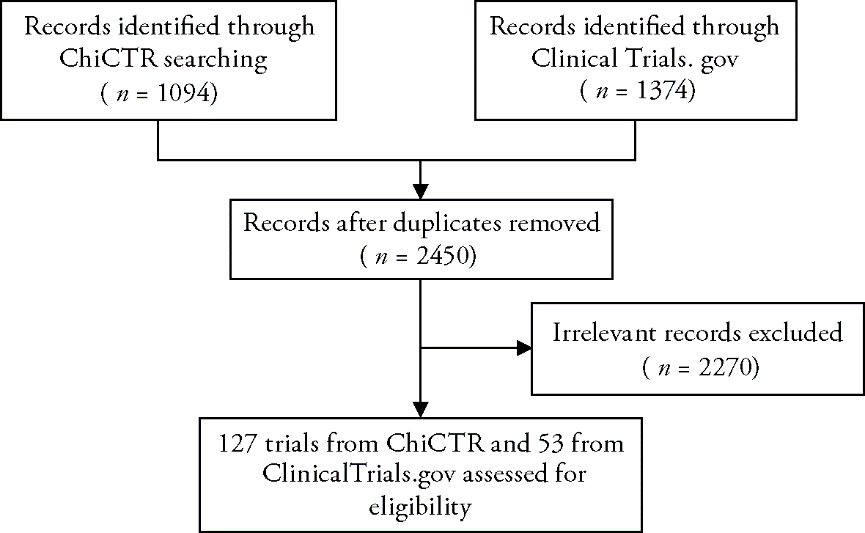
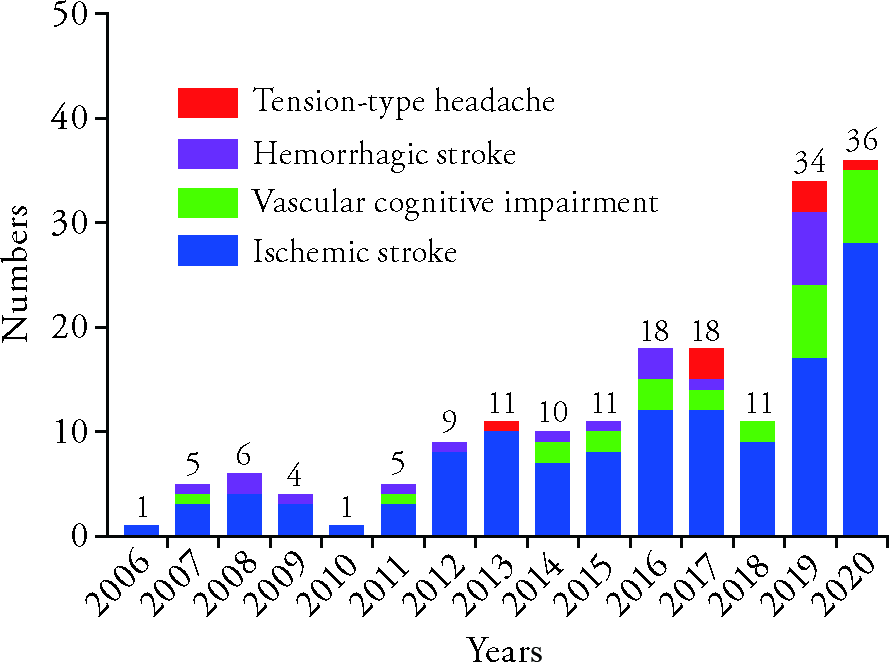
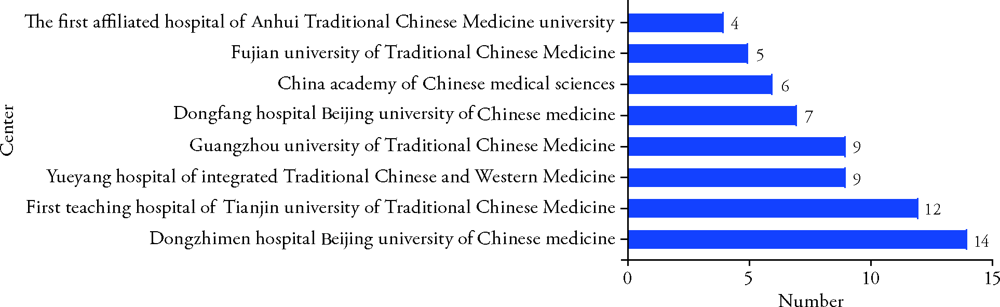
Journal of Traditional Chinese Medicine ›› 2022, Vol. 42 ›› Issue (1): 148-153.DOI: 10.19852/j.cnki.jtcm.2022.01.010
• Review • Previous Articles
Registration of intervention trials of Traditional Chinese Medicine for four neurological diseases on Chinese Clinical Trial Registry and ClinicalTrials.gov: a narrative review
Jingjing WEI1,2,3, Rongjuan GUO4, Guojing FU3, Xiao LIANG3, Zhenmin XU1, Min JIA3, Zixiu ZENG1, Wanqing DU1, Weiwei JIAO3, Linjuan SUN3, Hongmei LIU3, Chunli GUO3, Chenguang TONG3, Yunling ZHANG3( ), Xing LIAO2(
), Xing LIAO2( )
)
- 1 Graduate school, Beijing University of Chinese Medicine, Beijing 10029, China
2 Center for Evidence Based Chinese Medicine, Institute of Basic Research in Clinical Medicine, China Academy of Chinese Medical Sciences, Beijing 100700, China
3 Department of Neurology, Xiyuan Hospital, China Academy of Chinese Medical Sciences, Beijing 100091, China
4 Department of Neurology, Dongfang Hospital, Beijing University of Chinese Medicine, Beijing 100078, China
-
Received:2021-02-12Accepted:2021-05-13Online:2022-02-15Published:2022-01-25 -
Contact:Yunling ZHANG,Xing LIAO -
About author:Prof. LIAO Xing, Center for Evidence Based Chinese Medicine, Institute of Basic Research in Clinical Medicine, China Academy of Chinese Medical Sciences, Beijing 100700, China. Email: okfrom2008@hotmail.com.
Prof. ZHANG Yunling, Xiyuan Hospital, China Academy of Chinese Medical Sciences, Beijing 100091, China. yunlingzhang2004@126.com;
-
Supported by:NATCM TCM Inheritance and Innovation Hundred-Thousand-Ten Thousand “Talents Project QiHuang Scholar) – National TCM Leading Personnel Support Program (NATCM Personnel and Education Department [2018] No. 12)”;Research on optimizing construction of evidence-based ability of traditional Chinese medicine encephalopathy, Special fund for basic scientific research business expenses of central public welfare scientific research institutes(ZZ13-024-3)
Cite this article
Jingjing WEI, Rongjuan GUO, Guojing FU, Xiao LIANG, Zhenmin XU, Min JIA, Zixiu ZENG, Wanqing DU, Weiwei JIAO, Linjuan SUN, Hongmei LIU, Chunli GUO, Chenguang TONG, Yunling ZHANG, Xing LIAO. Registration of intervention trials of Traditional Chinese Medicine for four neurological diseases on Chinese Clinical Trial Registry and ClinicalTrials.gov: a narrative review[J]. Journal of Traditional Chinese Medicine, 2022, 42(1): 148-153.
share this article
| Item | Detail | Record [n (%)] |
|---|---|---|
| Primary sponsor | Hospital | 131 (72.8) |
| University | 37 (20.6) | |
| Research institute | 6 (3.3) | |
| Government | 3 (1.6) | |
| Individual | 1 (0.6) | |
| Industry | 2 (1.1) | |
| Assignment | Single arm | 4 (2.2) |
| Parallel | 163 (90.6) | |
| Crossover | 3 (1.7) | |
| Othersa | 6 (3.3) | |
| Not reported | 4 (2.2) | |
| Method of allocation | randomized | 172 (95.6) |
| Non-randomized | 4 (2.2) | |
| Not applicable | 4 (2.2) | |
| Masking | Single blind | 21 (11.7) |
| Double blind | 49 (27.2) | |
| Triple blind | 26 (14.4) | |
| Open label | 14 (7.8) | |
| Not reported | 70 (38.9) | |
| Trial participating center | Multi-center | 73 (40.5) |
| Single center | 102 (56.7) | |
| Not reported | 5 (2.8) | |
| Sample size | ≤ 200 | 103 (57.2) |
| 201-400 | 41 (22.8) | |
| 401-1000 | 24 (13.3) | |
| 1000+ | 12 (6.7) | |
| Recruitment status | Completed | 46 (25.6) |
| Recruiting | 49 (27.2) | |
| Not yet recruiting | 76 (42.2) | |
| Unknown | 9 (5.0) | |
| Ethical approval | Yes | 112 (62.2) |
| Unclear | 68 (37.8) | |
| Study phase | 0 | 23 (12.8) |
| Ⅰ | 7 (3.9) | |
| Ⅱ | 12 (6.7) | |
| Ⅲ | 4 (2.2) | |
| Ⅳ | 50 (27.7) | |
| New treatment measure clinical study | 12 (6.7) | |
| Not applicable | 72 (40.0) | |
| Publication | Yes | 38 (21.1) |
| No | 142 (78.9) | |
| Intervention | acupuncture | 68 (37.8) |
| Chinese herbal medicinesb | 99 (55.0) | |
| Othersc | 9 (5.0) | |
| Combinationd | 4 (2.2) |
Table 1 Characteristics of the registered clinical trials
| Item | Detail | Record [n (%)] |
|---|---|---|
| Primary sponsor | Hospital | 131 (72.8) |
| University | 37 (20.6) | |
| Research institute | 6 (3.3) | |
| Government | 3 (1.6) | |
| Individual | 1 (0.6) | |
| Industry | 2 (1.1) | |
| Assignment | Single arm | 4 (2.2) |
| Parallel | 163 (90.6) | |
| Crossover | 3 (1.7) | |
| Othersa | 6 (3.3) | |
| Not reported | 4 (2.2) | |
| Method of allocation | randomized | 172 (95.6) |
| Non-randomized | 4 (2.2) | |
| Not applicable | 4 (2.2) | |
| Masking | Single blind | 21 (11.7) |
| Double blind | 49 (27.2) | |
| Triple blind | 26 (14.4) | |
| Open label | 14 (7.8) | |
| Not reported | 70 (38.9) | |
| Trial participating center | Multi-center | 73 (40.5) |
| Single center | 102 (56.7) | |
| Not reported | 5 (2.8) | |
| Sample size | ≤ 200 | 103 (57.2) |
| 201-400 | 41 (22.8) | |
| 401-1000 | 24 (13.3) | |
| 1000+ | 12 (6.7) | |
| Recruitment status | Completed | 46 (25.6) |
| Recruiting | 49 (27.2) | |
| Not yet recruiting | 76 (42.2) | |
| Unknown | 9 (5.0) | |
| Ethical approval | Yes | 112 (62.2) |
| Unclear | 68 (37.8) | |
| Study phase | 0 | 23 (12.8) |
| Ⅰ | 7 (3.9) | |
| Ⅱ | 12 (6.7) | |
| Ⅲ | 4 (2.2) | |
| Ⅳ | 50 (27.7) | |
| New treatment measure clinical study | 12 (6.7) | |
| Not applicable | 72 (40.0) | |
| Publication | Yes | 38 (21.1) |
| No | 142 (78.9) | |
| Intervention | acupuncture | 68 (37.8) |
| Chinese herbal medicinesb | 99 (55.0) | |
| Othersc | 9 (5.0) | |
| Combinationd | 4 (2.2) |
| [1] | Feigin VL, Nichols E, Alam T, et al. Global, regional, and national burden of neurological disorders, 1990-2016: a systematic analysis for the global burden of disease study 2016. Lancet Neurol 2019;18:459-80. |
| [2] | Wu SM, Wu B, Liu M, et al. Stroke in China: advances and challenges in epidemiology, prevention, and management. Lancet Neurol 2019;18:394-405. |
| [3] | Wang LD, Liu JM, Yang Y, et al. The prevention and treatment of stroke still face huge challenges — brief report on stroke prevention and treatment in China, 2018. Zhong Guo Xun Huan Za Zhi 2019;34:105-19. |
| [4] | Czakó C, Kovács T, Ungvari Z, et al. Retinal biomarkers for Alzheimer's disease and vascular cognitive impairment and dementia (VCID): implication for early diagnosis and prognosis. GeroScience 2020;42:1499-525. |
| [5] | O'Brien JT, Thomas A. Vascular dementia. Lancet 2015;386:1698-706. |
| [6] | Jia LF, Du YF, Chu L, et al. Prevalence, risk factors, and management of dementia and mild cognitive impairment in adults aged 60 years or older in China: a cross-sectional study. Lancet Public Health 2020;5:e661-71. |
| [7] | Stovner LJ, Nichols e, Steiner TJ, et al. Global, regional, and national burden of migraine and tension-type headache, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 2018;17:954-76. |
| [8] | James SL, Abate D, Abate AH, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden Disease Study 2017. Lancet 2018;392:1789-858. |
| [9] | Wöber-Bingöl Ç, Wöber C, Uluduz D, et al. The global burden of headache in children and adolescents-developing a questionnaire and methodology for a global study. J Headache Pain 2014;15:86. |
| [10] | Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd ed. Cephalalgia 2018;38:1-211. |
| [11] | Ni XJ, Lin H, Li H, et al. Evidence‐based practice guideline on integrative medicine for stroke 2019. J Evid Based Med 2020;13:137-52. |
| [12] | Zhou XX, Huang JS, Xie M, Wu CH. Expert consensus for TCM preventive treatment of diseases: vascular mild cognitive impairment. Zhong Guo Zhong Yi Yao Xin Xi Za Zhi 2020;27:1-5. |
| [13] | Yu SY, Zhang MJ, Zhou JY, Liu RZ, Wan Q, Li YS. Headache care in China. Headache 2014;54:601-9. |
| [14] | Wang B, Chen D, Zhou HE, Shi HX, Xie Q. Influence of clinical pathways used the hospitals of Traditional Chinese Medicine on patients hospitalized with stroke: a systematic review and Meta-analysis. J Tradit Chin Med 2017;37:159-64. |
| [15] | Cai YY, Jiang WM. Research progress of Traditional Chinese Medicine intervention vascular cognitive impairment. Zhong Guo Zhong Yao Za Zhi 2017;42:1837-41. |
| [16] | Hu WY, Han ZY, Ma HP, Lin JF, Chang Z. Systematic evaluation and Meta-analysis of efficacy and safety of Tianzhi granules in treatment of vascular cognitive impairment. Zhong Guo Zhong Yao Za Zhi 2020;45:4766-75. |
| [17] | Gou J, Yang HX, Yu Y, Guo RJ. Systematic review and Meta-analysis of efficacy and safety of compound Congrong Yizhi capsules on vascular cognitive impairment. Zhong Guo Zhong Yao Za Zhi 2020;45:1924-32. |
| [18] | Coeytaux RR, Befus D. Role of acupuncture in the treatment or prevention of migraine, tension-type headache, or chronic headache disorders. Headache 2016;56:1-3. |
| [19] | Bendtsen L, Evers S, Linde M, Mitsikostas DD, Sandrini G, Schoenen J. EFNS guideline on the treatment of tension-type headache — report of an EFNS task force. Eur J Neurol 2010;17:1318-25. |
| [20] | Wu TX, Li YP, Li J, Zhong ZH, Jia WN. Chinese clinical trial registration and publishing system, and explanation. Zhong Guo Xun Zheng Yi Xue Za Zhi 2006;6:395-6. |
| [21] | WHO. WHO. International Clinical Trials Registry Platform (ICTRP), Trial registration. https://www.who.int/clinical-trials-registry-platform/network/trial-registration. (Accessed 20th January 2021). |
| [22] | Zhang X, Tian R, Yang Z, et al. Quality assessment of clinical trial registration with Traditional Chinese Medicine in WHO registries. BMJ Open 2019;9:e025218. |
| [23] | The Central People’s Government of the People’s Republic of China, China and India join the WHO international clinical trial registration platform. http://www.gov.cn/jrzg/2007-07/25/content_697236.htm (2007, accessed 7th October 2020). |
| [24] | National Administration of Traditional Chinese Medicine of China, Notice on the issuance of TCM diagnosis and treatment plans for 8 diseases of encephalopathy and psychiatry. http://yzs.satcm.gov.cn/zhengcewenjian/2018-03-24/3203.html. (21st January 2011, accessed 7th October 2020). |
| [25] | Wu TX, Li YP, Yao X, Li J. Clinical trial registration: to improve the quality of clinical research in China. Zhong Guo Xun Zheng Yi Xue Za Zhi 2006;3:153-6. |
| [26] | WHO. WHO International Clinical Trials Registry Platform (ICTRP), About ICTRP. https://www.who.int/ictrp/about/en/. (Accessed 7th October 2020). |
| [27] | Wang YY, Huang LQ. Taking a broad and long-term view to establish China Center for Evidence Based Traditional Chinese Medicine (CCEBTCM). Zhong Guo Xun Zheng Yi Xue Za Zhi 2019;19:1131-7. |
| [28] | Li YP, Wu TX, Li J, Zhong ZH, Jia WN. Joint statement of establishing Chinese clinical trial registration and publishing system. Zhong Xi Yi Jie He Xue Bao 2006;4:331-2. |
| [29] | Ross JS, Gross CP, Krumholz HM. Promoting transparency in pharmaceutical industry-sponsored research. Am J Public Health 2012;102:72-80. |
| [30] | Pearn J. Publication: an ethical imperative. BMJ 1995;310:1313-5. |
| [31] | Breil T, Boettcher M, Hoffmann G F, Ries M. Publication status of completed registered studies in pediatric appendicitis: a cross-sectional analysis. BMJ Open 2018;8:e021684. |
| [32] | Li J, Jin X, He YY, Fang YX, Wei JM. Practical explorations of multi-center clinical research organized by medical scientific periodicals. Bian Ji Xue Bao 2015;1:47-9. |
| [33] | Fisher M, Amarenco P, Aronowski J, et al. International collaborations are essential for stroke. Stroke 2019;50:2993-4. |
| [34] | Webster TJ. Think international collaborations are safe? Think again. Int J Nanomedicine 2019;14:6933-5. |
| [35] | Cheng CW, Wu TX, Shang HC, et al. CONSORT extension for Chinese herbal medicine formulas 2017: recommendations, explanation, and elaboration. Ann Intern Med 2017;167:112-21. |
| [1] | ZHOU Xianshi, ZHONG Minlin, XI Xiaotu, LI Jun, TANG Guanghua. Efficacy and safety of Chinese herbal medicine as adjunctive therapy in sepsis patients with bloodstream infection: a propensity-matched analysis [J]. Journal of Traditional Chinese Medicine, 2024, 44(1): 197-204. |
| [2] | REN Ping, WANG Quanwu, BAI Wei, SUN Miao, LIU Zheling, GAO Ming, WANG Liang, PENG Bo, XU Liguang. Identifying the effective combination of acupuncture and traditional Chinese medicinal herbs for postmenopausal osteoporosis therapy through studies of their molecular regulation of bone homeostasis [J]. Journal of Traditional Chinese Medicine, 2024, 44(1): 212-219. |
| [3] | WANG Jiabao, ZHANG Lishuang, NIU Baihan, YU Yajun, YANG Fengwen, MIAO Lin, CHAI Lijuan, DING Xinya, SUN Yingjie, WANG Yujing, WANG Lin, ZHANG Han, WANG Yi, LI Lin. Efficacy and safety of Weichang’ an pill (胃肠安丸) combined with Western Medicine on gastrointestinal diseases: a systematic review and Meta-analysis [J]. Journal of Traditional Chinese Medicine, 2023, 43(6): 1057-1067. |
| [4] | WANG Yaqi, ZHAO Weibo, WANG Yixing, ZHAO Haihong, ZHOU Yaoyao, YAN Yun, WU Taotao, LUO Bin, WANG Ji. Traditional Chinese Medicine constitution among patients with allergic rhinitis and its correlation with anxiety and depression [J]. Journal of Traditional Chinese Medicine, 2023, 43(6): 1252-1258. |
| [5] | DAI Zeqi, LIAO Xing, GUAN Yueyue, ZENG Zixiu, TANG Jun, HU Jing. Bloodletting puncture in the treatment of acute ischemic stroke: protocol for a mixed-method study of a multi-center randomized controlled trial and focus group [J]. Journal of Traditional Chinese Medicine, 2023, 43(6): 1259-1267. |
| [6] | MEI Pingping, FENG Wenzhe, SHI Peng, ZHANG Wenxiu, ZHUANG Yu. Clinical research progress in Traditional Chinese Medicine in treating wound healing after anal fistula surgery [J]. Journal of Traditional Chinese Medicine, 2023, 43(5): 1047-1054. |
| [7] | WANG Chao, WU Qiong, LI Ping, WANG Zhigang, LOU Xusheng, LI Yuanyuan, ZHANG Lin. Effect of Traditional Chinese Medicine combined with Western Medicine on blood lipid levels and inflammatory factors in patients with angina pectoris in coronary heart disease identified as intermingled phlegm and blood stasis syndrome: a network Meta-analysis [J]. Journal of Traditional Chinese Medicine, 2023, 43(4): 640-649. |
| [8] | LI Xingjie, LIU Qiqi, XIA Rui, LIU Jun, WANG Dan, SHI Jiao, KUANG Yuxing, DAI Yalan, HUANG Haoyu, TANG Wei, CHEN Shangjie. Moxibustion modulates working memory in patients with amnestic mild cognitive impairment: a functional magnetic resonance imaging study [J]. Journal of Traditional Chinese Medicine, 2023, 43(4): 801-808. |
| [9] | CAO Wencong, LIAO Shaojun, ZHANG Yuanwen, ZHOU Li, LI Geng, OUYANG Wenwei, WEN Zehuai. Effectiveness and safety of Xuefu Zhuyu oral liquid (血府逐瘀口服液) on Qi-stagnation and blood-stasis pattern in patients with stable angina, tension-type headache and primary dysmenorrhea: rationale and design of a master protocol [J]. Journal of Traditional Chinese Medicine, 2023, 43(4): 815-823. |
| [10] | LI He, SU Wenquan, LI Shanshan, JI Hanrui, CHENG Jiangyan, CUI Fangyuan, TANG Lu, ZHOU Li, GAO Ying, DONG Xinglu. Supplementing Qi and activating blood circulation method to treat vertebrobasilar dolichoectasia with posterior circulatory watershed infarction: a case report of two patients [J]. Journal of Traditional Chinese Medicine, 2023, 43(4): 824-828. |
| [11] | LUO Xin, XIE Jing, HUANG Li, GAN Wenfan, CHEN Ming. Efficacy and safety of activating blood circulation and removing blood stasis of Traditional Chinese Medicine for managing renal fibrosis in patients with chronic kidney disease: a systematic review and Meta-analysis [J]. Journal of Traditional Chinese Medicine, 2023, 43(3): 429-440. |
| [12] | HOU Chao, ZHANG Yusen, YANG Die, LI Yifei, ZHANG Xiaochun, LIU Yanqing. Effects of Traditional Chinese Medicine on the survival of patients with stage I gastric cancer and high-risk factors: a real-world retrospective study [J]. Journal of Traditional Chinese Medicine, 2023, 43(3): 568-573. |
| [13] | XU Guihua, CHEN Feifei, ZHANG Wei, WU Yingen, CHEN Xiaorong, SHI Kehua, WANG Zhenwei, SHI Miaoyan, ZHANG Xing, LU Yunfei, YUAN Weian, LYU Hua, CHEN Xuan. Effectiveness of Traditional Chinese Medicine on coronavirus disease 2019 in 92 patients: a retrospective study [J]. Journal of Traditional Chinese Medicine, 2023, 43(3): 582-587. |
| [14] | HU Xingyao, LIU Hongning, YAN Xiaojun, CHEN Zhong, FU Liu, LIU Ge, CHEN Xuan, SHANG Guangbin. Liver metabolomic characteristics in three different rat models of Yin deficiency based on ultra-performance liquid chromatography coupled to quadrupole time-of-flight mass spectrometry [J]. Journal of Traditional Chinese Medicine, 2023, 43(2): 274-285. |
| [15] | WANG Yanan, YUAN Li, WANG Li, XU Zhiyuan, RUAN Hua, CHENG Xiangdong, DONG Changwu. Microbial community structure of different tongue fur types in patients with chronic gastritis [J]. Journal of Traditional Chinese Medicine, 2023, 43(2): 365-373. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||