Journal of Traditional Chinese Medicine ›› 2024, Vol. 44 ›› Issue (5): 916-925.DOI: 10.19852/j.cnki.jtcm.20240719.001
Previous Articles Next Articles
Huaiyu pill (槐榆片) alleviates inflammatory bowel disease in mice via blocking toll like receptor 4/ myeloid differentiation primary response gene 88/ nuclear factor kappa B subunit 1 pathway
YANG Chunyan1, LUO Jia1, PENG Weijie2, DAI Weibo1( )
)
- 1 Department of Pharmacy, Zhongshan Hospital of Traditional Chinese Medicine, Guangzhou University of Traditional Chinese Medicine, Zhongshan 528400, China
2 Department of Pharmacy, Shenshan Medical Center Memorial Hospital of Sun Yat-Sen University Sun Yat-Sen University, Shanwei 516600, China
-
Received:2024-01-22Accepted:2024-05-15Online:2024-10-15Published:2024-07-19 -
Contact:DAI Weibo, Department of Pharmacy, Zhongshan Hospital of Traditional Chinese Medicine Affiliated to Guangzhou University of Traditional Chinese Medicine, Zhongshan 528400, China. daiweibo007@163.com Telephone: +86-15014506263 -
Supported by:Mechanistic Study on the Regulation of Mucin 2/interleukin 6-signal Transducer and Activator of Transcription 3 Signaling Pathway by Active Saponin Ardisiacrispin B in Improving Ulcerative Colitis Intestinal Mucosal Barrier Dysfunction(A2022479);Mechanistic Study on the Regulation of Inflammatory Microenvironment and Improvement of Ulcerative Colitis by Lingnan Traditional Medicine Ficus Quercifolia through Wilms' tumor 1-associating Protein-Mediated RNA Methyltransferase Promoting Toll Like Receptor 4 m6A Modification(2023A1515011699)
Cite this article
YANG Chunyan, LUO Jia, PENG Weijie, DAI Weibo. Huaiyu pill (槐榆片) alleviates inflammatory bowel disease in mice via blocking toll like receptor 4/ myeloid differentiation primary response gene 88/ nuclear factor kappa B subunit 1 pathway[J]. Journal of Traditional Chinese Medicine, 2024, 44(5): 916-925.
share this article
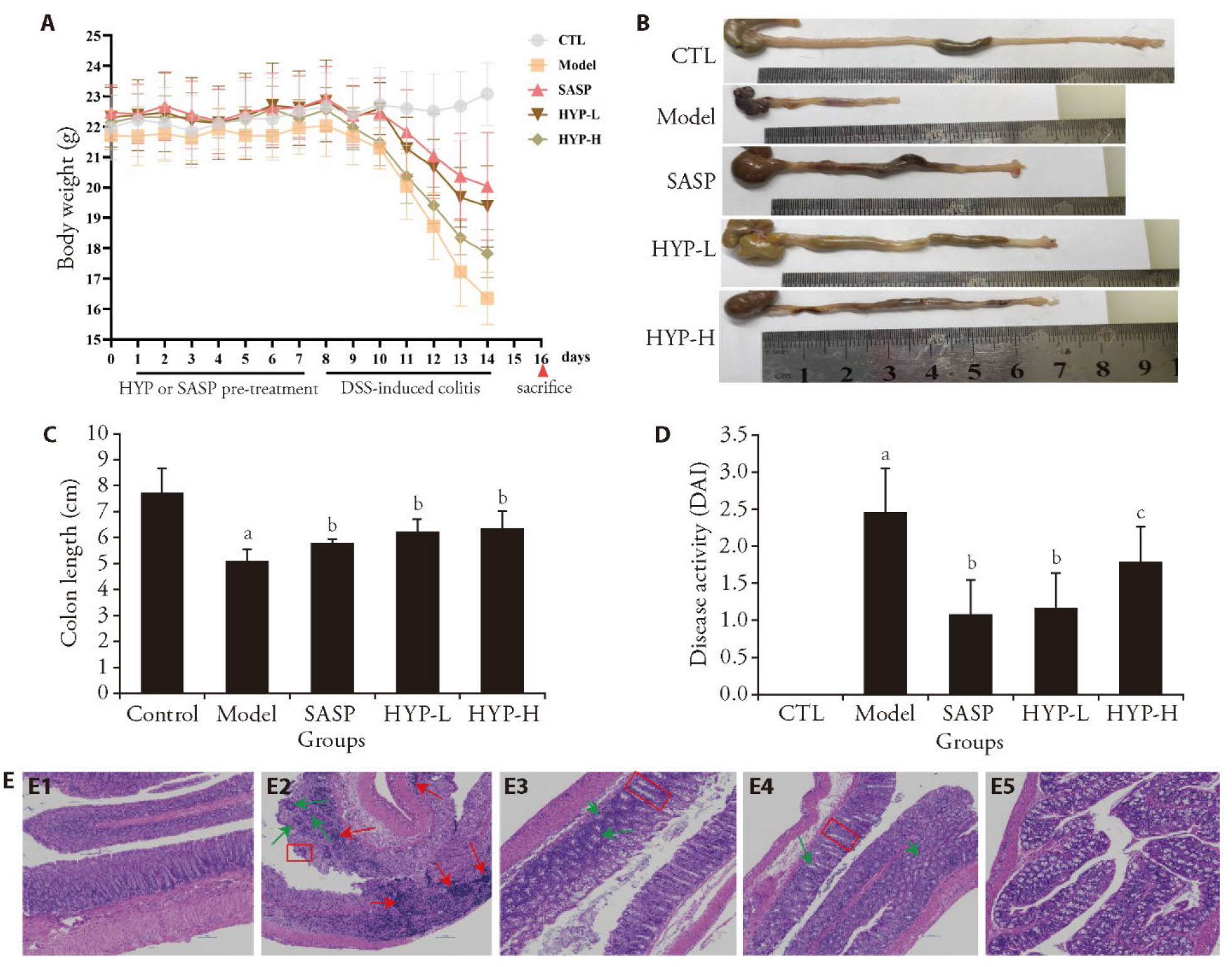
Figure 1 HYP relieved DSS-induced IBD symptoms in mice A: experimental design of the animal study and the time-course of changes in body weight. B: representative colon images in each group. C: the colon length in each group. D: the DAI in different group. E: colon tissue with hematoxylin-eosin staining (× 200, scale bars = 100 μm, the red box indicates the intestinal crypt structure, the red arrow indicates the inflammatory cells infiltration, and the green arrow indicates the glandular structure); E1: colon tissues of the control group; E2: colon tissues of the model group; E3: colon tissues of the SASP group; E4: colon tissues of the HYP low-dose group; E5: colon tissues of the HYP high-dose group. CTL: normal control group (fed on standard chow); Model: model group (DSS + standard chow); SASP: positive control group (DSS + 40 mg/20 mL/kg SASP); HYP-L: low-dose group (DSS + 25 g/20 mL/kg HYP); HYP-H: high-dose HYP group (DSS + 50 g/20 mL/kg HYP). HYP: Huaiyu pill; DSS: dextran sulfate sodium; IBD: inflammatory bowel disease; SASP: sulfasalazine. Student t-test and one-way analysis of variance were used for comparison analysis. Statistical significance between the groups was determined by Dunnett's T3 analysis. The data were illustrate d in mean ± standard deviation (n = 8). aP<0.01 compared with the control group; bP<0.01 compared with the model group; cP<0.05 compared with the model group.
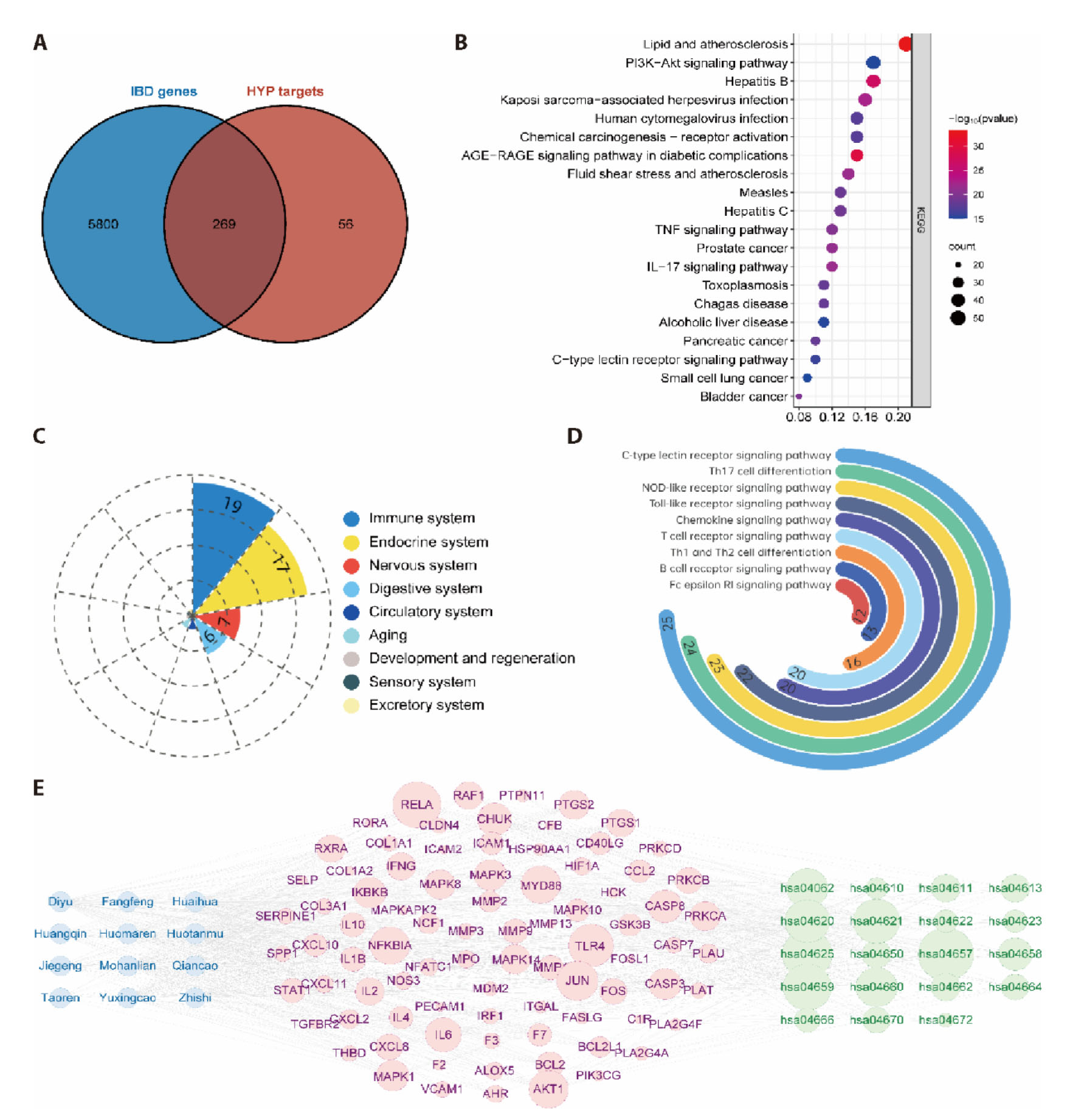
Figure 2 Examination of the molecular mechanisms underlying the therapeutic efficacy of HYP A: venn diagram illustrates the shared targets between HYP and IBD. B: KEGG enrichment analysis of shared targets; C: functional module statistics for KEGG enrichment results; D: the pathways enriched for the top 10 number of gene counts within the immune system; E: compounds-targets-pathways network in IBD. HYP: Huaiyu pill; IBD: inflammatory bowel disease; KEGG: Kyoto Encyclopedia of Genes and Genomes.
| Group | n | IL-1β | IL-6 | IL-17 | TNF-α |
|---|---|---|---|---|---|
| CTL | 8 | 6±5 | 15±4 | 31±9 | 25±5 |
| Model | 8 | 24±13a | 28±7a | 68±12a | 50±11a |
| SASP | 8 | 11±4b | 18±4b | 31±5b | 27±5b |
| HYP-L | 8 | 16±7c | 20±7b | 31±4b | 33±8b |
| HYP-H | 8 | 13±7b | 17±5b | 31±6b | 28±9b |
Table 1 Effect of HYP on mice colon tissues inflammatory cytokines levels (pg/mg, $\bar{x}±s$)
| Group | n | IL-1β | IL-6 | IL-17 | TNF-α |
|---|---|---|---|---|---|
| CTL | 8 | 6±5 | 15±4 | 31±9 | 25±5 |
| Model | 8 | 24±13a | 28±7a | 68±12a | 50±11a |
| SASP | 8 | 11±4b | 18±4b | 31±5b | 27±5b |
| HYP-L | 8 | 16±7c | 20±7b | 31±4b | 33±8b |
| HYP-H | 8 | 13±7b | 17±5b | 31±6b | 28±9b |

Figure 3 HYP attenuates intestinal damage caused by increased intestinal permeability in the DSS-induced IBD mouse model A: representative western blotting brands of ZO-1 and occludin in the colon tissues; B: normalized against β-actin; B1: quantitative analysis of ZO-1; B2: quantitative analysis of occluding; C: the contents of ET-1 and EPO in colon tissues were measured by ELISA; C1: the contents of ET-1 in colon tissues; C2: the contents of EPO in colon tissues; D: Mean density of MUC2 protein in colon tissues; E: Representative images of MUC2 immunostaining of colon tissues in each group (× 200, bar = 100 μm); E1: Colon tissues of the control group; E2: Colon tissues of the model group; E3: Colon tissues of the SASP group; E4: Colon tissues of the HYP low-dose group; E5: Colon tissues of the HYP high-dose group. CTL: normal control group (fed on standard chow); Model: model group (DSS + standard chow); SASP: positive control group (DSS + 40 mg/20 mL/kg SASP); HYP-L: low-dose group (DSS + 25 g/20 mL/kg HYP); HYP-H: high-dose HYP group (DSS + 50 g/20 mL/kg HYP). HYP: Huaiyu pill; DSS: dextran sulfate sodium; SASP: sulfasalazine; ZO-1: occludens 1; ET-1: endothelin 1; EPO: erythropoietin; MUC2: mucin 2. Student t-test and one-way analysis of variance were used for comparison analysis. Statistical significance between the groups was determined by the least significant difference method. The data was illustrated in mean ± standard deviation (n = 3). aP<0.01 and dP<0.05 compared with the control group; bP<0.01 and cP<0.05 compared with the model group.

Figure 4 HYP suppresses intestinal inflammation and damage by inhibiting TLR4/MyD88/NF-κB signaling pathway A: the expressions of TLR4, MYD88 and NF-κB p65 in colon tissue of mice by western blotting; B: normalized against β-actin; B1: quantitative analysis of TLR4; B2: quantitative analysis of MYD88; B3: quantitative analysis of NF-κB p65; C: mean density of TLR4, NF-κB p65 and MYD88 protein in colon tissues; C1: mean density of TLR4 protein in colon tissues. C2: mean density of MYD88 protein in colon tissues. C3: mean density of NF-κB p65 protein in colon tissues; D: representative images of TLR4 immunostaining of colon tissues in each group (× 200, bar = 100 μm); E: representative images of MYD88 immunostaining of colon tissues in each group (× 200, bar = 100 μm); F: representative images of NF-κB p65 immunostaining of colon tissues in each group (× 200, bar = 100 μm); D1, E1, F1: colon tissues of the control group; D2, E2, F2: colon tissues of the model group; D3, E3, F3: colon tissues of the SASP group; D4, E4, F4: colon tissue of the HYP low-dose group; D5, E5, F5: colon tissues of the HYP high-dose group. CTL: normal control group (fed on standard chow); Model: model group (DSS + standard chow); SASP: positive control group (DSS + 40 mg/20 mL/kg SASP); HYP-L: low-dose group (DSS + 25 g/20 mL/kg HYP); HYP-H: high-dose HYP group (DSS + 50 g/20 mL/kg HYP). HYP: Huaiyu pill; DSS: dextran sulfate sodium; SASP: sulfasalazine; TLR4: toll like receptor 4; MYD88: myeloid differentiation primary response gene 88; NF-κB p65: nuclear factor kappa B p65 subunit. Student t-test and one-way analysis of variance were used for comparison analysis. Statistical significance between the groups was determined by the least significant difference method. The data was illustrated in mean ± standard deviation (n = 3). aP<0.01 compared with the control group; bP<0.01 and cP<0.05 compared with the model group.
| 1. | Wang HH, He Y, Wang HX, et al. Comparison of TPMT and NUDT15 polymorphisms in Chinese patients with inflammatory bowel disease. World J Gastroenterol 2018; 24: 941-8. |
| 2. | Li L, Sun LL, Qiu Y, Zhu WJ, Hu KY, Mao JQ. Protective effect of stachydrine against cerebral ischemia-reperfusion injury by reducing inflammation and apoptosis through P65 and JAK2/STAT3 signaling pathway. Front Pharmacol 2020; 18: 64. |
| 3. |
Dmochowska N, Tieu W, Keller MD, et al. Zr-89-pro-MMP-9 F(ab ')(2) detects colitis induced intestinal and kidney fibrosis. Sci Rep 2020; 10: 20372.
DOI PMID |
| 4. | Hou X, Zhu FF, Zheng WW, et al. Protective effect of schistosoma japonicum eggs on TNBS-induced colitis is associated with regulating Treg/Th 17 balance and reprogramming glycolipid metabolism in mice. Front Cell Infect Mi 2022; 11: 1028899. |
| 5. | Agista AZ, Rusbana TB, Islam J, et al. Fermented rice bran sup-plementation prevents the development of intestinal fibrosis due to DSS-induced inflammation in mice. Nutrients 2021; 13: 1869. |
| 6. | Lefevre PLC, Vande CN. Clinical pharmacology of janus kinase inhibitors in inflammatory bowel disease. J Crohns Colitis 2020; 14: 725-36. |
| 7. | Bajic JE, Eden GL, Lampton LS, et al. Rhubarb extract partially improves mucosal integrity in chemotherapy-induced intestinal mucositis. World J Gastroenterol 2016; 22: 8322-33. |
| 8. | Wan F, Wang M, Zhong R, et al. Supplementation with Chinese medicinal plant extracts from lonicera hypoglauca and scutellaria baicalensis mitigates colonic inflammation by regulating oxidative stress and gut microbiota in a colitis mouse model. Front Cell Infect Microbiol 2021; 11: 798052. |
| 9. | Liu Y, Huang W, Ji SY, Wang J, Luo JY, Lu BY. Sophora japonica flowers and their main phytochemical, rutin, regulate chemically induced murine colitis in association with targeting the NF-κB signaling pathway and gut microbiota. Food Chem 2022; 393: 133395. |
| 10. | Chen JF, Tan L, Ju F, et al. Phenolic glycosides from sanguisorba officinalis and their anti-inflammatory effects. Nat Prod Res 2022; 36: 2097-104. |
| 11. | Shen JH, Ma XH, He YB, Wang YJ, Zhong TH, Zhang YH. Anti-inflammatory and anti-oxidant properties of melianodiol on DSS-induced ulcerative colitis in mice. PeerJ 2022; 10: 14209. |
| 12. | Zhou SZ, Yang Z, Liu JX, Ran MJ. TIPE-2 ameliorates inflammatory bowel disease in mice via inhibiting STAT3 and NF-kB activation. Immunol Lett 2023; 255: 32-9. |
| 13. | Dai Y, Lu Q, Li P, et al. Xianglian pill attenuates ulcerative colitis through TLR4/MyD88/NF-κB signaling pathway. J Ethnopharmacol 2023; 10: 115690. |
| 14. | Ojha D, Mukherjee H, Mondal S, et al. Anti-inflammatory activity of odina wodier roxb, an Indian folk remedy, through inhibition of toll-like receptor 4 signaling pathway. PLoS One 2023; 18: 288420. |
| 15. |
Zhu L, Gu P, Shen H. Protective effects of berberine hydrochloride on DSS-induced ulcerative colitis in rats. Int Immunopharmacol 2019; 68: 242-51.
DOI PMID |
| 16. |
Rashidian A, Muhammadnejad A, Dehpour AR, et al. Atorvastatin attenuates TNBS-induced rat colitis: the involvement of the TLR4/NF-kB signaling pathway. Inflammopharmacology 2016; 24: 109-18.
DOI PMID |
| 17. |
Chen T, Hu SH, Zhang HW, Guan QF, Yang YH, Wang XM. Anti-inflammatory effects of dioscorea alata L. anthocyanins in a TNBS-induced colitis model. Food Funct 2017; 8: 659-69.
DOI PMID |
| 18. | Novak G, Parker CE, Pai RK, et al. Histologic scoring indices for evaluation of disease activity in crohn's disease. Cochrane Database Syst Rev 2017; 7: 12351. |
| 19. |
Shimizu H, Suzuki K, Watanabe M, Okamoto R. Stem cell-based therapy for inflammatory bowel disease. Intest Res 2019; 17: 311-6.
DOI PMID |
| 20. | Koetzner L, Grover G, Boulet J, Jacoby HI. Plant-derived polysaccharide supplements inhibit dextran sulfate sodium-induced colitis in the rat. Dig Dis Sci 2020; 55: 1278-85. |
| 21. |
Strubberg AM, Madison BB. MicroRNAs in the etiology of colorectal cancer: pathways and clinical implications. Dis Model Mech 2017; 10: 197-214.
DOI PMID |
| 22. | Yu SC, Zhang MX, Ye ZF, Wang YL, Wang X, Chen YG. Development of a 32-gene signature using machine learning for accurate prediction of inflammatory bowel disease. Cell Regen 2023; 12: 8. |
| 23. | Hwang YJ, Nam SJ, Chun WJ, et al. Anti-inflammatory effects of apocynin on dextran sulfate sodium-induced mouse colitis model. PLoS One 2019; 14: 217642. |
| 24. | Wang Y, Lin JX, Cheng ZY, Wang TC, Chen J, Long M. Bacillus coagulans TL3 Inhibits LPS-induced caecum damage in rat by regulating the TLR4/MyD88/NF-kappaB and Nrf2 signal pathways and modulating intestinal microflora. Oxid Med Cell Longev 2022; 2022: 5463290. |
| 25. | Liu F, Zhang XS, Ji Y. Total flavonoid extract from hawthorn (Crataegus pinnatifida) improves inflammatory cytokines-evoked epithelial barrier deficit. Med Sci Monit 2020; 26: 920170. |
| 26. | Lu QQ, Li JX, Ding P, et al. Qingchang wenzhong eecoction alleviates DSS-induced inflammatory bowel disease by inhibiting M1 macrophage polarization in vitro and in vivo. Biomed Res Int 2022; 2022: 9427076. |
| 27. | Zhang JL, Chen YT, Chen GD, Wang T, Zhang JX, Zeng QY. Glucose-insulin-potassium alleviates intestinal mucosal barrier injuries involving decreased expression of uncoupling protein 2 and NLR family-pyrin domain-containing 3 inflammasome in polymicrobial sepsis. Biomed Res Int 2017; 2017: 4702067. |
| 28. | Shen Q, Wei XM, Hu JN, et al. Saponins from platycodon grandiflorum reduces cisplatin-induced intestinal toxicity in mice through endoplasmic reticulum stress-activated apoptosis. Am J Chin Med 2022; 50: 1927-44. |
| 29. |
Zhang LL, Wang F, Wang JJ, Wang YS, Fang Y. Intestinal fatty acid-binding protein mediates atherosclerotic progress through increasing intestinal inflammation and permeability. J Cell Mol Med 2020; 24: 5205-12.
DOI PMID |
| 30. |
Huda MN, Ahmad SM, Kalanetra KM, et al. Neonatal vitamin a supplementation and vitamin a status are associated with gut microbiome composition in bangladeshi infants in early infancy and at 2 years of age. J Nutr 2019; 149: 1075-88.
DOI PMID |
| 31. | Sang XQ, Wang QY, Ning YY, et al. Age-related mucus barrier dysfunction in mice is related to the changes in muc2 mucin in the colon. Nutrients 2023; 15: 1830. |
| 32. |
Vahid AF, Lagace DC, Albert PR. Persistent post-stroke depression in mice following unilateral medial prefrontal cortical stroke. Transl Psychiat 2016; 6: 863.
DOI PMID |
| 33. |
Wang ZG, Khor SN, Cai DS. Regulation of muscle and metabolic physiology by hypothalamic erythropoietin independently of its peripheral action. Mol Metab 2020; 32: 56-68.
DOI PMID |
| 34. |
Yang L, Cao H, Sun D, et al. Bone marrow mesenchymal stem cells combine with normothermic machine perfusion to improve rat donor liver quality-the important role of hepatic microcirculation in donation after circulatory death. Cell Tissue Res 2020; 381: 239-54.
DOI PMID |
| 35. | Wang YK, Zhu HB, Wang XJ, Yu Y, Xie JH. Natural food polysaccharides ameliorate inflammatory bowel disease and its mechanisms. Foods 2021; 10: 1288. |
| 36. | Li YP, Huang BH, Yang H, et al. Latexin deficiency in mice up-regulates inflammation and aggravates colitis through HECTD1/Rps3/NF-kappa B pathway. Sci Rep 2020; 10: 9868. |
| 37. | Li M, Wang F, Zhang C, et al. Integrated systematic pharmacology analysis and experimental validation to reveal the mechanism of action of Semen aesculi on inflammatory bowel diseases. J Ethnopharmacol 2022; 298: 115627. |
| 38. | Wang YG, Dou YQ, Feng J, Xu CY, Wang Q. Efficacy of Liangxue Guyuan decoction on radiation-induced intestinal injury in rats via the toll-like receptor 4/myeloid differentiation primary response 88/ nuclear factor-kappa B pathway. J Tradit Chin Med 2021; 41: 254-61. |
| 39. |
Bao X, Li L, Xue X. Flavonoids from scutellaria barbata inhibit activation of tumor-associated macrophages by blocking the toll-like receptor 4/myeloid differentiation factor 88/nuclear factor-κB signaling pathway. J Tradit Chin Med 2019; 39: 160-5.
PMID |
| 40. | Huang XQ, Zhu JQ, Jiang YY, et al. SU5416 attenuated lipopolysaccharide-induced acute lung injury in mice by modulating properties of vascular endothelial cells. Drug Des Devel Ther 2019; 13: 1763-72. |
| 41. | Medunjanin S, Putzier M, Nothen T, et al. DNA-PK: gatekeeper for IKKgamma/NEMO nucleocytoplasmic shuttling in genotoxic stress-induced NF-kappa B activation. Cell Mol Life Sci 2020; 77: 4133-42. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||

