; 37: 6383-98. |
| 40. | Cha JH, Yang WH, Xia W, et al. Metformin promotes antitumor immunity via endoplasmic-reticulum-associated degradation of PD-L1. Mol Cell 2018; 71: 606-20.e7. |
| [1] | WANG Yiying, LIU Jianjun, XIONG Yongjian, ZHANG Yongli, WEN Yuqi, XUE Mengli, GUO Huishu, QIU Juanjuan. Analysis of composition of gut microbial community in a rat model of functional dyspepsia treated with Simo Tang (四磨汤) [J]. Journal of Traditional Chinese Medicine, 2024, 44(6): 1168-1176. |
| [2] | LIAO Mengting, LI Tao, CHU Fuhao, CHEN Yan, LOU Ni, ZHUANG Yuan, BO Rongqiang, DING Xia. Weichang’ an pill (胃肠安丸) alleviates functional dyspepsia through modulating brain-gut peptides and gut microbiota [J]. Journal of Traditional Chinese Medicine, 2024, 44(6): 1177-1186. |
| [3] | ZHANG Guangshun, XU Xiaonan, XU Chuyun, LIAO Guanghui, XU Hao, LOU Zhaohuan, ZHANG Guangji. Actinidia chinensis polysaccharide interferes with the epithelial-mesenchymal transition of gastric cancer by regulating the nuclear transcription factor-κB pathway to inhibit invasion and metastasis [J]. Journal of Traditional Chinese Medicine, 2024, 44(5): 896-905. |
| [4] | SUN Chuanbo, XU Guangpei, JIANG Ping, HUANG Shipping, CHEN Cunwu, HE Yanfei. Protective effect of Zhizi Huangqi Shanzha formula (栀子黄芪山楂方) on aflatoxin poisoning in mice and its effect on intestinal flora [J]. Journal of Traditional Chinese Medicine, 2024, 44(5): 926-933. |
| [5] | JIANG Zhaojian, CAI Hongfei, YUAN Cheng, CAO Lin, XU Wendong, HAN Yaming, ZHANG Qin, LI Jing, WANG Qin, LIU Juyan. Ganoderma Lucidum Spore Oil enhances the effect of cyclophosphamide via inhibiting programmed death-1 and prolongs the survival of H22 tumor-bearing mice [J]. Journal of Traditional Chinese Medicine, 2024, 44(4): 652-659. |
| [6] | GUO Yuxi, LI Ze, CHENG Nan, JIA Xuemei, WANG Jie, MA Hongyu, ZHAO Runyuan, LI Bolin, XUE Yucong, CAI Yanru, YANG Qian. High-throughput sequencing analysis of differential microRNA expression in the process of blocking the progression of chronic atrophic gastritis to gastric cancer by Xianglian Huazhuo formula (香连化浊方) [J]. Journal of Traditional Chinese Medicine, 2024, 44(4): 703-712. |
| [7] | REN Li, HAI Yang, YANG Xue, LUO Xianqin. Yemazhui (Herba Eupatorii Lindleyani) ameliorates lipopolysaccharide-induced acute lung injury via modulation of the toll-like receptor 4/nuclear factor kappa-B/nod-like receptor family pyrin domain-containing 3 protein signaling pathway and intestinal flora in rats [J]. Journal of Traditional Chinese Medicine, 2024, 44(2): 303-314. |
| [8] | LI Chaoran, YANG Yan, FENG Chuwen, LI Heng, QU Yuanyuan, WANG Yulin, WANG Delong, WANG Qingyong, GUO Jing, SHI Tianyu, SUN Xiaowei, WANG Xue, HOU Yunlong, SUN Zhongren, YANG Tiansong. Integrated 'omics analysis for the gut microbiota response to moxibustion in a rat model of chronic fatigue syndrome [J]. Journal of Traditional Chinese Medicine, 2023, 43(6): 1176-1189. |
| [9] | ZHOU Jun, WANG Junhua, LI Xiaobing, WAN Chenyi, LI Fangjun, Lü Yanni, CHEN Hao, SUN Meiying. Efficacy of Heshouwu (Radix Polygoni Multiflori) on gut mircobiota in mice with autoimmune encephalomyelitis [J]. Journal of Traditional Chinese Medicine, 2023, 43(4): 676-685. |
| [10] | JIANG Yiqian, ZHOU Xibin, PU Wenyuan, ZHOU Chunxiang. Sanwu Baisan decoction (三物白散) inhibits colorectal cancer progression in mice by remodeling gut microbiota and tumorigenesis [J]. Journal of Traditional Chinese Medicine, 2023, 43(3): 466-473. |
| [11] | HOU Chao, ZHANG Yusen, YANG Die, LI Yifei, ZHANG Xiaochun, LIU Yanqing. Effects of Traditional Chinese Medicine on the survival of patients with stage I gastric cancer and high-risk factors: a real-world retrospective study [J]. Journal of Traditional Chinese Medicine, 2023, 43(3): 568-573. |
| [12] | YANG Yang, YUAN Haining, JIA Hongxiao, NING Yanzhe, WANG Di, ZHANG Lei, YAN Kaijuan, GUO Yumeng, WANG Fei, SUN Weishuang, CHEN Pei. Therapy of replenishing Yin and regulating Yang for manic episode in bipolar disorder: study protocol for a prospective, double-blind, randomized controlled trial [J]. Journal of Traditional Chinese Medicine, 2023, 43(3): 594-601. |
| [13] | DING Yajie, LIU Feng, LI Zhaoyan, Xu Yan, CAO Nida, ZHANG Guangao, WANG Rui, ZHAO Aiguang. Efficacy of luteolin on the human gastric cancer cell line MKN45 and underlying mechanism [J]. Journal of Traditional Chinese Medicine, 2023, 43(1): 34-41. |
| [14] | XU Qing, WANG Yang, LI Zhongyu, YAN Jiaxing, ZHAO Yingpan, WANG Ping, WEN Yandong. Therapeutic mechanisms of integrated traditional Chinese and conventional medicine underlying its treatment of precancerous lesions of gastric cancer [J]. Journal of Traditional Chinese Medicine, 2022, 42(6): 1023-1028. |
| [15] | SUN Mengzhu, ZHANG Yujie, SONG Yafang, GUO Jing, ZHAO Tingting, WANG Yuhang, PEI Lixia, SUN Jianhua. Electroacupuncture at Tianshu (ST25) and Zusanli (ST36) alleviates stress-induced irritable bowel syndrome in mice by modulating gut microbiota and corticotropin-releasing factor [J]. Journal of Traditional Chinese Medicine, 2022, 42(5): 732-740. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||

