Journal of Traditional Chinese Medicine ›› 2024, Vol. 44 ›› Issue (5): 1058-1066.DOI: 10.19852/j.cnki.jtcm.20240423.001
• Reviews • Previous Articles
Neuro- and immuno-modulation mediated by the cardiac sympathetic nerve: a novel insight into the anti-ischemic efficacy of acupuncture
XI Hanqing, LI Xia, ZHANG Ziyi, CUI Xiang, JING Xianghong, ZHU Bing, GAO Xinyan( )
)
- Institute of Acupuncture and Moxibustion, China Academy of Chinese Medical Sciences, Beijing 100700, China
-
Received:2024-01-12Accepted:2024-04-15Online:2024-10-15Published:2024-04-23 -
Contact:GAO Xinyan, Institute of Acupuncture and Moxibustion, China Academy of Chinese Medical Sciences, Beijing 100700, China. gaoxy@mail.cintcm.ac.cn Telephone: +86-10-64089420 -
Supported by:Research on the Specificity and Systemic Effects of Acupoints on Target Organs(2022YFC3500702);Autonomic Neural Modulation underlying “One Acupoint for Multi-Organ Comorbidity” via Propriospinal Interaction(82330127)
Cite this article
XI Hanqing, LI Xia, ZHANG Ziyi, CUI Xiang, JING Xianghong, ZHU Bing, GAO Xinyan. Neuro- and immuno-modulation mediated by the cardiac sympathetic nerve: a novel insight into the anti-ischemic efficacy of acupuncture[J]. Journal of Traditional Chinese Medicine, 2024, 44(5): 1058-1066.
share this article
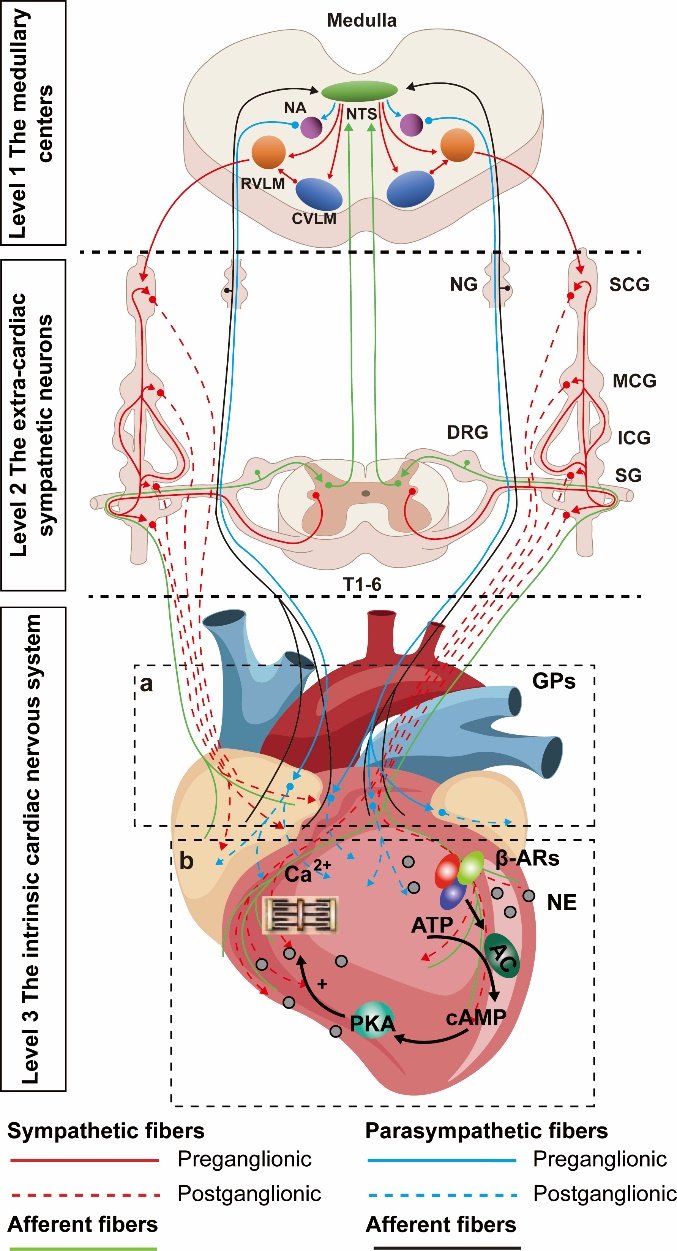
Figure 1 Sympathetic and parasympathetic innervations transmit neuromodulations to the heart a: ganglion plexus; b: sympathetic nerve endings release NE to regulate myocardial activity. NTS: nucleus tractus solitarius; NA: nucleus ambiguous; CVLM: caudal ventrolateral medulla; RVLM: rostral ventrolateral medulla; SG: stellate ganglion; DRG: dorsal root ganglion; ICNS: intrinsic cardiac nervous system; GPs: ganglion plexus; NE: norepinephrine; β-AR: β-adrenergic receptor; ATP: adenosine triphosphate; AC: adenylyl cyclase; Camp: cyclic adenosine monophosphate; PKA: protein kinase A system. Adobe Illustrator CC 2021 (Adobe, San Jose, CA, USA) was used for plotting.
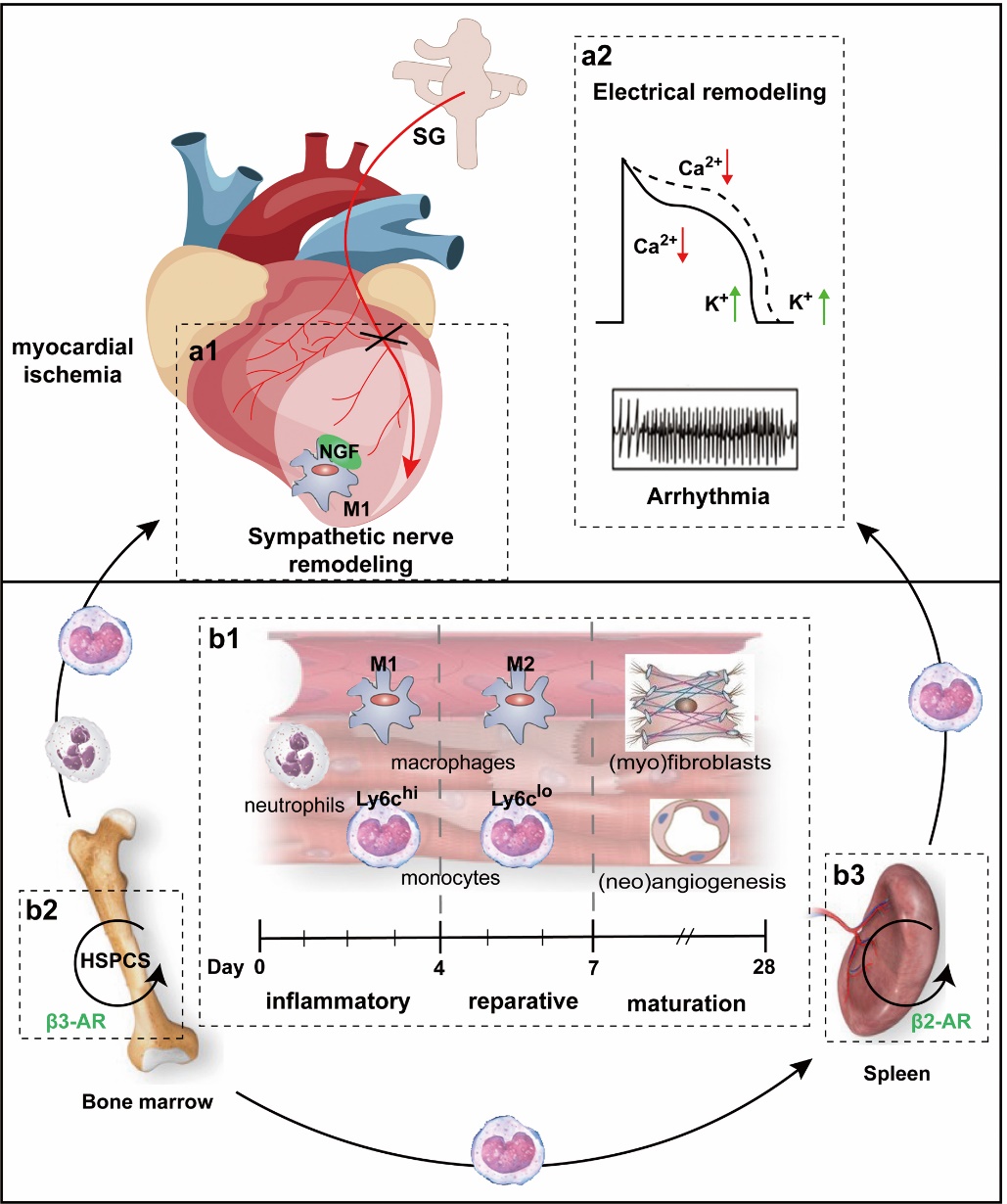
Figure 2 Sympathetic nerve remodeling and immunoinflammatory responses after MI A: sympathetic nerve remodeling after myocardial ischemia. a1: anatomically, sympathetic nerve remodeling is characterized by the sprouting of fiber endings in the surrounding tissues of the ischemic myocardium and denervation of the ischemic myocardium in situ. a2: functionally, sympathetic remodeling, known as electrical remodeling, is accompanied by altered electrophysiologic properties. B: immunoinflammatory response after myocardial ischemia. b1: the three overlapping immunoinflammatory response phases during MI repair and changes at the cellular level. b2: NE activates HSPC mobilization and inhibits its release into circulation by β3-AR in the bone marrow. b3: the high expression of β2-AR on splenic immune cells promotes the entry of monocytes stored in the spleen into the circulation and recruitment to the infarcted myocardium. SG: stellate ganglion; NGF: nerve growth factor; HSPCS: hematopoietic stem cell and progenitor cell. Adobe Illustrator CC 2021 (Adobe, San Jose, CA, USA) was used for plotting.
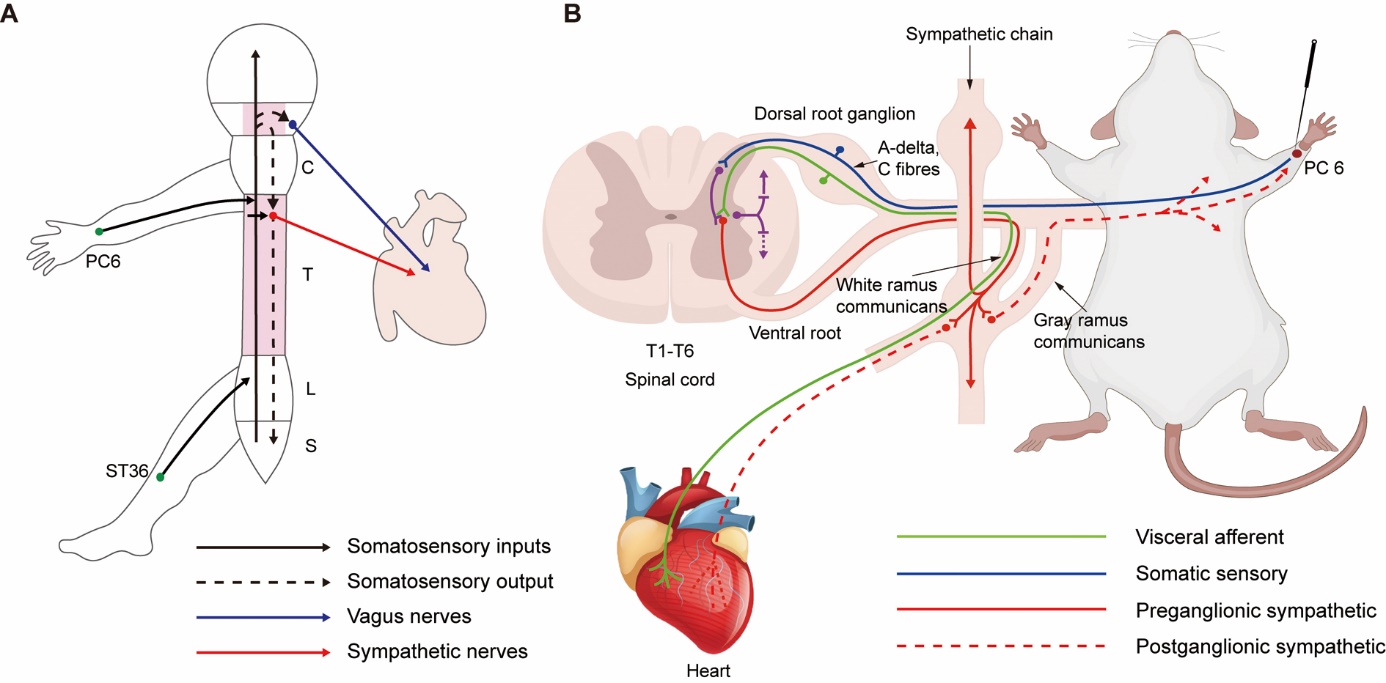
Figure 3 Acupuncture regulates cardiac function and promotes cardiac autor-epair via neuroanatomic pathways mediating somato-cardiac reflexes A: the somatic-autonomic reflex of the heart involves both the spinal and central pathways. After acupuncture at the Neiguan (PC6) acupoint in the same segment as the heart or at the Zusanli (ST36) acupoint in the nonsegmental region, the afferent information (solid black line) can trigger both central (vagus nerves, blue) and spinal (sympathetic nerves, red) reflexes. In animals with intact central nervous systems, spinal reflexes are affected by descending brain regulation (black dashed line). The spinal thoracolumbar segmental reflex is dominated by sympathetic regulation, while central descending regulation results from the sympathetic-parasympathetic balance. B: sympathetic-sensory coupling and the Neiguan (PC6)-cardiac pathway in cardiac pathology. The heart (T1-T6) has the same spinal nerve innervation as the left chest and the medial upper limb, which is due to the convergent and reverse activation of sensory information between the body surface and the viscera, which is also the most common site of cardiogenic referred pain. Stimulation of points such as Neiguan (PC6) in the same segment of the heart can activate the sympathetic nerves through the synaptic connection between the dorsal horn or directly with the sympathetic preganglionic neurons in the lateral horn, resulting in increased heart rate. C: cervical cord; T: thoracic cord; L: lumbar cord; S: sacral cord. Adobe Illustrator CC 2021 (Adobe, San Jose, CA, USA) was used for plotting.
| 1. | Stătescu C, Anghel L, Benchea LC, et al. A systematic review on the risk modulators of myocardial infarction in the "Young"-implications of lipoprotein (a). Int J Mol Sci 2023; 24: 5927. |
| 2. |
Gulati R, Behfar A, Narula J, et al. Acute myocardial infarction in young individuals. Mayo Clin Proc 2020; 95: 136-56.
DOI PMID |
| 3. | Zhou M, Wang H, Zeng X, et al. Mortality, morbidity, and risk factors in China and its provinces, 1990-2017: a systematic analysis for the Global Burden of Disease Study 201. Lancet 2019; 394: 1145-58. |
| 4. |
Garcia R, Marijon E, Karam N, et al. Ventricular fibrillation in acute myocardial infarction: 20-year trends in the FAST-MI study. Eur Heart J 2022; 43: 4887-96.
DOI PMID |
| 5. |
Chatterjee NA, Singh JP. Autonomic modulation and cardiac arrhythmias: old insights and novel strategies. Europace 2021; 23: 1708-21.
DOI PMID |
| 6. |
Chatzidou S, Kontogiannis C, Tsilimigras DI, et al. Propranolol versus metoprolol for treatment of electrical storm in patients with implantable cardioverter-defibrillator. J Am Coll Cardiol 2018; 71: 1897-906.
DOI PMID |
| 7. | Liang C, Zhang C, Gan S, Chen X, Tan Z. Long-term effect of β-blocker use on clinical outcomes in postmyocardial infarction patients: a systematic review and Meta-analysis. Front Cardiovasc Med 2022; 9: 779462. |
| 8. |
Adamo L, Rocha-Resende C, Prabhu SD, Mann DL. Reappraising the role of inflammation in heart failure. Nat Rev Cardiol 2020; 17: 269-85.
DOI PMID |
| 9. |
Carnevale D. Neuroimmune axis of cardiovascular control: mechanisms and therapeutic implications. Nat Rev Cardiol 2022; 19: 379-94.
DOI PMID |
| 10. | Yang L, Chen Y, Lou F, Zhao X, Zhou J. Efficacy of dialectical comprehensive treatment of Traditional Chinese Medicine in patients with chronic stable heart failure: a randomized controlled trial. Cardiol Res Pract 2022; 2022: 5408063. |
| 11. |
Wang K, Zhou J, Cui S, et al. Electroacupuncture ameliorates cardiac dysfunction in myocardial ischemia model rats: a potential role of the hypothalamic-pituitary-adrenal axis. J Tradit Chin Med 2023; 43: 944-54.
DOI |
| 12. | Xin JJ, Dai QF, Lu FY, et al. Antihypertensive and antifibrosis effects of acupuncture at PC 6 acupoints in spontaneously hypertensive rats and the underlying mechanisms. Front Physiol 2020; 11: 734. |
| 13. | Ye Y, Birnbaum Y, Widen SG, et al. Acupuncture reduces hypertrophy and cardiac fibrosis, and improves heart function in mice with diabetic cardiomyopathy. Cardiovasc Drugs Ther 2020; 34: 835-48. |
| 14. | Hong H, Cao X, Deng T, et al. Acupuncture at Neiguan suppresses PVCs occurring post-myocardial infarction by alleviating inflammation and fibrosis. Chin Med 2022; 17: 52. |
| 15. | Manolis AA, Manolis TA, Apostolopoulos EJ, Apostolaki NE, Melita H, Manolis AS. The role of the autonomic nervous system in cardiac arrhythmias: the neuro-cardiac axis, more foe than friend? Trends Cardiovasc Med 2021; 31: 290-302. |
| 16. |
Fukuda K, Kanazawa H, Aizawa Y, Ardell JL, Shivkumar K. Cardiac innervation and sudden cardiac death. Circ Res 2015; 116: 2005-19.
DOI PMID |
| 17. |
Holstein GR, Friedrich VL Jr, Martinelli GP. Projection neurons of the vestibulo-sympathetic reflex pathway. J Comp Neurol 2014; 522: 2053-74.
DOI PMID |
| 18. | Moreira TS, Sato MA, Takakura AC, Menani JV, Colombari E. Role of pressor mechanisms from the NTS and CVLM in control of arterial pressure. Am J Physiol Regul Integr Comp Physiol 2005; 289: R1416-25. |
| 19. |
Marina N, Tang F, Figueiredo M, et al. Purinergic signalling in the rostral ventro-lateral medulla controls sympathetic drive and contributes to the progression of heart failure following myocardial infarction in rats. Basic Res Cardiol 2013; 108: 317.
DOI PMID |
| 20. | Koganezawa T, Shimomura Y, Terui N. The role of the RVLM neurons in the viscero-sympathetic reflex: a mini review. Auton Neurosci 2008; 142: 17-9. |
| 21. | Coote JH, Chauhan RA. The sympathetic innervation of the heart: important new insights. Auton Neurosci 2016; 199: 17-23. |
| 22. | Winter J, Tanko AS, Brack KE, Coote JH, Ng GA. Differential cardiac responses to unilateral sympathetic nerve stimulation in the isolated innervated rabbit heart. Auton Neurosci 2012; 166: 4-14. |
| 23. |
Scridon A, Şerban RC, Chevalier P. Atrial fibrillation: neurogenic or myogenic? Arch Cardiovasc Dis 2018; 111: 59-69.
DOI PMID |
| 24. | Beaumont E, Salavatian S, Southerland EM, et al. Network interactions within the canine intrinsic cardiac nervous system: implications for reflex control of regional cardiac function. J Physiol 2013; 591: 4515-33. |
| 25. | Singh K, Xiao L, Remondino A, Sawyer DB, Colucci WS. Adrenergic regulation of cardiac myocyte apoptosis. J Cell Physiol 2001; 189: 257-65. |
| 26. | Brodde OE. Beta-adrenoceptors in cardiac disease. Pharmacol Ther 1993; 60: 405-30. |
| 27. |
Bers DM. Calcium cycling and signaling in cardiac myocytes. Annu Rev Physiol 2008; 70: 23-49.
PMID |
| 28. |
Landstrom AP, Dobrev D, Wehrens XHT. Calcium signaling and cardiac arrhythmias. Circ Res 2017; 120: 1969-93.
DOI PMID |
| 29. | Colucci WS, Wright RF, Braunwald E. New positive inotropic agents in the treatment of congestive heart failure. Mechanisms of action and recent clinical developments. 2. N Engl J Med 1986; 314: 349-58. |
| 30. | Zekios KC, Mouchtouri ET, Lekkas P, Nikas DN, Kolettis TM. Sympathetic activation and arrhythmogenesis after myocardial infarction: where do we stand? J Cardiovasc Dev Dis 2021; 8: 57. |
| 31. |
Kimura K, Ieda M, Kanazawa H, et al. Cardiac sympathetic rejuvenation: a link between nerve function and cardiac hypertrophy. Circ Res 2007; 100: 1755-64.
DOI PMID |
| 32. |
Snider WD. Functions of the neurotrophins during nervous system development: what the knockouts are teaching us. Cell 1994; 77: 627-38.
PMID |
| 33. |
Kaye DM, Vaddadi G, Gruskin SL, Du XJ, Esler MD. Reduced myocardial nerve growth factor expression in human and experimental heart failure. Circ Res 2000; 86: E80-4.
DOI PMID |
| 34. |
Zhou S, Chen LS, Miyauchi Y, et al. Mechanisms of cardiac nerve sprouting after myocardial infarction in dogs. Circ Res 2004; 95: 76-83.
DOI PMID |
| 35. | Kimura K, Kanazawa H, Ieda M, et al. Norepinephrine-induced nerve growth factor depletion causes cardiac sympathetic denervation in severe heart failure. Auton Neurosci 2010; 156: 27-35. |
| 36. |
Inoue H, Zipes DP. Results of sympathetic denervation in the canine heart: supersensitivity that may be arrhythmogenic. Circulation 1987; 75: 877-87.
PMID |
| 37. |
Pinto JM, Boyden PA. Electrical remodeling in ischemia and infarction. Cardiovasc Res 1999; 42: 284-97.
PMID |
| 38. |
Hammond HK, Roth DA, Ford CE, Stamnas GW, Ziegler MG, Ennis C. Myocardial adrenergic denervation supersensitivity depends on a postreceptor mechanism not linked with increased cAMP production. Circulation 1992; 85: 666-79.
PMID |
| 39. |
Kammerling JJ, Green FJ, Watanabe AM, et al. Denervation supersensitivity of refractoriness in noninfarcted areas apical to transmural myocardial infarction. Circulation 1987; 76: 383-93.
PMID |
| 40. |
Tribulova N, Szeiffova Bacova B, Benova T, Viczenczova C. Can we protect from malignant arrhythmias by modulation of cardiac cell-to-cell coupling? J Electrocardiol 2015; 48: 434-40.
DOI PMID |
| 41. | Sanchez-Alonso JL, Loucks A, Schobesberger S, et al. Nanoscale regulation of L-type calcium channels differentiates between ischemic and dilated cardiomyopathies. EBioMedicine 2020; 57: 102845. |
| 42. | Pluijmert NJ, Atsma DE, Quax PHA. Post-ischemic myocardial inflammatory response: A complex and dynamic process susceptible to immunomodulatory therapies. Front Cardiovasc Med 2021; 8: 647785. |
| 43. | Nahrendorf M, Swirski FK, Aikawa E, et al. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med 2007; 204: 3037-47. |
| 44. |
Sager HB, Hulsmans M, Lavine KJ, et al. Proliferation and recruitment contribute to myocardial macrophage expansion in chronic heart failure. Circ Res 2016; 119: 853-64.
DOI PMID |
| 45. |
Lee WW, Marinelli B, van der Laan AM, et al. PET/MRI of inflammation in myocardial infarction. J Am Coll Cardiol 2012; 59: 153-63.
DOI PMID |
| 46. |
Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of monocytes, macrophages, and dendritic cells. Science 2010; 327: 656-61.
DOI PMID |
| 47. | Robbins CS, Chudnovskiy A, Rauch PJ, et al. Extramedullary hematopoiesis generates Ly-6C (high) monocytes that infiltrate atherosclerotic lesions. Circulation 2012; 125: 364-74. |
| 48. |
Nahrendorf M, Swirski FK. Monocyte and macrophage heterogeneity in the heart. Circ Res 2013; 112: 1624-33.
DOI PMID |
| 49. |
Peet C, Ivetic A, Bromage DI, Shah AM. Cardiac monocytes and macrophages after myocardial infarction. Cardiovasc Res 2020; 116: 1101-12.
DOI PMID |
| 50. | Sager HB, Dutta P, Dahlman JE, et al. RNAi targeting multiple cell adhesion molecules reduces immune cell recruitment and vascular inflammation after myocardial infarction. Sci Transl Med 2016; 8: 342ra80. |
| 51. | Sreejit G, Nooti SK, Jaggers RM, et al. Retention of the NLRP3 inflammasome-primed neutrophils in the bone marrow is essential for myocardial infarction-induced granulopoiesis. Circulation 2022; 145: 31-44. |
| 52. | Leuschner F, Rauch PJ, Ueno T, et al. Rapid monocyte kinetics in acute myocardial infarction are sustained by extramedullary monocytopoiesis. J Exp Med 2012; 209: 123-37. |
| 53. |
Swirski FK, Nahrendorf M, Etzrodt M, et al. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science 2009; 325: 612-6.
DOI PMID |
| 54. | Dutta P, Courties G, Wei Y, et al. Myocardial infarction accelerates atherosclerosis. Nature 2012; 487: 325-9. |
| 55. |
Spiegel A, Shivtiel S, Kalinkovich A, et al. Catecholaminergic neurotransmitters regulate migration and repopulation of immature human CD34+ cells through Wnt signaling. Nat Immunol 2007; 8: 1123-31.
DOI PMID |
| 56. |
Zhao Y, Liu M, Chan XY, et al. Uncovering the mystery of opposite circadian rhythms between mouse and human leukocytes in humanized mice. Blood 2017; 130: 1995-2005.
DOI PMID |
| 57. |
Katayama Y, Battista M, Kao WM, et al. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell 2006; 124: 407-21.
DOI PMID |
| 58. | Méndez-Ferrer S, Lucas D, Battista M, Frenette PS. Haematopoietic stem cell release is regulated by circadian oscillations. Nature 2008; 452: 442-7. |
| 59. |
Heusch G. The spleen in myocardial infarction. Circ Res 2019; 124: 26-8.
DOI PMID |
| 60. |
Grisanti LA, Gumpert AM, Traynham CJ, et al. Leukocyte-expressed β2-adrenergic receptors are essential for survival after acute myocardial injury. Circulation 2016; 134: 153-67.
DOI PMID |
| 61. |
Wernli G, Hasan W, Bhattacherjee A, van Rooijen N, Smith PG. Macrophage depletion suppresses sympathetic hyperinnervation following myocardial infarction. Basic Res Cardiol 2009; 104: 681-93.
DOI PMID |
| 62. |
Hasan W, Jama A, Donohue T, et al. Sympathetic hyperinnervation and inflammatory cell NGF synthesis following myocardial infarction in rats. Brain Res 2006; 1124: 142-54.
DOI PMID |
| 63. |
Lyu J, Wang M, Kang X, et al. Macrophage-mediated regulation of catecholamines in sympathetic neural remodeling after myocardial infarction. Basic Res Cardiol 2020; 115: 56.
DOI PMID |
| 64. | Yin J, Wang Y, Hu H, et al. P2X7 receptor inhibition attenuated sympathetic nerve sprouting after myocardial infarction via the NLRP3/IL-1β pathway. J Cell Mol Med 2017 21: 2695-710. |
| 65. | Yin J, Hu H, Li X, et al. Inhibition of Notch signaling pathway attenuates sympathetic hyperinnervation together with the augmentation of M2 macrophages in rats post-myocardial infarction. Am J Physiol Cell Physiol 2016; 310: C41-53. |
| 66. |
Ziegler KA, Ahles A, Wille T, Kerler J, Ramanujam D, Engelhardt S. Local sympathetic denervation attenuates myocardial inflammation and improves cardiac function after myocardial infarction in mice. Cardiovasc Res 2018; 114: 291-9.
DOI PMID |
| 67. |
Sun X, Wei Z, Li Y, et al. Renal denervation restrains the inflammatory response in myocardial ischemia-reperfusion injury. Basic Res Cardiol 2020; 115: 15.
DOI PMID |
| 68. | Chen PS, Chen LS, Fishbein MC, Lin SF, Nattel S. Role of the autonomic nervous system in atrial fibrillation: pathophysiology and therapy. Circ Res 2014; 114: 1500-15. |
| 69. |
Chen J, Ren YL, Tang Y, Li ZJ, Liang FR. Acupuncture therapy for angina pectoris: a systematic review. J Tradit Chin Med 2012; 32: 494-501.
PMID |
| 70. |
Wei XT, Li LY, Zhang YT, et al. Electroacupuncture preconditioning alleviates myocardial ischemia-reperfusion injury through the hypothalamic paraventricular nucleus- interposed nucleus nerve pathway. J Tradit Chin Med 2022; 42: 379-88.
DOI |
| 71. |
Wang K, Zhou J, Cui S, et al. Electroacupuncture ameliorates cardiac dysfunction in myocardial ischemia model rats: a potential role of the hypothalamic-pituitary-adrenal axis. J Tradit Chin Med 2023; 43: 944-54.
DOI |
| 72. | Gao X, Zhao Y, Su Y, et al. β1/ 2 or M2/3 receptors are required for different gastrointestinal motility responses induced by acupuncture at heterotopic or homotopic acupoints. PLoS One 2016; 11: e0168200. |
| 73. | Su Y, Huang J, Sun S, et al. Restoring the autonomic balance in an atrial fibrillation rat model by electroacupuncture at the Neiguan point. Neuromodulation 2022; S1094-7159: 01366-6. |
| 74. | Ren BB, Yu Zh, Wang YL, et al. Regulatory effect of electroacupuncture on heart and stomach of rats. Zhong Guo Zhong Xi Yi Jie He Za Zhi 2014; 34: 1212-5. |
| 75. | Li YQ, Zhu B, Rong PJ, et al. Neural mechanism of acupuncture-modulated gastric motility. World J Gastroenterol 2007; 13: 709-16. |
| 76. | Lee DY, Jiu YR, Hsieh CL. Metabolism modulation in rat tissues in response to point specificity of electroacupuncture. Sci Rep 2022; 12: 210. |
| 77. |
Kimura A, Sato A, Sato Y, Suzuki H. A- and C-reflexes elicited in cardiac sympathetic nerves by single shock to a somatic afferent nerve include spinal and supraspinal components in anesthetized rats. Neurosci Res 1996; 25: 91-6.
PMID |
| 78. | Li YW, Li W, Wang ST, et al. The autonomic nervous system: a potential link to the efficacy of acupuncture. Front Neurosci 2022; 16: 1038945. |
| 79. | Sato A. Neural mechanisms of autonomic responses elicited by somatic sensory stimulation. Neurosci Behav Physiol 1997; 27: 610-21. |
| 80. |
Sato A, Sato Y, Schmidt RF. Heart rate changes reflecting modifications of efferent cardiac sympathetic outflow by cutaneous and muscle afferent volleys. J Auton Nerv Syst 1981; 4: 231-47.
PMID |
| 81. | Li P, Tjen-A-Looi SC, Guo ZL, Fu LW, Longhurst JC. Long-loop pathways in cardiovascular electroacupuncture responses. J Appl Physiol (1985) 2009; 106: 620-30. |
| 82. | Tjen-A-Looi SC, Guo ZL, Li M, Longhurst JC. Medullary GABAergic mechanisms contribute to electroacupuncture modulation of cardiovascular depressor responses during gastric distention in rats. Am J Physiol Regul Integr Comp Physiol 2013; 304: R321-32. |
| 83. |
Liu J, Li SN, Liu L, et al. Conventional acupuncture for cardiac arrhythmia: a systematic review of randomized controlled trials. Chin J Integr Med 2018; 24: 218-26.
DOI PMID |
| 84. | Lu SF, Wang JM, Yuan J, et al. Electroacupuncture improves cardiac function and reduces infarct size by modulating cardiac autonomic remodeling in a mouse model of myocardial ischemia. Acupunct Med 2021; 39: 681-90. |
| 85. | Zhao TT, Liu JJ, Zhu J, et al. SDF-1/CXCR4-mediated stem cell mobilization involved in cardioprotective effects of electroacupuncture on mouse with myocardial infarction. Oxid Med Cell Longev 2022; 2022: 4455183. |
| 86. | Qi W, Li X, Ren Y, et al. Downregulation of lncRNA Miat contributes to the protective effect of electroacupuncture against myocardial fibrosis. Chin Med 2022; 17: 57. |
| 87. | Xin JJ, Gao JH, Liu Q, Zhao YX, Zhou C, Yu XC. Involvement of interleukin-1 β/insulin-like growth factor 1 in ameliorating effects of electroacupuncture on myocardial fibrosis induced by essential hypertension. Chin J Integr Med 2023; 29: 162-9. |
| 88. | Gao J, Zhang L, Wang Y, et al. Antiarrhythmic effect of acupuncture pretreatment in rats subjected to simulative global ischemia and reperfusion-involvement of adenylate cyclase, protein kinase A, and L-type Ca2+ channel. J Physiol Sci 2008; 58: 389-96. |
| 89. |
Hong H, Cao X, Meng XM, et al. Comparative study of different acupoints for treating acute myocardial ischemia in mice. J Cardiovasc Transl Res 2023; 16: 644-61.
DOI PMID |
| 90. |
Cui X, Sun G, Cao H, et al. Referred somatic hyperalgesia mediates cardiac regulation by the activation of sympathetic nerves in a rat model of myocardial ischemia. Neurosci Bull 2022; 38: 386-402.
DOI PMID |
| 91. | Shi JJ, Yang JL, Ma NQ, et al. Electroacupuncture influences macrophage M2 polarization and TLR4 and MyD88 expression in myocardial tissue of myocardial ischemia injury mice. Nanjing Zhong Yi Yao Da Xue Xue Bao 2023; 39: 319-27. |
| 92. | Li N, Guo Y, Gong Y, et al. The anti-inflammatory actions and mechanisms of acupuncture from acupoint to target organs via neuro-immune regulation. J Inflamm Res 2021; 14: 7191-224. |
| 93. |
Spengler RN, Chensue SW, Giacherio DA, Blenk N, Kunkel SL. Endogenous norepinephrine regulates tumor necrosis factor-alpha production from macrophages in vitro. J Immunol 1994; 152: 3024-31.
PMID |
| 94. | Huang JL, Zhang YL, Wang CC, et al. Enhanced phosphorylation of MAPKs by NE promotes TNF-α production by macrophage through α adrenergic receptor. Inflammation 2012; 35: 527-34. |
| 95. |
Yu J, Xiao K, Chen X, et al. Neuron-derived neuropeptide Y fine-tunes the splenic immune responses. Neuron 2023; 111: 1346-47.
DOI PMID |
| 96. |
Liu S, Wang ZF, Su YS, et al. Somatotopic organization and intensity dependence in driving distinct NPY-expressing sympathetic pathways by electroacupuncture. Neuron 2020; 108: 436-50.e7.
DOI PMID |
| 97. | Liu S, Wang Z, Su Y, et al. A neuroanatomical basis for electroacupuncture to drive the vagal-adrenal axis. Nature 2021; 598: 641-5. |
| 98. | Ye Y, Birnbaum Y, Widen SG, et al. Acupuncture reduces hypertrophy and cardiac fibrosis, and improves heart function in mice with diabetic cardiomyopathy. Cardiovasc Drugs Ther 2020; 34: 835-48. |
| 99. | Li X, Wang L, Ying X, et al. Electroacupuncture pre-treatment alleviates sepsis-induced cardiac inflammation and dysfunction by inhibiting the calpain-2/STAT3 pathway. Front Physiol 2022; 13: 961909. |
| 100. | Zhang T, Yang WX, Wang YL, et al. Electroacupuncture preconditioning attenuates acute myocardial ischemia injury through inhibiting NLRP 3 inflammasome activation in mice. Life Sci 2020; 248: 117451. |
| 101. | Ma Q. Somatotopic organization of autonomic reflexes by acupuncture. Curr Opin Neurobiol 2022; 76: 102602. |
| 102. | Yang WX, Chen LY, Yang JL, et al. Effect of different electrical current intensities of electroacupuncture preconditioning on cardiac function and macrophage polarization in mice with acute myocardial ischemia. Zhen Ci Yan Jiu 2022; 47: 955-61. |
| [1] | WANG Bingyu, JIN Fangfang, GAO Jiawei, YANG Liuxin, ZHANG Yali, YUAN Xingxing, ZHANG Yang. Acupuncture reduces sedative and anaesthetic consumption and improves pain tolerance in patients undergoing colonoscopy: a Meta-analysis [J]. Journal of Traditional Chinese Medicine, 2024, 44(6): 1091-1103. |
| [2] | ZHANG Boyang, ZHOU Yang, FENG Liyuan, SUI Dan, HE Lei, TONG Dan, WANG Ruoyu, SUI Xue, SONG Jing, WANG Dongyan. A neural regulation mechanism of head electroacupuncture on brain network of patients with stroke related sleep disorders [J]. Journal of Traditional Chinese Medicine, 2024, 44(6): 1268-1276. |
| [3] | DONG Yingying, GUO Qin, GAO Yuan, WANG Huanhuan, BAI Dong. Revealing the scientific connotation of compatibility of Chinese medicine medica based on self-assembly technology [J]. Journal of Traditional Chinese Medicine, 2024, 44(6): 1288-1295. |
| [4] | XU Yingshan, WU Chunxiao, YU Wei, GUO Hongji, LU Liming, XU Nenggui, TANG Chunzhi. Systematic review and Meta-analysis of brain plasticity associated with electroacupuncture in experimental ischemic stroke [J]. Journal of Traditional Chinese Medicine, 2024, 44(5): 859-870. |
| [5] | DENG Yasheng, HAN Siyin, XI Lanhua, HUANG Hui, LIANG Tianwei, ZHENG Yiqing, FAN Yanping, LIN Jiang. Traditional Chinese Medicine in the treatment of recurrent respiratory tract infections in children: an overview of systematic reviews and Meta-analyses [J]. Journal of Traditional Chinese Medicine, 2024, 44(5): 871-884. |
| [6] | ZHENG Peng, MENG Ying, LIU Meijun, YU Di, LIU Huiying, WANG Fuchun, XU Xiaohong. Electroacupuncture inhibits hippocampal oxidative stress and autophagy in sleep-deprived rats through the protein kinase B and mechanistic target of rapamycin signaling pathway [J]. Journal of Traditional Chinese Medicine, 2024, 44(5): 974-980. |
| [7] | ZHANG Fang, YAN Cuina, WENG Zhijun, WU Luyi, QI Li, ZHAO Min, XIN Yuhu, WU Huangan, LIU Huirong. Regulatory role of electroacupuncture on satellite glial cell activity in the colon and dorsal root ganglion of rats with irritable bowel syndrome [J]. Journal of Traditional Chinese Medicine, 2024, 44(5): 981-990. |
| [8] | CHEN Dandan, JIN Qianhong, SHEN Yuanjuan, WANG Qing, DAI Zhengxiang. Scraping therapy for knee osteoarthritis: a systematic review and Meta-analysis [J]. Journal of Traditional Chinese Medicine, 2024, 44(4): 633-641. |
| [9] | LI Zhongzheng, ZHAO Yadan, Ma Weigang, Zhang Yonglong, XU Zhifang, XI Qiang, LI Yanqi, QIN Siru, ZHANG Zichen, WANG Songtao, ZHAO Xue, LIU Yangyang, GUO Yi, GUO Yongming. Adenosine triphosphate mediates the pain tolerance effect of manual acupuncture at Zusanli (ST36) in mice [J]. Journal of Traditional Chinese Medicine, 2024, 44(4): 660-669. |
| [10] | YAN Jing, FENG Huimin, QIU Fang, WANG Haijun, YIN Luyun, JIN Xiaofei, ZHAO Jiyu, WANG Hongyang, YAN Xiaoqin. Effect on serum metabolomics of rats with premature ovarian insufficiency by Zhibian (BL54) through Shuidao (ST28) acupuncture [J]. Journal of Traditional Chinese Medicine, 2024, 44(4): 722-733. |
| [11] | CHEN Yonglin, OUYANG Ling, MENG Lingling, WU Bufan, PENG Rou, LIU Sitong, HOU Dan, WANG Yaling, JING Xinyue, LU Shengfeng, FU Shuping. Electroacupuncture ameliorates blood-brain barrier disruption after ischemic stroke through histone acetylation regulation at the matrix metalloproteinase 9 and tissue inhibitor of metalloproteinase 2 genes [J]. Journal of Traditional Chinese Medicine, 2024, 44(4): 734-744. |
| [12] | SUN Junjian, XIE Henghui, LI Huanhuan, TIAN Xiangming, FANG Yigong, ZHOU Wenhui. Acupuncture improves the live birth of patients with repeated implantation failure: a retrospective cohort study [J]. Journal of Traditional Chinese Medicine, 2024, 44(4): 830-838. |
| [13] | ZHU Wenting, GUO Changqing, DU Mei, MA Yunxuan, CUI Yongqi, CHEN Xilin, GUO Changqing. Acupotomy alleviates knee osteoarthritis in rabbit by regulating chondrocyte mitophagy via Pink1-Parkin pathway [J]. Journal of Traditional Chinese Medicine, 2024, 44(3): 468-477. |
| [14] | YU Wenxi, TANG Lina, LI Hongtao, WANG Yonggang, SHEN Zan. Neiguan (PC6) acupoint stimulation for preventing chemotherapy-induced nausea and vomiting: a cost-effective supplement in guideline-inconsistent chemotherapy-induced nausea and vomiting prophylaxis subgroup [J]. Journal of Traditional Chinese Medicine, 2024, 44(3): 581-585. |
| [15] | Shikha Agrawal, Adarsh Kumar, Ankit Kumar Singh, Harshwardhan Singh, Suresh Thareja, Pradeep Kumar. A comprehensive review on pharmacognosy, phytochemistry and pharmacological activities of 8 potent Prunus species of southeast Asia [J]. Journal of Traditional Chinese Medicine, 2024, 44(3): 620-628. |
| Viewed | ||||||||||||||||||||||||||||||||||||||||||||||||||
|
Full text 116
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||
|
Abstract 73
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||

