Journal of Traditional Chinese Medicine ›› 2024, Vol. 44 ›› Issue (5): 926-933.DOI: 10.19852/j.cnki.jtcm.2024.05.003
Previous Articles Next Articles
Protective effect of Zhizi Huangqi Shanzha formula (栀子黄芪山楂方) on aflatoxin poisoning in mice and its effect on intestinal flora
SUN Chuanbo, XU Guangpei, JIANG Ping, HUANG Shipping, CHEN Cunwu, HE Yanfei( )
)
- College of Biotechnology and Pharmaceutical Engineering, West Anhui University, Lu’an 237012, China
-
Received:2023-03-12Accepted:2023-07-14Online:2024-10-15Published:2024-09-11 -
Contact:HE Yanfei, College of Biotechnology and Pharmaceutical Engineering, West Anhui University, Lu’an 237012, China. 341281a00ek.cdb@sina.cn Telephone: +86-15956792864 -
Supported by:Provincial Natural Science Foundation of Anhui Province-Quantitative Constitutive Effect and Mechanism of Action of Dendrobium Polysaccharides against Tumours based on the Tumour Microenvironment(2208085QH273);Natural Science Research Project for Anhui Universities-mechanism of Anti-bacterial Diarrhea Effect of Probiotic-fermented Portulaca Oleracea in Targeting and Regulating Amino acid Metabolism of Intestinal Flora and Screening of Anti-tumor Active Fraction of Paris polyphylla and its Effect on the Tumour Immune Microenvironment(2023AH052634);Natural Science Research Project for Anhui Universities-mechanism of Anti-bacterial Diarrhea Effect of Probiotic-fermented Portulaca Oleracea in Targeting and Regulating Amino acid Metabolism of Intestinal Flora and Screening of Anti-tumor Active Fraction of Paris polyphylla and its Effect on the Tumour Immune Microenvironment(2022AH051668)
Cite this article
SUN Chuanbo, XU Guangpei, JIANG Ping, HUANG Shipping, CHEN Cunwu, HE Yanfei. Protective effect of Zhizi Huangqi Shanzha formula (栀子黄芪山楂方) on aflatoxin poisoning in mice and its effect on intestinal flora[J]. Journal of Traditional Chinese Medicine, 2024, 44(5): 926-933.
share this article
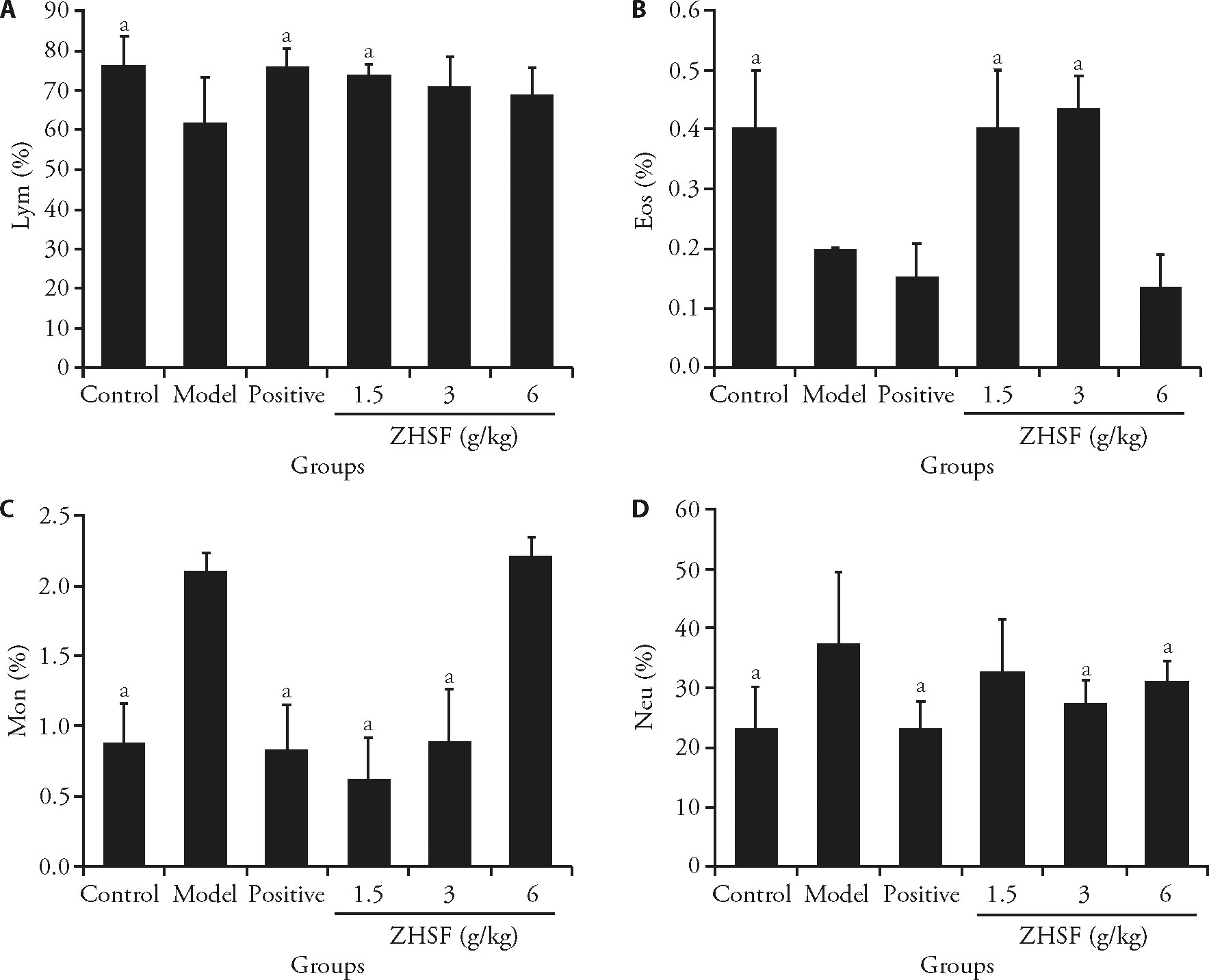
Figure 1 Effect of ZHSF on the blood cell composition of mice poisoned with AFB1 A: percentage of lymphocyte in the groups. B: percentage of eosinophils in the groups. C: percentage of monocyte in the groups. D: percentage of neutrophils in the groups. Control group: mice were orally administered (p.o.) with saline for 10 d. Model group: mice were orally administered (p.o.) with AFB1 of 0.4 mg/kg from the first day to the third day and orally administered (p.o.) with saline for 7 d before the sample harvesting. AFB1 + ZHSF (1.5 g/kg) group: mice were orally administered (p.o.) with AFB1 of 0.4 mg/kg from the first day to the third day and orally administered (p.o.) with ZHSF (1.5 g/kg) for 7 d before the sample harvesting. AFB1 + ZHSF (3.0 g/kg) group: mice were orally administered (p.o.) with AFB1 of 0.4 mg/kg from the first day to the third day and orally administered (p.o.) with ZHSF (3.0 g/kg) for 7 d before the sample harvesting. AFB1 + ZHSF (6.0 g/kg) group: mice were orally administered (p.o.) with AFB1 of 0.4 mg/kg from the first day to the third day and orally administered (p.o.) with ZHSF (6.0 g/kg) for 7 d before the sample harvesting. Blood routine analysis was used to analyze the blood cell composition in control group, model group, positive group and ZHSF groups. ZHSF: Zhizi Huangqi Shanzha formula; AFB1: aflatoxin B1; Lym: lymphocyte; Mon: monocyte; Eos: eosinophils; Neu: neutrophils. All data was measured by one-way analysis, and Newman-Keuls test was performed for inter-group comparisons. All data was presented as mean ± standard deviation (n = 5). aP<0.05 compared to the model group.

Figure 2 Effect of ZHSF on liver function and oxidative stress of AFB1 poisoning mice A: hematoxylin-eosin staining was used to analyze morphological and histological of liver in control group, model group, positive group and ZHSF groups. A1: Control group; A2: Model group; A3: Positive group; A4: ZHSF (1.5 g/kg) group; A5: ZHSF (3.0 g/kg) group; A6: ZHSF (6.0 g/kg) group. B: result of ALT; C: result of AST; D: result of SOD; E: result of MDA. Control group: Mice were orally administered (p.o.) with saline for 10 d. Model group: mice were orally administered (p.o.) with AFB1 of 0.4 mg/kg from the first day to the third day and orally administered (p.o.) with saline for 7 d before the sample harvesting. AFB1 + ZHSF (1.5 g/kg) group: mice were orally administered (p.o.) with AFB1 of 0.4 mg/kg from the first day to the third day and orally administered (p.o.) with ZHSF (1.5 g/kg) for 7 d before the sample harvesting. AFB1 + ZHSF (3.0 g/kg) group: mice were orally administered (p.o.) with AFB1 of 0.4 mg/kg from the first day to the third day and orally administered (p.o.) with ZHSF (3.0 g/kg) for 7 d before the sample harvesting. AFB1 + ZHSF (6.0 g/kg) group: mice were orally administered (p.o.) with AFB1 of 0.4 mg/kg from the first day to the third day and orally administered (p.o.) with ZHSF (6.0 g/kg) for 7 d before the sample harvesting. ZHSF: Zhizi Huangqi Shanzha formula; AFB1: aflatoxin B1; ALT: alanine aminotransferase; AST: aspartate aminotransferase; SOD: superoxide dismutase; MDA: malondialdehyde. All data was measured by one-way analysis, and Newman-Keuls test was performed for inter-group comparisons. All data was presented as mean ± standard deviation (n = 5). aP<0.05 compared to the model group.
| Item | Control group | Model group | Positive group | ZHSF (g/kg) | ||
|---|---|---|---|---|---|---|
| 1.5 | 3 | 6 | ||||
| IgG (μg/mL) | 1556±65a | 1259±118 | 1625±96a | 1508±31a | 1323±106a | 1325±144 |
| IgM (μg/mL) | 223±37a | 92±21 | 129±31 | 159±13a | 117±16a | 128±12a |
Table 1 Effect of ZHSF on the content of serum IgG and IgM mice poisoned with AFB1
| Item | Control group | Model group | Positive group | ZHSF (g/kg) | ||
|---|---|---|---|---|---|---|
| 1.5 | 3 | 6 | ||||
| IgG (μg/mL) | 1556±65a | 1259±118 | 1625±96a | 1508±31a | 1323±106a | 1325±144 |
| IgM (μg/mL) | 223±37a | 92±21 | 129±31 | 159±13a | 117±16a | 128±12a |
| 1. |
Wang Q, Yang Q, Wu W. Progress on structured biosensors for monitoring Aflatoxin B1 from biofilms: a review. Front Microbiol 2020; 11: 408.
DOI PMID |
| 2. | Ma X, Wang W, Chen X, et al. Selection, identification, and application of Aflatoxin B1 aptamer. Eur Food Res Technol 2014; 238: 919-25. |
| 3. | Huang WY, Cao Z, Yao QC, et al. Mitochondrial damage are involved in Aflatoxin B-1-induced testicular damage and spermatogenesis disorder in mice. Sci Total Environ 2020; 701: 135077. |
| 4. | Xu FB, Wang PY, Yao QC, et al. Lycopene alleviates AFB1-induced immunosuppression by inhibiting oxidative stress and apoptosis in the spleen of mice. Food Funct 2019; 10: 3868-79. |
| 5. | Guo Y, Zhao L, Ma Q, et al. Novel strategies for degradation of aflatoxins in food and feed: a review. Food Res Int 2020; 140: 109878. |
| 6. | Yan H, Ge J, Gao H, et al. Melatonin attenuates AFB1-induced cardiotoxicity via the NLRP3 signaling pathway. J Int Med Res 2020; 48:1-13. |
| 7. |
Rushing BR, Selim MI. Aflatoxin B1: a review on metabolism, toxicity, occurrence in food, occupational exposure, and detoxification methods. Food Chem Toxicol 2019; 124: 81-100.
DOI PMID |
| 8. | Guo C, Liu Y, Wang Y, et al. PINK1/Parkin-mediated mitophagy is activated to protect against AFB1-induced immunosuppression in mice spleen. Toxicol Lett 2022; 366: 33-44, 2022. |
| 9. |
Rushing BR, Selim MI. Aflatoxin B1: a review on metabolism, toxicity, occurrence in food, occupational exposure, and detoxification methods. Food Chem Toxicol 2019; 124: 81-100.
DOI PMID |
| 10. | Li H, Li S, Yang H, et al. L-Proline alleviates kidney injury caused by AFB1 and AFM1 through regulating excessive apoptosis of kidney cells. Toxins (Basel) 2019; 11: 226. |
| 11. |
Ge J, Yu H, Li J, et al. Assessment of aflatoxin B1 myocardial toxicity in rats: mitochondrial damage and cellular apoptosis in cardiomyocytes induced by aflatoxin B1. J Int Med Res 2017; 45: 1015-23.
DOI PMID |
| 12. | Sun LH. Selenium deficiency aggravates aflatoxin B1-induced immunotoxicity in chick spleen by regulating 6 selenoprotein genes and redox/inflammation/apoptotic signaling. J Nut 2019; 149: 894-901. |
| 13. |
Souto NS, Claudia Monteiro Braga A, Lutchemeyer de Freitas M, et al. Aflatoxin B1 reduces non-enzymatic antioxidant defenses and increases protein kinase C activation in the cerebral cortex of young rats. Nutr Neurosci 2018; 21: 268-75.
DOI PMID |
| 14. | Ahmad KA, Ze H, Chen J, et al. The protective effects of a novel synthetic beta-elemene derivative on human umbilical vein endothelial cells against oxidative stress-induced injury: Involvement of antioxidation and PI3k/Akt/eNOS/NO signaling pathways. Biomed Pharmacother 2018; 106: 1734-41. |
| 15. | Xu F, Wang P, Yao Q, et al. Lycopene alleviates AFB1-induced immunosuppression by inhibiting oxidative stress and apoptosis in the spleen of mice. Food Funct 2019; 10: 3868-79. |
| 16. | Li M, Kong Y, Guo W, et al. Dietary aflatoxin B 1 caused the growth inhibition, and activated oxidative stress and endoplasmic reticulum stress pathway, inducing apoptosis and inflammation in the liver of northern snakehead (Channa argus). Sci Tot Enviro 2022; 850: 157997. |
| 17. | Tian J, Qin S, Han J, et al. A review of the ethnopharmacology, phytochemistry, pharmacology and toxicology of Fructus Gardeniae (Zhi-zi). J Ethnopharmacol 2022; 289: 114984. |
| 18. | Chang X, Chen X, Guo Y, et al. Advances in chemical composition, extraction techniques, analytical methods, and biological activity of Astragali Radix. Molecules 2022; 27: 1058. |
| 19. | Li H, Yang B. Studies on processing of Fructus Crataegi. Zhong Guo Zhong Yao Za Zhi 2004; 29: 501-4. |
| 20. | Sun CB, Zhang N, Xu GP, et al. Anti-tumor and immunomodulation activity of polysaccharides from Dendrobium officinale in S 180 tumor-bearing mice. J Funct Foods 2022; 94: 105105. |
| 21. |
Caporaso JG, Kuczynski J, Stombaugh J, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods 2010; 7: 335-6.
DOI PMID |
| 22. |
Fukui H. Leaky Gut and Gut-Liver axis in liver cirrhosis: clinical studies update. Gut Liver 2021; 15: 666-76.
DOI PMID |
| 23. | Chen J, Zhang YL, Liu ZJ. Progress in the study of mechanisms related to the hepatic-intestinal axis. J Mod Med Health 2014; 30: 3405-8. |
| 24. | Cheng Z, Yang L, Chu H. The gut microbiota: a novel player in autoimmune hepatitis. Front Cell Infect Microbiol 2022; 12: 947382. |
| 25. | Li Y, Liu WC, Chang B. Intestinal virome: an important research direction for alcoholic and nonalcoholic liver diseases. World J Gastroenterol 2022; 28: 3279-81. |
| 26. | Wang T, Rong X, Zhao C. Circadian rhythms coordinated with gut microbiota partially account for individual differences in hepatitis B-related cirrhosis. Front Cell Infect Mi 2022; 12: 936815. |
| 27. | Li S, Han W, He Q. Relationship between intestinal microflora and hepatocellular cancer based on gut-liver axis theory. Contrast Media Mol 2022; 6533628. |
| 28. | Xia T, Fang B, Kang C, et al. Hepatoprotective mechanism of ginsenoside Rg 1 against alcoholic liver damage based on gut microbiota and network pharmacology. Oxid Med Cell Longev 2022; 2022: 5025237. |
| 29. | Gu D, Zhou S, Yao L, et al. Effects of Shenling Baizhu San supplementation on gut microbiota and oxidative stress in rats with ulcerative colitis. Evid-Based Compl Alt 2021; 2021: 3960989. |
| [1] | XIA Xichao, XUE Shipeng, SONG Guoying, LI Bin, WANG Huiping, QIU Ju, Wang Jihong, LIU Qingchun, MA Yuhong, OUYANG Jingfeng. Anti-oxidative and immunological role of Cyclocarya paliurus polysaccharide on the liver injury of diabetic rats [J]. Journal of Traditional Chinese Medicine, 2024, 44(6): 1146-1152. |
| [2] | WANG Yiying, LIU Jianjun, XIONG Yongjian, ZHANG Yongli, WEN Yuqi, XUE Mengli, GUO Huishu, QIU Juanjuan. Analysis of composition of gut microbial community in a rat model of functional dyspepsia treated with Simo Tang (四磨汤) [J]. Journal of Traditional Chinese Medicine, 2024, 44(6): 1168-1176. |
| [3] | LIAO Mengting, LI Tao, CHU Fuhao, CHEN Yan, LOU Ni, ZHUANG Yuan, BO Rongqiang, DING Xia. Weichang’ an pill (胃肠安丸) alleviates functional dyspepsia through modulating brain-gut peptides and gut microbiota [J]. Journal of Traditional Chinese Medicine, 2024, 44(6): 1177-1186. |
| [4] | HUANG Xiaona, LI Yuzhen, ZHU Chenyang, ZHU Hengzhou, JIANG Chenyu, ZHU Xiaodan, ZHANG Chencen, JIN Chunhui. Weitiao No. 3 (微调3号方) enhances the efficacy of anti-programmed cell death protein-1 immunotherapy by modulating the intestinal microbiota in an orthotopic model of gastric cancer mice [J]. Journal of Traditional Chinese Medicine, 2024, 44(5): 906-915. |
| [5] | SUN Linjuan, LI Chengfu, LIU Jiangang, LI Nannan, HAN Fuhua, QIAO Dandan, TAO Zhuang, ZHAN Min, CHEN Wenjie, ZHANG Xiaohui, TONG Chenguang, CHEN Dong, Qi Jiangxia, LIU Yang, LIANG Xiao, ZHENG Xiaoying, ZHANG Yunling. Efficacy of Sailuotong (塞络通) on neurovascular unit in amyloid precursor protein/presenilin-1 transgenic mice with Alzheimer’s disease [J]. Journal of Traditional Chinese Medicine, 2024, 44(2): 289-302. |
| [6] | REN Li, HAI Yang, YANG Xue, LUO Xianqin. Yemazhui (Herba Eupatorii Lindleyani) ameliorates lipopolysaccharide-induced acute lung injury via modulation of the toll-like receptor 4/nuclear factor kappa-B/nod-like receptor family pyrin domain-containing 3 protein signaling pathway and intestinal flora in rats [J]. Journal of Traditional Chinese Medicine, 2024, 44(2): 303-314. |
| [7] | LI Chaoran, YANG Yan, FENG Chuwen, LI Heng, QU Yuanyuan, WANG Yulin, WANG Delong, WANG Qingyong, GUO Jing, SHI Tianyu, SUN Xiaowei, WANG Xue, HOU Yunlong, SUN Zhongren, YANG Tiansong. Integrated 'omics analysis for the gut microbiota response to moxibustion in a rat model of chronic fatigue syndrome [J]. Journal of Traditional Chinese Medicine, 2023, 43(6): 1176-1189. |
| [8] | ZHOU Jun, WANG Junhua, LI Xiaobing, WAN Chenyi, LI Fangjun, Lü Yanni, CHEN Hao, SUN Meiying. Efficacy of Heshouwu (Radix Polygoni Multiflori) on gut mircobiota in mice with autoimmune encephalomyelitis [J]. Journal of Traditional Chinese Medicine, 2023, 43(4): 676-685. |
| [9] | JIANG Yiqian, ZHOU Xibin, PU Wenyuan, ZHOU Chunxiang. Sanwu Baisan decoction (三物白散) inhibits colorectal cancer progression in mice by remodeling gut microbiota and tumorigenesis [J]. Journal of Traditional Chinese Medicine, 2023, 43(3): 466-473. |
| [10] | YANG Yang, YUAN Haining, JIA Hongxiao, NING Yanzhe, WANG Di, ZHANG Lei, YAN Kaijuan, GUO Yumeng, WANG Fei, SUN Weishuang, CHEN Pei. Therapy of replenishing Yin and regulating Yang for manic episode in bipolar disorder: study protocol for a prospective, double-blind, randomized controlled trial [J]. Journal of Traditional Chinese Medicine, 2023, 43(3): 594-601. |
| [11] | SUN Mengzhu, ZHANG Yujie, SONG Yafang, GUO Jing, ZHAO Tingting, WANG Yuhang, PEI Lixia, SUN Jianhua. Electroacupuncture at Tianshu (ST25) and Zusanli (ST36) alleviates stress-induced irritable bowel syndrome in mice by modulating gut microbiota and corticotropin-releasing factor [J]. Journal of Traditional Chinese Medicine, 2022, 42(5): 732-740. |
| [12] | YU Zeyue, HAO Liyu, LI Zongyuan, SUN Jianhui, CHEN Hongying, HUO Hairu, LI Xiaoqin, SHAN Zhongchao, LI Hongmei. Correlation between slow transit constipation and spleen Qi deficiency, and gut microbiota: a pilot study [J]. Journal of Traditional Chinese Medicine, 2022, 42(3): 353-363. |
| [13] | Huixiang ZHANG, Limei WANG, Jipeng GUO, Jiai WANG, Qianqian ZHANG, Yutao WANG, Xun LIU, Lihuan ZHANG, Lanlan SHI, Hongxiang WU, Xue CAO. Gut microbiota and differential genes-maintained homeostasis is key to maintaining health of individuals with Yang-deficiency constitution [J]. Journal of Traditional Chinese Medicine, 2022, 42(1): 96-101. |
| [14] | PAN Lijia, MA Shuya, WEN Jing, ZHANG Xiaoqi, XING Haijiao, JIA Chunsheng. Direct contact moxibustion promotes apoptosis of gastric cancer cells in rats by regulating intestinal flora [J]. Journal of Traditional Chinese Medicine, 2021, 41(6): 943-952. |
| [15] | Abdelouafi Benmouloud. In vivo anti-diarrheal activity of jujube honey on castor oil-induced diarrhea in mice [J]. Journal of Traditional Chinese Medicine, 2021, 41(6): 900-908. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||

