Journal of Traditional Chinese Medicine ›› 2025, Vol. 45 ›› Issue (4): 829-835.DOI: 10.19852/j.cnki.jtcm.20241226.001
• Original Articles • Previous Articles Next Articles
Efficacy and safety of Tuomin Zhiti decoction (脱敏止嚏汤) on patients with seasonal allergic rhinitis: a randomized, double-blind, placebo-controlled trial
ZHAO Weibo1, WANG Yaqi2, KONG Lingyao3, WANG Tianyi4, ZHAO Haihong1, ZHANG Ying3, LUO Bin1, WANG Ji1( ), WANG Qi1(
), WANG Qi1( )
)
- 1 National Institute of TCM Constitution and Preventive Medicine, Beijing University of Chinese Medicine, Beijing 100029, China
2 College of Basic Medical Sciences, Zhejiang Chinese Medical University, Hangzhou 310053, China
3 Centre for Evidence-Based Chinese Medicine, Beijing University of Chinese Medicine, Beijing 100029, China
4 Beijing University of Chinese Medicine International School, Beijing 100029, China
-
Received:2024-08-22Accepted:2024-12-12Online:2025-08-15Published:2024-12-26 -
Contact:WANG Ji,WANG Qi -
About author:WANG Qi, National Institute of TCM Constitution and Preventive Medicine, Beijing University of Chinese Medicine, Beijing 100029, China. wangqi710@126.com,Telephone: +86-17801016686
WANG Ji, National Institute of TCM Constitution and Preventive Medicine, Beijing University of Chinese Medicine, Beijing 100029, China. doctorwang2009@126.com;
-
Supported by:Fundamental Research Funds for the Central Universities(2023-JYB-JBZD-009);High level Key Discipline of National Administration of Traditional Chinese Medicine-Traditional Chinese Constitutional Medicine(zyyzdxk-2023251);General program of National Natural Science Foundation of China: Study on the Mechanism of "the Simultaneous Prevention of Different Diseases" of Allergic Constitution Regulating Formula based on DNA Trap Mediated Eosinophil-Derived Dendritic Cells Cell Crosstalk(82174243);General Project of Beijing Natural Science Foundation: Study on the Mechanism of Guominkang for Treating Allergic Rhinitis through Regulating Body Constitution based on Vacuolating Cytotoxin A-mediated Eosinophil-derived Dendritic Cell Cell immune microenvironment(7242227)
Cite this article
ZHAO Weibo, WANG Yaqi, KONG Lingyao, WANG Tianyi, ZHAO Haihong, ZHANG Ying, LUO Bin, WANG Ji, WANG Qi. Efficacy and safety of Tuomin Zhiti decoction (脱敏止嚏汤) on patients with seasonal allergic rhinitis: a randomized, double-blind, placebo-controlled trial[J]. Journal of Traditional Chinese Medicine, 2025, 45(4): 829-835.
share this article
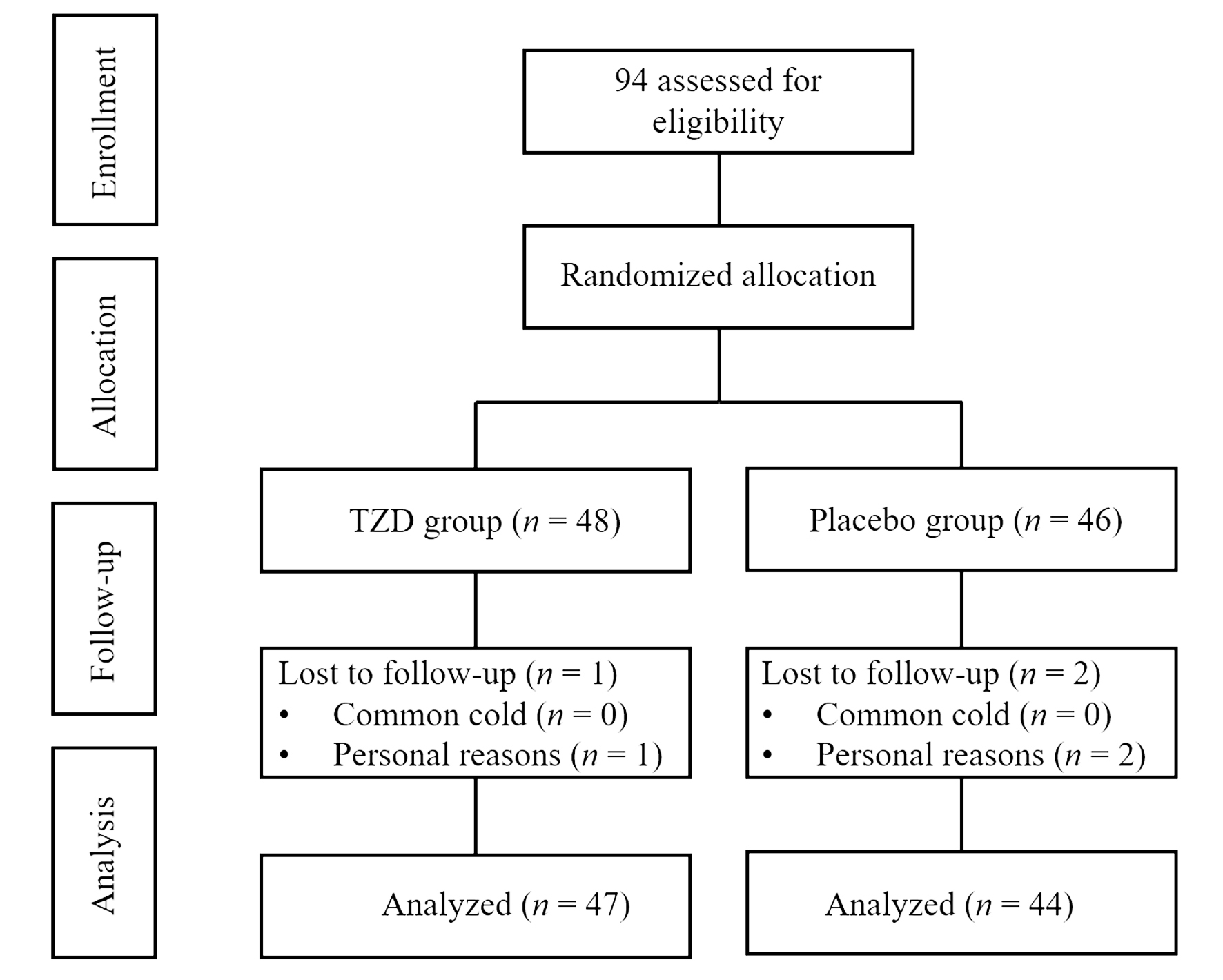
Figure 1 Study participant flow diagram TZD: Tuominzhiti decoction. TZD group (7 g/pack, po, 1 pack/time, bid, for 2 weeks), placebo group (7 g/pack, po, 1 pack/time, bid, for 2 weeks).
| Characteristic | TZD group (n = 47) | placebo group (n = 44) | P value |
|---|---|---|---|
| Age (years) | 38.1±10.5 | 37.6±8.4 | 0.773a |
| Male/female | 23/24 | 21/23 | 0.909a |
| TNSS | |||
| Sneezing | 2.4±0.7 | 2.4±0.7 | 0.881a |
| Runny nose | 2.1±0.8 | 2.2±0.7 | 0.898a |
| Itchy nose | 2.4±0.7 | 2.3±0.9 | 0.199a |
| Nasal obstruction | 2.0±0.9 | 2.0±1.0 | 0.357a |
| TOSS | |||
| Itching/redness | 2.0±0.8 | 2.3±0.9 | 0.133a |
| Tearing | 1.3±1.0 | 1.5±1.1 | 0.325a |
| Overall | 12.0±3.5 | 12.5±3.7 | 0.200b |
| mini RQLQ score | |||
| Overall | 44.8±15.5 | 49.2±17.2 | 0.122b |
| Activity limitation | 9.2±4.2 | 10.8±4.4 | 0.055a |
| Practical problems | 8.2±2.6 | 8.5±3.1 | 0.588b |
| Nasal symptoms | 11.3±4.0 | 12.0±4.0 | 0.115a |
| Eye symptoms | 9.9±4.1 | 10.1±5.3 | 0.507b |
| Other symptoms | 7.0±4.3 | 7.7±4.4 | 0.256b |
Table 1 Homogeneity test for general characteristics and measurement variables at baseline ($\bar{x} \pm s$)
| Characteristic | TZD group (n = 47) | placebo group (n = 44) | P value |
|---|---|---|---|
| Age (years) | 38.1±10.5 | 37.6±8.4 | 0.773a |
| Male/female | 23/24 | 21/23 | 0.909a |
| TNSS | |||
| Sneezing | 2.4±0.7 | 2.4±0.7 | 0.881a |
| Runny nose | 2.1±0.8 | 2.2±0.7 | 0.898a |
| Itchy nose | 2.4±0.7 | 2.3±0.9 | 0.199a |
| Nasal obstruction | 2.0±0.9 | 2.0±1.0 | 0.357a |
| TOSS | |||
| Itching/redness | 2.0±0.8 | 2.3±0.9 | 0.133a |
| Tearing | 1.3±1.0 | 1.5±1.1 | 0.325a |
| Overall | 12.0±3.5 | 12.5±3.7 | 0.200b |
| mini RQLQ score | |||
| Overall | 44.8±15.5 | 49.2±17.2 | 0.122b |
| Activity limitation | 9.2±4.2 | 10.8±4.4 | 0.055a |
| Practical problems | 8.2±2.6 | 8.5±3.1 | 0.588b |
| Nasal symptoms | 11.3±4.0 | 12.0±4.0 | 0.115a |
| Eye symptoms | 9.9±4.1 | 10.1±5.3 | 0.507b |
| Other symptoms | 7.0±4.3 | 7.7±4.4 | 0.256b |
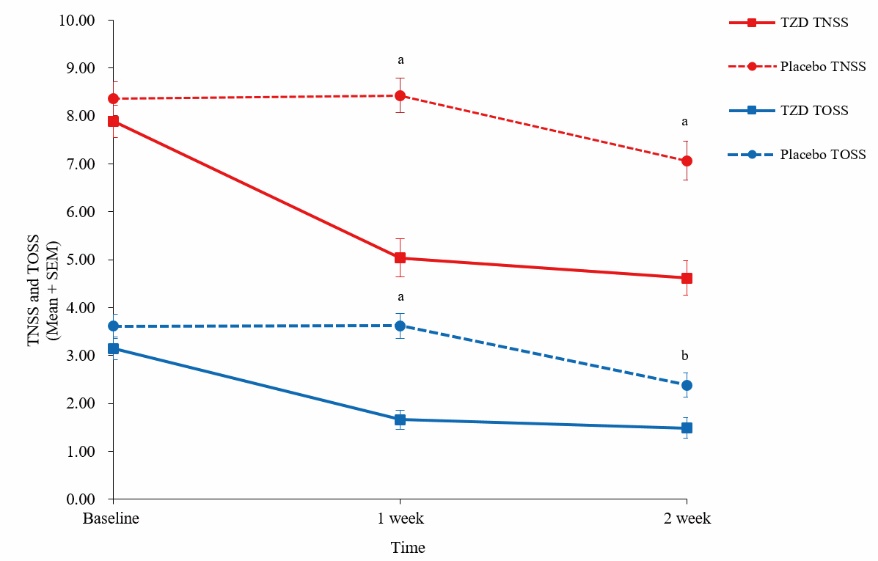
Figure 2 TNSS and TOSS for 1- and 2-week treatment periods TZD group (n = 47) (7 g/pack, po, 1 pack/time, bid, for 2 weeks), placebo group (n = 44) (7 g/pack, po,1 pack/time, bid, for 2 weeks). TNSS: total nasal symptom score; TOSS: total ocular symptom score. Difference between groups was tested by independent sample t-test or non-parametric test. Data are presented as mean ± standard deviation. Compared with baseline, aP < 0.001, bP < 0.01.
| Variable | TZD group (n = 47) | placebo group (n = 44) | P value |
|---|---|---|---|
| TNSS | |||
| Sneezing | 1.3±0.7 | 1.9±0.8 | 0.001a |
| Runny nose | 1.2±0.8 | 1.9±0.8 | 0.000b |
| Itchy nose | 1.1±0.8 | 1.6±1.0 | 0.002a |
| Nasal obstruction | 1.1±0.9 | 1.7±0.9 | 0.001a |
| TOSS | |||
| Itching/redness | 1.0±0.9 | 1.5±1.0 | 0.024c |
| Tearing | 0.5±0.8 | 0.9±0.9 | 0.014c |
| Overall | 6.1±3.7 | 9.5±4.0 | 0.000b |
Table 2 Effect of treatments on total nasal symptom score ($\bar{x} \pm s$)
| Variable | TZD group (n = 47) | placebo group (n = 44) | P value |
|---|---|---|---|
| TNSS | |||
| Sneezing | 1.3±0.7 | 1.9±0.8 | 0.001a |
| Runny nose | 1.2±0.8 | 1.9±0.8 | 0.000b |
| Itchy nose | 1.1±0.8 | 1.6±1.0 | 0.002a |
| Nasal obstruction | 1.1±0.9 | 1.7±0.9 | 0.001a |
| TOSS | |||
| Itching/redness | 1.0±0.9 | 1.5±1.0 | 0.024c |
| Tearing | 0.5±0.8 | 0.9±0.9 | 0.014c |
| Overall | 6.1±3.7 | 9.5±4.0 | 0.000b |
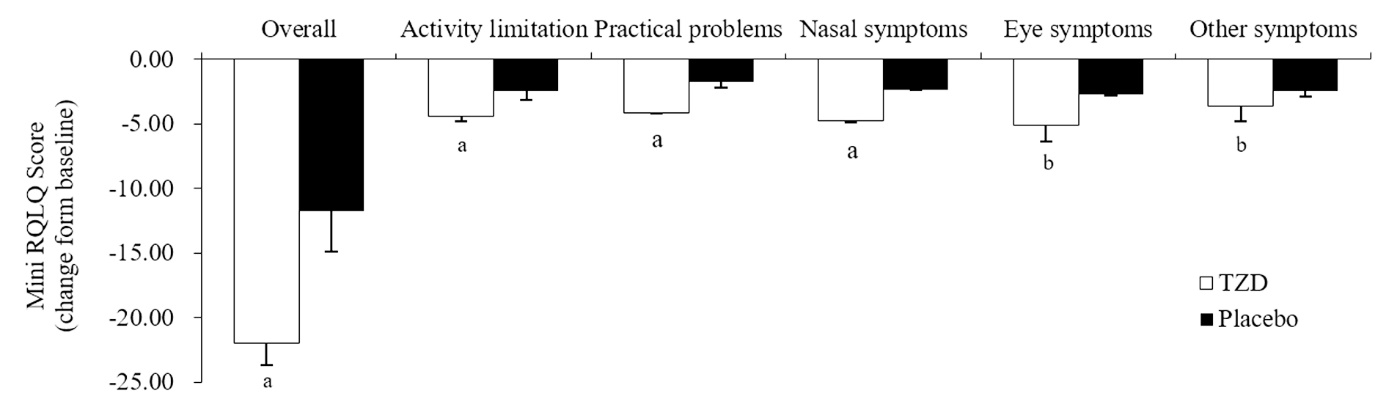
Figure 3 Mean mini RQLQ score change for 2-week treatment period TZD group (n = 47) (7 g/pack, po, 1 pack/time, bid, for 2 weeks), placebo group (n = 44) (7 g/pack, po, 1 pack/time, bid, for 2 weeks). RQLQ: rhinoconjunctivitis quality-of-life questionnaire; TZD: Tuominzhiti decoction. Differences between groups were compared using independent-sample t-tests or nonparametric tests. Data are presented as mean ± standard deviation. Compared with baseline, aP < 0.001; compared with baseline, bP < 0.01.
| 1. | Rondón C, Fernandez J, Canto G, Blanca M. Local allergic rhinitis: concept, clinical manifestations, and diagnostic approach. J Investig Allergol Clin Immunol 2010; 20: 364-71; quiz 2 p following 371. |
| 2. |
Brożek JL, Bousquet J, Agache I, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines-2016 revision. J Allergy Clin Immunol 2017; 140: 950-8.
DOI PMID |
| 3. | Tang R, Lei S, Zhu L, Lyu Y, Li H. Prevention of omalizumab for seasonal allergic rhinoconjunctivitis: a retrospective cohort study. Front Immunol 2022; 13: 913424. |
| 4. |
Bousquet J, Schünemann HJ, Togias A, et al. Next-generation Allergic Rhinitis and Its Impact on Asthma (ARIA) guidelines for allergic rhinitis based on Grading of Recommendations Assessment, Development and Evaluation (GRADE) and real-world evidence. J Allergy Clin Immunol 2020; 145: 70-80.e3.
DOI PMID |
| 5. | Spector SL. Overview of comorbid associations of allergic rhinitis. J Allergy Clin Immunol 1997; 99: S773-80. |
| 6. | Chan RY, Chien WT. The effects of two Chinese herbal medicinal formulae vs placebo controls for treatment of allergic rhinitis: a randomised controlled trial. Trials 2014; 15: 261. |
| 7. |
Zhao J, Yan X, Gai J, et al. Efficacy of Bimin decoction for patients with perennial allergic rhinitis: an open-label non-inferiority randomized controlled trial. Trials 2019; 20: 802.
DOI PMID |
| 8. | Yang SH, Yu CL, Chen YL, Chiao SL, Chen ML. Traditional Chinese Medicine, Xinyi San, reduces nasal symptoms of patients with perennial allergic rhinitis by its diverse immunomodulatory effects. Int Immunopharmacol 2010; 10: 951-8. |
| 9. | Luo Q, Zhang CS, Yang L, et al. Potential effectiveness of Chinese herbal medicine Yuping Feng san for adult allergic rhinitis: a systematic review and Meta-analysis of randomized controlled trials. BMC Complement Altern Med 2017; 17: 485. |
| 10. | Cheng L, Chen J, Fu Q, et al. Chinese society of allergy guidelines for diagnosis and treatment of allergic rhinitis. Allergy Asthma Immunol Res 2018; 10: 300- 53. |
| 11. | Cheng J, Zhang M, Zheng Y, Wang J, Wang Q. Integrative analysis of network pharmacology and proteomics to identify key targets of Tuomin-Zhiti-decoction for allergic rhinitis. J Ethnopharmacol 2022; 296: 115448. |
| 12. | Yung TY, Zhang H, Tang LC, et al. Acupuncture and herbal moxibustion for the treatment of 'BiQiu' (allergic rhinitis symptoms) in a Hong Kong Chinese medicine clinic: a randomized controlled trial. Chin Med 2019; 14: 50. |
| 13. |
Pfaar O, Demoly P, Gerth van Wijk R, et al. Recommendations for the standardization of clinical outcomes used in allergen immunotherapy trials for allergic rhinoconjunctivitis: an EAACI Position Paper. Allergy 2014; 69: 854-67.
DOI PMID |
| 14. |
Juniper EF, Thompson AK, Ferrie PJ, Roberts JN. Development and validation of the mini rhinoconjunctivitis quality of life questionnaire. Clin Exp Allergy 2000; 30: 132-40.
DOI PMID |
| 15. | Zhang Y, Xi L, Gao Y, et al. Omalizumab is effective in the preseasonal treatment of seasonal allergic rhinitis. Clin Transl Allergy 2022; 12: e12094. |
| 16. |
Okubo K, Suzuki T, Tanaka A, Aoki H. Long-term safety and efficacy of rupatadine in Japanese patients with perennial allergic rhinitis: a 52-week open-label clinical trial. J Drug Assess 2019; 8: 104-14.
DOI PMID |
| 17. | Jung JW, Kang HR, Ji GE, et al. Therapeutic effects of fermented red ginseng in allergic rhinitis: a randomized, double-blind, placebo-controlled study. Allergy Asthma Immunol Res 2011; 3: 103-10. |
| 18. |
Mösges R, Bachert C, Panzner P, et al. Short course of grass allergen peptides immunotherapy over 3 weeks reduces seasonal symptoms in allergic rhinoconjunctivitis with/without asthma: a randomized, multicenter, double-blind, placebo-controlled trial. Allergy 2018; 73: 1842-50.
DOI PMID |
| 19. |
Welch MJ, Meltzer EO, Simons FE. H1-antihistamines and the central nervous system. Clin Allergy Immunol 2002; 17: 337-88.
PMID |
| 20. |
Wang J, Wang T, Li YS, Li LR, Zheng YF, Wang Q. Allergic constitution theory of Chinese medicine and its assessment criterion and related studies. Chin J Integr Med 2015; 21: 716-20.
DOI PMID |
| 21. |
Zhao Y, Woo KS, Ma KH, et al. Treatment of perennial allergic rhinitis using Shi-Bi-Lin, a Chinese herbal formula. J Ethnopharmacol 2009; 122: 100-5.
DOI PMID |
| 22. | Zhang Y, Chen Z, Chen L, et al. Astragali Radix (Huangqi): a time-honored nourishing herbal medicine. Chin Med 2024; 19: 119. |
| 23. | Li Y, Fu CM, Ren B, et al. Study on attenuate and synergistic mechanism between aconiti lateralis praeparata radix and glycyrrhizae radix for toxicity reduction based on metabonomic of MI-RI mouse cardiomyocytes. Zhong Guo Zhong Yao Za Zhi 2014; 39: 3166-71. |
| [1] | CHEN Ziying, ZHAO Xiaoping, FAN Xiaoxuan, TANG Didi, SUN Wen, LYU Jing, HUANG Lan, QI Fan. Seven Traditional Chinese Medicine external treatments combined with rehabilitation training on the functional recovery of limbs in patients with cerebral hemorrhage: a network Meta-analysis [J]. Journal of Traditional Chinese Medicine, 2025, 45(4): 711-719. |
| [2] | LI Yuxuan, LI Yan, WANG Wujiao, CUI Xiaoyun, WAN Jie, ZHOU Kun, LU Jinjin, LIU Jing, LIN Qian, LI Dong. Clinical study of Yiqi Liangxue Shengji prescription (益气凉血生肌方) for improving cardiac function after myocardial ischemia reperfusion injury in patients with acute myocardial infarction: a randomized, double-blind, placebo-controlled trial [J]. Journal of Traditional Chinese Medicine, 2025, 45(4): 836-844. |
| [3] | ZHENG Ruwen, DONG Xu, WANG Tianyi, FENG Liyuan, ZHANG Hongyan, HUO Hong, ZHANG Ying, ZHANG Qianshi, ZHU Xingyan, WANG Dongyan. Electroacupuncture versus conventional acupuncture of scalp motor area for post-stroke wrist dyskinesia and its effect on muscle function: a randomized, controlled clinical trial [J]. Journal of Traditional Chinese Medicine, 2025, 45(4): 852-859. |
| [4] | XIAO Jing, SONG Danlei, LIANG Caiming, HE Yinuo, ZHENG Weifang, WU Xiaqiu. Efficacy of Jianpi formulas (健脾剂) in reducing the recurrence of colorectal adenoma after polypectomy: a systematic review and Meta-analysis [J]. Journal of Traditional Chinese Medicine, 2025, 45(2): 225-233. |
| [5] | LAI Xiaolei, SHANG Juju, LIU Hongxu, HU Jing, LI Xiang, ZHANG Zhenmin, XING Wenlong. Clinical efficacy of Angong Jiangya pill (安宫降压丸) for grade 2 hypertension with liver-fire hyperactivity syndrome: a randomized, double-blind, placebo-controlled, multicenter trial [J]. Journal of Traditional Chinese Medicine, 2025, 45(2): 422-429. |
| [6] | CHENG Jianping, FAN Chanjuan, ZHAI Lili, WANG Hui, XIE Dongling, CAI Yong, LI Zhen, HUANG Kun, BAI Qixuan. Efficacy and safety of Qingwei Zhitong pellets (清胃止痛微丸)-containing quadruple therapy for Helicobacter pylori eradication: a prospective, single-center, randomized trial [J]. Journal of Traditional Chinese Medicine, 2025, 45(2): 430-436. |
| [7] | YE Wujie, YANG Yawei, ZHANG Da, TANG Ling, CUI Minying, FU Bin, ZHANG Meng, HU Xingang, ZHAO Yan. Effectiveness of combining Qingyanyin formulated granules (轻燕饮配方颗粒) with press needles in treating abdominal obesity: a multicenter randomized controlled trial [J]. Journal of Traditional Chinese Medicine, 2025, 45(1): 107-114. |
| [8] | YU Manshu, JIANG Chunchun, YAO Min, HUA Jianwu, JIANG Yan, HUANG Min, LIU Jianjing, ZHOU Yan, WANG Yuan, SHENG Meixiao. Yunpiqiangshen gel (运脾强肾浸膏) improves quality of life in dialysis patients [J]. Journal of Traditional Chinese Medicine, 2025, 45(1): 123-131. |
| [9] | LANG Jiawang, JIN Lingqing, LUO Jianchang, LANG Boxu. Effects of acupuncture combined with bone-setting therapy to treat tourette syndrome: a three-arm randomized controlled trial [J]. Journal of Traditional Chinese Medicine, 2025, 45(1): 176-183. |
| [10] | Emre Bulut, Didem Özkal Eminoğlu, Yasemin Çayır. Effect of electroacupuncture on pain after periodontal flap surgery: a randomized controlled trial [J]. Journal of Traditional Chinese Medicine, 2025, 45(1): 184-191. |
| [11] | WU Qiaomin, GUAN Xuanke, LIU Jinfeng, WANG Yanli, CHANG Xing, LIU Zhiming, LIU Ruxiu. Compound Tongyang Fumai decoction (通阳复脉方) improves quality of life in sick sinus syndrome: a randomized controlled study [J]. Journal of Traditional Chinese Medicine, 2024, 44(6): 1247-1253. |
| [12] | QIAN Jianan, XU Yan, HU Hongyi, ZHAO Aiguang. Clinical efficacy and safety evaluation of Buzhongyiqi pills (补中益气丸) on appetite improvement in patients with colorectal cancer receiving chemotherapy: a pilot randomized cross-over clinical trial [J]. Journal of Traditional Chinese Medicine, 2024, 44(6): 1254-1267. |
| [13] | DENG Yasheng, HAN Siyin, XI Lanhua, HUANG Hui, LIANG Tianwei, ZHENG Yiqing, FAN Yanping, LIN Jiang. Traditional Chinese Medicine in the treatment of recurrent respiratory tract infections in children: an overview of systematic reviews and Meta-analyses [J]. Journal of Traditional Chinese Medicine, 2024, 44(5): 871-884. |
| [14] | GU Xiangchen, QIU Meisi, XIE Lin, CHEN Min, DENG Yueyi, ZHANG Changming, JIAN Guihua, WANG Chen, WANG Yi. Individualized Traditional Chinese Medicine treatment vs antibiotics for recurrent urinary tract infections: a multicenter, randomized controlled study [J]. Journal of Traditional Chinese Medicine, 2024, 44(3): 524-529. |
| [15] | TIAN Haolin, YANG Yuanbin, ZHANG Hu, ZHAO Wenjing, ZHOU Jing, TIAN Jingfeng, HE Long, LI Xuechao, SHEN Qinxuan, SHUAI Mei. Efficacy of Daoyin combined with lower limb robot as a comprehensive rehabilitation intervention for stroke patients: a randomized controlled trial [J]. Journal of Traditional Chinese Medicine, 2024, 44(3): 530-536. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||

