Journal of Traditional Chinese Medicine ›› 2024, Vol. 44 ›› Issue (6): 1177-1186.DOI: 10.19852/j.cnki.jtcm.2024.06.006
• Research Articles • Previous Articles Next Articles
Weichang’ an pill (胃肠安丸) alleviates functional dyspepsia through modulating brain-gut peptides and gut microbiota
LIAO Mengting1, LI Tao2, CHU Fuhao2,3, CHEN Yan2, LOU Ni1, ZHUANG Yuan1, BO Rongqiang1, DING Xia2( )
)
- 1 Department of Internal Medicine, Dongzhimen Hospital, Beijing University of Chinese Medicine, Beijing 100010, China
2 School of Traditional Chinese Medicine, Beijing University of Chinese Medicine, Beijing 100010, China
3 Beijing Research Institute of Chinese Medicine, Beijing University of Chinese Medicine, Beijing 100010, China
-
Received:2024-02-18Accepted:2024-08-21Online:2024-12-15Published:2024-11-12 -
Contact:Prof. Xia Ding, School of Traditional Chinese Medicine, Beijing University of Chinese Medicine, Beijing 100010, China. dingx@bucm.edu.cn Telephone: +86-18811016240 -
Supported by:Horizontal Development Foundation of Beijing University of Chinese Medicine: Research and development of Weicang’an pill in the treatment of functional dyspepsia(2018110031010092)
Cite this article
LIAO Mengting, LI Tao, CHU Fuhao, CHEN Yan, LOU Ni, ZHUANG Yuan, BO Rongqiang, DING Xia. Weichang’ an pill (胃肠安丸) alleviates functional dyspepsia through modulating brain-gut peptides and gut microbiota[J]. Journal of Traditional Chinese Medicine, 2024, 44(6): 1177-1186.
share this article

Figure 1 WCAP improves gastrointestinal motility in FD rats A: representative hematoxylin-eosin staining of stomach samples from each group, Magnification × 200, Scar bar = 100 μm. A1: Control group; A2: Model group; A3: WC1 group; A4: WC2 group; A5: WC3 group; A6: WC4 group; A7: WC5 group; A8: Dom group. B: representative appearance of small intestine in each group. B1: Control group; B2: Model group; B3: WC1 group; B4: WC2 group; B5: WC3 group; B6: WC4 group; B7: WC5 group; B8: Dom group. Control group and Model group were given normal saline for seven consecutive days; WC1 group: FD rat treated with 21.60 mg·kg-1·d-1 WCAP for seven days; WC2 group: FD rat treated with 43.20 mg·kg-1·d-1 WCAP for seven days; WC3 group: FD rat treated with 64.80 mg·kg-1·d-1 WCAP for seven days; WC4 group: FD rat treated with 86.40 mg·kg-1·d-1 WCAP for seven days; WC5 group: FD rat treated with 108.00 mg·kg-1·d-1 WCAP for seven days; Dom group: FD rat treated with 2.70 mg·kg-1·d-1 domperidone for seven days. FD: Functional dyspepsia; WCAP: Weichang’an pill; WC1: WCAP1; WC2: WCAP2; WC3: WCAP3; WC4: WCAP4; WC5: WCAP5.
| Group | n | Gastric residual rates | Intestinal propulsion rate |
|---|---|---|---|
| Control | 10 | 54±6 | 81±5 |
| Model | 10 | 79±6a | 67±5a |
| WC1 | 10 | 68±12b | 74±5b |
| WC2 | 10 | 67±4b | 74±5b |
| WC3 | 10 | 63±14c | 80±5d |
| WC4 | 10 | 67±12b | 76±8c |
| WC5 | 10 | 64±13c | 76±9c |
| Dom | 10 | 63±11c | 77±11c |
Table 1 Gastric residual rate and Intestinal propulsion rate of rats in each group (%,$\bar{x}±s$)
| Group | n | Gastric residual rates | Intestinal propulsion rate |
|---|---|---|---|
| Control | 10 | 54±6 | 81±5 |
| Model | 10 | 79±6a | 67±5a |
| WC1 | 10 | 68±12b | 74±5b |
| WC2 | 10 | 67±4b | 74±5b |
| WC3 | 10 | 63±14c | 80±5d |
| WC4 | 10 | 67±12b | 76±8c |
| WC5 | 10 | 64±13c | 76±9c |
| Dom | 10 | 63±11c | 77±11c |
| Group | n | MTL | GAS | SS | VIP |
|---|---|---|---|---|---|
| Control | 10 | 225±19 | 160±19 | 54±5 | 124±16 |
| Model | 10 | 138±34a | 99±29a | 84±10a | 231±19a |
| WC1 | 10 | 191±39b | 115±27 | 80±6 | 201±24c |
| WC2 | 10 | 193±31b | 117±32 | 76±8d | 187±24b |
| WC3 | 10 | 204±31b | 127±21c | 69±8b | 199±19b |
| WC4 | 10 | 193±27b | 123±18d | 65±9b | 186±18b |
| WC5 | 10 | 194±23b | 140±20b | 62±7b | 162±15b |
| Dom | 10 | 208±34b | 142±16b | 63±8b | 178±18b |
Table 2 Secretion of BGPs in each group (pg/mL, $\bar{x}±s$)
| Group | n | MTL | GAS | SS | VIP |
|---|---|---|---|---|---|
| Control | 10 | 225±19 | 160±19 | 54±5 | 124±16 |
| Model | 10 | 138±34a | 99±29a | 84±10a | 231±19a |
| WC1 | 10 | 191±39b | 115±27 | 80±6 | 201±24c |
| WC2 | 10 | 193±31b | 117±32 | 76±8d | 187±24b |
| WC3 | 10 | 204±31b | 127±21c | 69±8b | 199±19b |
| WC4 | 10 | 193±27b | 123±18d | 65±9b | 186±18b |
| WC5 | 10 | 194±23b | 140±20b | 62±7b | 162±15b |
| Dom | 10 | 208±34b | 142±16b | 63±8b | 178±18b |
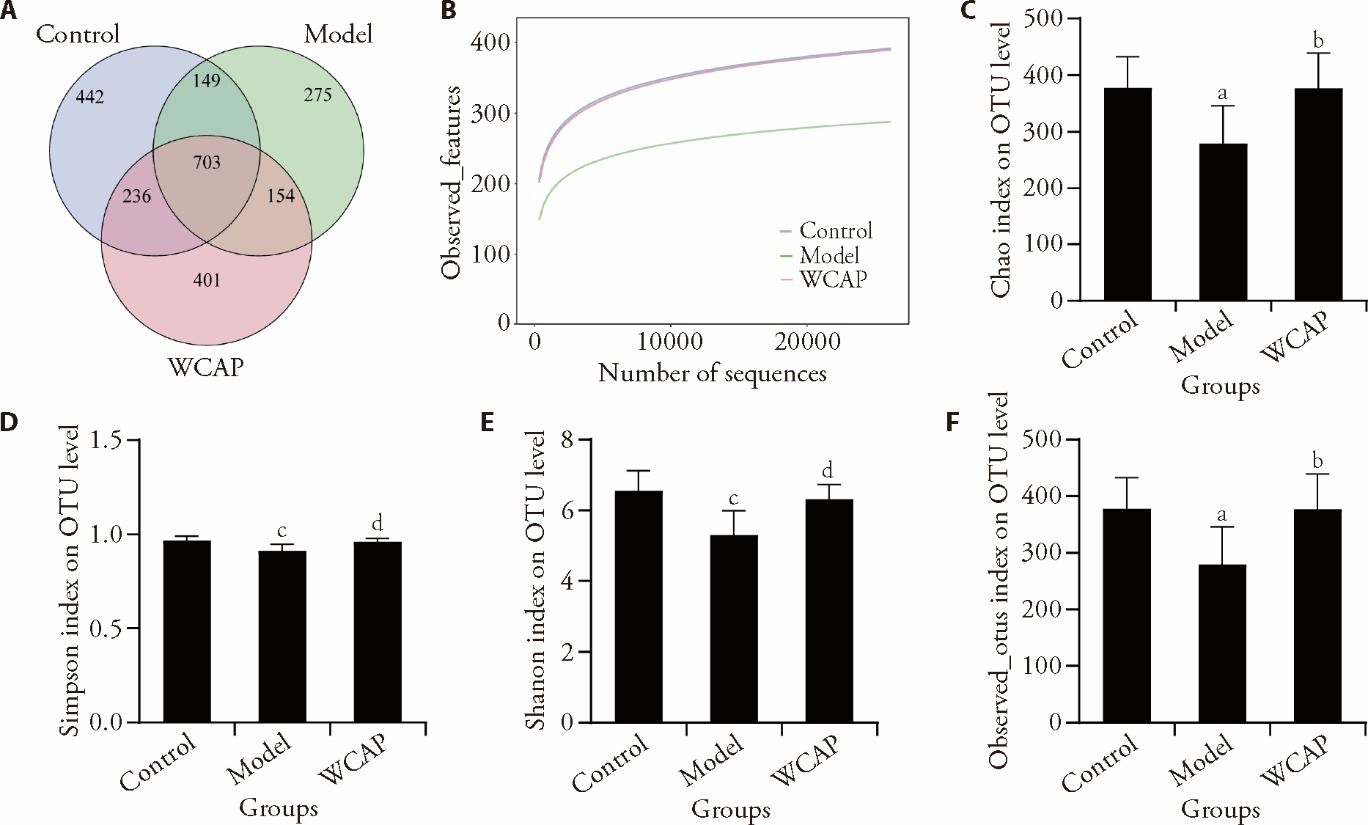
Figure 2 Effect of WCAP on the diversity of gut microbiota in rats with FD A: venn diagram; B: species accumulation curves; C: Chao diversity index; D: simpson diversity index; E: Shannon diversity index; F: observed-species diversity index. Control: FD rat with normal saline; Model: FD rat with normal saline; WCAP: FD rat treated with 64.80 mg·kg-1·d-1 WCAP for seven days. FD: functional dyspepsia; WCAP: Weichang’an pill; OTU: operational taxonomic units. Dates were analyzed by one-way analysis of variance (n = 10). aP < 0.01, cP < 0.001 versus the control group; bP < 0.01, dP < 0.001, versus the model group.

Figure 3 Effect of WCAP on the structure of the gut microbiota in rats with FD A: phylum level species barplot; B: ratio of Firmicutes/Bacteroidetes; C: class level species barplot; D: order level species barplot; E: genus level species barplot; F: partial least squares discriminant analysis diagram of three groups. Control: FD rat with normal saline; Model: FD rat with normal saline; WCAP: FD rat treated with 64.80 mg·kg-1·d-1 WCAP for seven days. FD: functional dyspepsia; WCAP: Weichang’an pill; PLS-DA: partial least squares discriminant analysis; F/B: Firmicutes/Bacteroidetes.
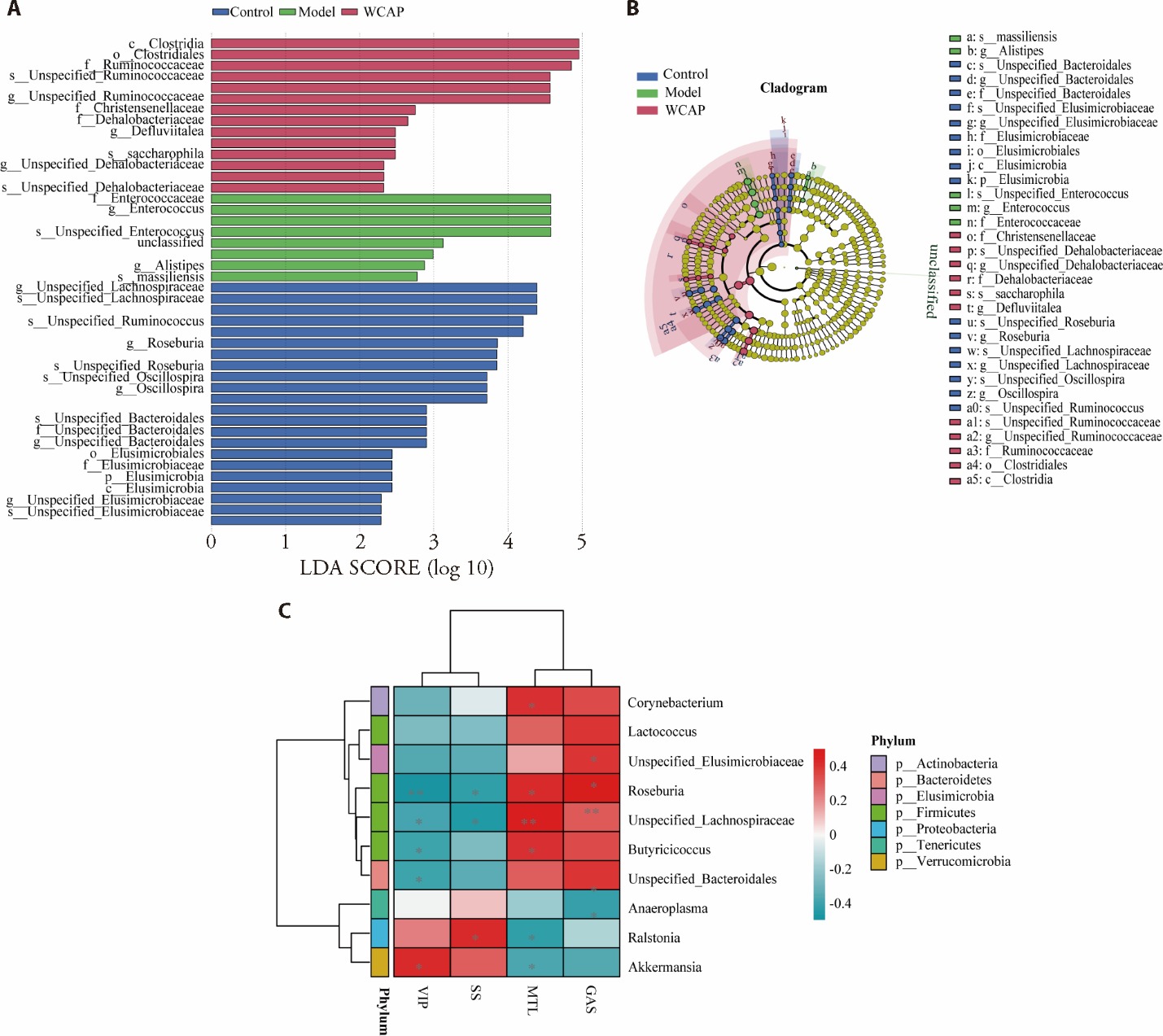
Figure 4 LEfSe analysis and spearman correlation analysis between gut microbiota and BGPs A: bar graph of LEfSe analysis among the control group, the model group and the WCAP group. B: cladogram graph of LEfSe analysis among the control group, the model group and the WCAP group. C: Spearman correlation analysis was performed between the relative abundance of differential bacteria and the levels of MTL, GAS, SS, VIP. Control: FD rat with normal saline; Model: FD rat with normal saline; WCAP: FD rat treated with 64.80 mg·kg-1·d-1 WCAP for seven days. FD: functional dyspepsia; WCAP: Weichang’an pill; MTL: motilin; GAS: gastrin; SS: somatostatin; VIP: vasoactive intestinal peptide; LEfSe: linear discriminant analysis effect size.
| 1. | Gabbard S, Vijayvargiya N. Functional dyspepsia: how to manage the burn and the bloat. Cleve Clin J Med 2024; 91: 301-7. |
| 2. | Miwa H, Nagahara A, Asakawa A, et al. Evidence-based clinical practice guidelines for functional dyspepsia. J Gastroenterol 2022; 57: 47-61. |
| 3. |
Mounsey A, Barzin A, Rietz A. Functional dyspepsia: evaluation and management. Am Fam Physician 2020; 101: 84-8.
PMID |
| 4. |
Sayuk GS, Gyawali CP. Functional dyspepsia: diagnostic and therapeutic approaches. Drugs 2020; 80: 1319-36.
DOI PMID |
| 5. | Labanski A, Langhorst J, Engler H, Elsenbruch S. Stress and the brain-gut axis in functional and chronic-inflammatory gastrointestinal diseases: a transdisciplinary challenge. Psychoneuroendocrinology 2020; 111: 104501. |
| 6. | Rupp SK, Stengel A. Bi-directionality of the microbiota-gut-brain axis in patients with functional dyspepsia: relevance of psychotherapy and probiotics. Front Neurosci 2022; 16: 844564. |
| 7. | Tziatzios G, Gkolfakis P, Papanikolaou IS, et al. Gut microbiota dysbiosis in functional dyspepsia. Microorganisms 2020; 8: 691. |
| 8. | Karakan T, Ozkul C, Küpeli Akkol E, et al. Gut-brain-microbiota axis: antibiotics and functional gastrointestinal disorders. Nutrients 2021; 13: 389. |
| 9. | Mayer EA, Nance K, Chen S. The Gut-brain axis. Annu Rev Med 2022; 73: 439-53. |
| 10. |
Ford AC, Mahadeva S, Carbone MF, Lacy BE, Talley NJ. Functional dyspepsia. Lancet 2020; 396: 1689-702.
DOI PMID |
| 11. | Ho L, Zhong CC, Wong CH, et al. Herbal medicine for functional dyspepsia: network Meta-analysis of placebo-controlled randomised trials. J Ethnopharmacol 2022; 283: 114665. |
| 12. | Luo XY, Wang L, Fang SS, et al. Chinese herbal medicine for functional dyspepsia with psychological disorders: a systematic review and Meta-analysis. Front Neurosci 2022; 16: 933290. |
| 13. | Wang JB, Zhang LS, Niu BH, et al. Efficacy and safety of Weichang’ an pill combined with Western Medicine on gastrointestinal diseases: a systematic review and Meta-analysis. J Tradit Chin Med 2023; 43: 1057-67. |
| 14. | Chen Y, Chu F, Lin J, et al. The mechanisms of action of Weichang’an pill (WCAP) treat diarrhoea-predominant irritable bowel syndrome (IBS-D) using network pharmacology approach and in vivo studies. J Ethnopharmacol 2021; 275: 114119. |
| 15. | Zhang GS, Xie S, Hu W, et al. Effects of electroacupuncture on interstitial cells of cajal (ICC) ultrastructure and connexin 43 protein expression in the gastrointestinal tract of functional dyspepsia (FD) rats. Med Sci Monit 2016; 22: 2021-27. |
| 16. | Pan XL, Zhou L, Wang D, et al. Electroacupuncture at Zusanli (ST36) promotes gastrointestinal motility possibly by suppres-sing excessive autophagy via AMPK/ULK1 signaling in rats with functional dyspepsia. Zhen Ci Yan Jiu 2019; 44: 486-91. |
| 17. |
Callahan BJ, McMurdie PJ, Rosen MJ, et al. DADA2: high-resolution sample inference from illumina amplicon data. Nat Methods 2016; 13: 581-3.
DOI PMID |
| 18. |
Bokulich NA, Kaehler BD, Rideout JR, et al. Optimizing taxonomic classification of marker-gene amplicon sequences with QIIME 2's q2-feature-classifier plugin. Microbiome 2018; 6: 90.
DOI PMID |
| 19. | Rohart F, Gautier B, Singh A, Lê Cao KA. mixOmics: an R package for’omics feature selection and multiple data integration. PLoS Comput Biol 2017; 13: e1005752. |
| 20. | Oshima T. Functional dyspepsia: current understanding and future perspective. Digestion 2024; 105: 26-33. |
| 21. |
Camilleri M, Stanghellini V. Current management strategies and emerging treatments for functional dyspepsia. Nat Rev Gastroenterol Hepatol 2013; 10: 187-94.
DOI PMID |
| 22. |
Agirman G, Yu KB, Hsiao EY. Signaling inflammation across the gut-brain axis. Science 2021; 374: 1087-92.
DOI PMID |
| 23. |
Collins SM, Surette M, Bercik P. The interplay between the intestinal microbiota and the brain. Nat Rev Microbiol 2012; 10: 735-42.
DOI PMID |
| 24. |
Schubert ML, Rehfeld JF. Gastric peptides-gastrin and somatostatin. Compr Physiol 2019; 10: 197-228.
DOI PMID |
| 25. |
Zhang Q, Li G, Zhao W, et al. Efficacy of bifidobacterium animalis subsp. lactis BL-99 in the treatment of functional dyspepsia: a randomized placebo-controlled clinical trial. Nat Commun 2024; 15: 227.
DOI PMID |
| 26. | Mori H, Verbeure W, Tanemoto R, Sosoranga ER, Jan Tack. Physiological functions and potential clinical applications of motilin. Peptides 2023; 160: 170905. |
| 27. | Kitazawa T, Kaiya H. Motilin comparative study: structure, distribution, receptors, and gastrointestinal motility. Front Endocrinol 2021; 12: 700884. |
| 28. | Scirocco A, Pallotta L, Rengo M, et al. Myogenic oxidative imbalance interferes with antral motility in obese subjects. Dig Liver Dis 2018; 50: 820-7. |
| 29. | Conlin VS, Wu X, Nguyen C, et al. Vasoactive intestinal peptide ameliorates intestinal barrier disruption associated with citrobacter rodentium-induced colitis. Am J Physiol Gastrointest Liver Physiol 2009; 297: 735-50. |
| 30. |
Van den Houte K, Scarpellini E, Verbeure W, et al. The role of GI peptides in functional dyspepsia and gastroparesis: a systematic review. Front Psychiatry 2020; 11: 172.
DOI PMID |
| 31. | Shen XY, Xie AJ, Li ZQ, et al. Research progress for probiotics regulating intestinal flora to improve functional dyspepsia: a review. Foods 2024; 13: 151. |
| 32. | Fujisaka S, Watanabe Y, Tobe K. The gut microbiome: a core regulator of metabolism. J Endocrinol 2023; 256: e220111. |
| 33. | Quaglio AEV, Grillo TG, De Oliveira ECS, Di Stasi LC, Sassaki LY. Gut microbiota, inflammatory bowel disease and colorectal cancer. World J Gastroenterol 2022; 28: 4053-60. |
| 34. |
Wahlström A, Sayin S I, Marschall H-U, Bäckhed F. Intestinal crosstalk between bile acids and microbiota and its impact on host metabolism. Cell Metab 2016; 24: 41-50.
DOI PMID |
| 35. | Li ZY, Zhang RR, Mu HN, et al. Oral administration of branched-chain amino acids attenuates atherosclerosis by inhibiting the inflammatory response and regulating the gut microbiota in apoe-deficient mice. Nutrients 2022; 14: 5065. |
| 36. | Kraimi N, Ross T, Pujo J, De Palma G. The gut microbiome in disorders of gut-brain interaction. Gut Microbes 2024; 16: 2360233. |
| [1] | WANG Yiying, LIU Jianjun, XIONG Yongjian, ZHANG Yongli, WEN Yuqi, XUE Mengli, GUO Huishu, QIU Juanjuan. Analysis of composition of gut microbial community in a rat model of functional dyspepsia treated with Simo Tang (四磨汤) [J]. Journal of Traditional Chinese Medicine, 2024, 44(6): 1168-1176. |
| [2] | HUANG Xiaona, LI Yuzhen, ZHU Chenyang, ZHU Hengzhou, JIANG Chenyu, ZHU Xiaodan, ZHANG Chencen, JIN Chunhui. Weitiao No. 3 (微调3号方) enhances the efficacy of anti-programmed cell death protein-1 immunotherapy by modulating the intestinal microbiota in an orthotopic model of gastric cancer mice [J]. Journal of Traditional Chinese Medicine, 2024, 44(5): 906-915. |
| [3] | SUN Chuanbo, XU Guangpei, JIANG Ping, HUANG Shipping, CHEN Cunwu, HE Yanfei. Protective effect of Zhizi Huangqi Shanzha formula (栀子黄芪山楂方) on aflatoxin poisoning in mice and its effect on intestinal flora [J]. Journal of Traditional Chinese Medicine, 2024, 44(5): 926-933. |
| [4] | REN Li, HAI Yang, YANG Xue, LUO Xianqin. Yemazhui (Herba Eupatorii Lindleyani) ameliorates lipopolysaccharide-induced acute lung injury via modulation of the toll-like receptor 4/nuclear factor kappa-B/nod-like receptor family pyrin domain-containing 3 protein signaling pathway and intestinal flora in rats [J]. Journal of Traditional Chinese Medicine, 2024, 44(2): 303-314. |
| [5] | WANG Jiabao, ZHANG Lishuang, NIU Baihan, YU Yajun, YANG Fengwen, MIAO Lin, CHAI Lijuan, DING Xinya, SUN Yingjie, WANG Yujing, WANG Lin, ZHANG Han, WANG Yi, LI Lin. Efficacy and safety of Weichang’ an pill (胃肠安丸) combined with Western Medicine on gastrointestinal diseases: a systematic review and Meta-analysis [J]. Journal of Traditional Chinese Medicine, 2023, 43(6): 1057-1067. |
| [6] | LI Chaoran, YANG Yan, FENG Chuwen, LI Heng, QU Yuanyuan, WANG Yulin, WANG Delong, WANG Qingyong, GUO Jing, SHI Tianyu, SUN Xiaowei, WANG Xue, HOU Yunlong, SUN Zhongren, YANG Tiansong. Integrated 'omics analysis for the gut microbiota response to moxibustion in a rat model of chronic fatigue syndrome [J]. Journal of Traditional Chinese Medicine, 2023, 43(6): 1176-1189. |
| [7] | ZHOU Jun, WANG Junhua, LI Xiaobing, WAN Chenyi, LI Fangjun, Lü Yanni, CHEN Hao, SUN Meiying. Efficacy of Heshouwu (Radix Polygoni Multiflori) on gut mircobiota in mice with autoimmune encephalomyelitis [J]. Journal of Traditional Chinese Medicine, 2023, 43(4): 676-685. |
| [8] | JIANG Yiqian, ZHOU Xibin, PU Wenyuan, ZHOU Chunxiang. Sanwu Baisan decoction (三物白散) inhibits colorectal cancer progression in mice by remodeling gut microbiota and tumorigenesis [J]. Journal of Traditional Chinese Medicine, 2023, 43(3): 466-473. |
| [9] | YANG Yang, YUAN Haining, JIA Hongxiao, NING Yanzhe, WANG Di, ZHANG Lei, YAN Kaijuan, GUO Yumeng, WANG Fei, SUN Weishuang, CHEN Pei. Therapy of replenishing Yin and regulating Yang for manic episode in bipolar disorder: study protocol for a prospective, double-blind, randomized controlled trial [J]. Journal of Traditional Chinese Medicine, 2023, 43(3): 594-601. |
| [10] | SUN Mengzhu, ZHANG Yujie, SONG Yafang, GUO Jing, ZHAO Tingting, WANG Yuhang, PEI Lixia, SUN Jianhua. Electroacupuncture at Tianshu (ST25) and Zusanli (ST36) alleviates stress-induced irritable bowel syndrome in mice by modulating gut microbiota and corticotropin-releasing factor [J]. Journal of Traditional Chinese Medicine, 2022, 42(5): 732-740. |
| [11] | YU Zeyue, HAO Liyu, LI Zongyuan, SUN Jianhui, CHEN Hongying, HUO Hairu, LI Xiaoqin, SHAN Zhongchao, LI Hongmei. Correlation between slow transit constipation and spleen Qi deficiency, and gut microbiota: a pilot study [J]. Journal of Traditional Chinese Medicine, 2022, 42(3): 353-363. |
| [12] | Huixiang ZHANG, Limei WANG, Jipeng GUO, Jiai WANG, Qianqian ZHANG, Yutao WANG, Xun LIU, Lihuan ZHANG, Lanlan SHI, Hongxiang WU, Xue CAO. Gut microbiota and differential genes-maintained homeostasis is key to maintaining health of individuals with Yang-deficiency constitution [J]. Journal of Traditional Chinese Medicine, 2022, 42(1): 96-101. |
| [13] | PAN Lijia, MA Shuya, WEN Jing, ZHANG Xiaoqi, XING Haijiao, JIA Chunsheng. Direct contact moxibustion promotes apoptosis of gastric cancer cells in rats by regulating intestinal flora [J]. Journal of Traditional Chinese Medicine, 2021, 41(6): 943-952. |
| [14] | Sun Zhigao, Hu Yazhuo, Wang Yuguo, Feng Jian, Dou Yongqi. Bupi Hewei decoction ameliorates 5-fluorouracil-induced intestinal dysbiosis in rats through T helper 17/T regulatory cell signaling pathway [J]. Journal of Traditional Chinese Medicine, 2020, 40(1): 38-48. |
| [15] | Peng Ying, Zhang Shuoying, Liu Zhiwei, Ji Jia, Wu Chunfu, Yang Jingyu, Li Xiaobo. Gut microbiota and Chinese medicine syndrome: altered fecal microbiotas in spleen(Pi)-deficient patients [J]. Journal of Traditional Chinese Medicine, 2020, 40(1): 137-143. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||

