Journal of Traditional Chinese Medicine ›› 2024, Vol. 44 ›› Issue (6): 1146-1152.DOI: 10.19852/j.cnki.jtcm.2024.06.005
• Research Articles • Previous Articles Next Articles
Anti-oxidative and immunological role of Cyclocarya paliurus polysaccharide on the liver injury of diabetic rats
XIA Xichao1( ), XUE Shipeng2(
), XUE Shipeng2( ), SONG Guoying1, LI Bin1, WANG Huiping1, QIU Ju1, Wang Jihong1, LIU Qingchun2, MA Yuhong2, OUYANG Jingfeng3
), SONG Guoying1, LI Bin1, WANG Huiping1, QIU Ju1, Wang Jihong1, LIU Qingchun2, MA Yuhong2, OUYANG Jingfeng3
- 1 Nursing College, Pingdingshan University, Pingdingshan 467000, China
2 Basic Medicine College of Nanyang Medical University, Nanyang 473041, China
3 Beijing Key Laboratory of Traditional Chinese Medicine Basic Research on Prevention and Treatment for Major Diseases, Experimental Research Center, China Academy of Chinese Medical Sciences, Beijing 100700, China
-
Received:2023-03-10Accepted:2023-06-16Online:2024-12-15Published:2024-11-12 -
Contact:Prof. XIA Xichao, Medcine College of Pingdingshan University, Pingdingshan 467000, China. xiaxichao8336@163.com Telephone: +86-15936167005
XUE Shipeng, Basic Medicine College of Nanyang Medical University, Nanyang 473041, China. 409195235@qq.com -
Supported by:Effect of Cyclocarya Paliurus Polysaccharides on the Diabetes Mellitus(212102310845);Effect of Kelening decoction on diabetic rats(242102310556)
Cite this article
XIA Xichao, XUE Shipeng, SONG Guoying, LI Bin, WANG Huiping, QIU Ju, Wang Jihong, LIU Qingchun, MA Yuhong, OUYANG Jingfeng. Anti-oxidative and immunological role of Cyclocarya paliurus polysaccharide on the liver injury of diabetic rats[J]. Journal of Traditional Chinese Medicine, 2024, 44(6): 1146-1152.
share this article
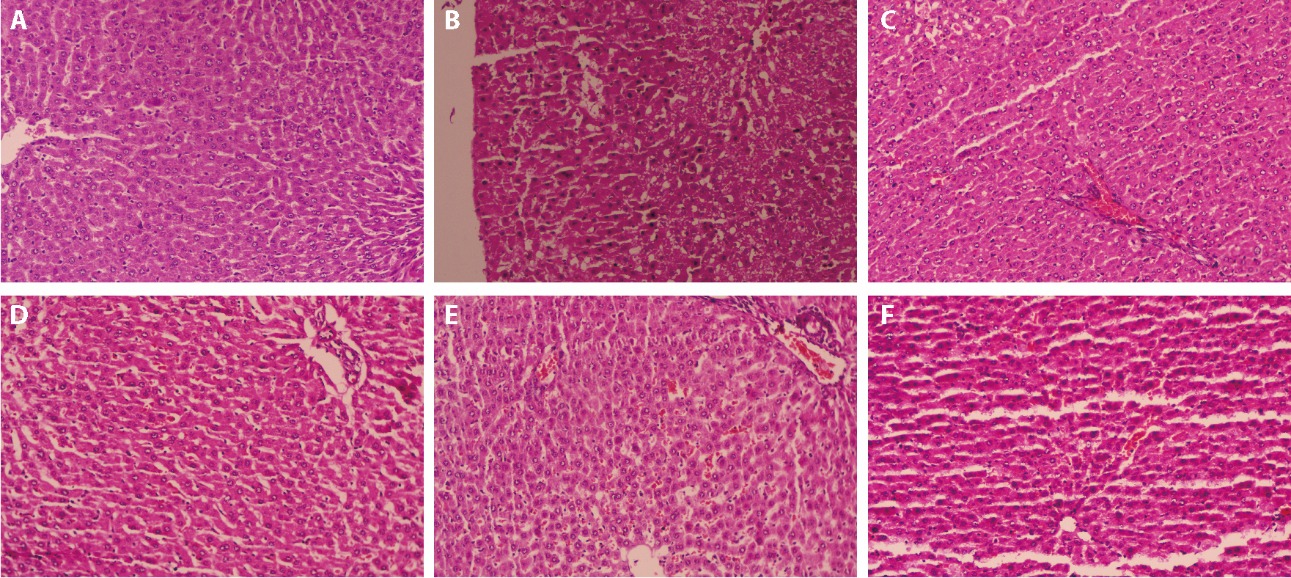
Figure 1 Light micrographs of liver tissue (hematoxilin and eosin, × 200) A: normal group, B: diabetic control group, C: Metformin group, D: C. paliurus polysaccharide low-dose group, E: C. paliurus polysaccharide middle-dose group, F: C. paliurus polysaccharide high-dose group. Metformin group was treated with 5 mg·kg-1·d-1. Group of low-dose, group of middle-dose and group of high-dose were treated with of C. paliurus polysaccharide at the concentration of 1, 10 and 100 mg·kg-1·d-1, respectively. C. paliurus: Cyclocarya paliurus.
| Group | n | Glucose (mmol/L) | Glycogen (mg/g) | Insulin (pg/mL) | ALT (U/L) | AST (U/L) | ALP (U/L) |
|---|---|---|---|---|---|---|---|
| Normal Diabetic control Metformin Low-dose Middle-dose High-dose | 10 10 10 10 10 10 | 4.47±0.73 27.42±5.18a 7.81±1.52bc 18.39±1.73ac 14.54±1.65ac 10.33±2.76bd | 28.51±1.39 7.66±3.87a 20.49±2.46bd 9.70±1.24a 12.42±1.38ac 19.28±3.66bd | 6.13±0.08 1.68±0.48a 4.08±0.24bd 2.04±0.14ac 3.43±0.23ad 3.82±0.63ad | 126.32±4.47 468.57±13.32a 201.63±9.75bd 440.85±15.77a 361.29±18.25ac 246.12±10.61bc | 253.24±7.27 586.95±11.35a 375.36±8.93bc 542.67±11.50a 407.31±16.92bc 330.74±6.38d | 72.64±5.14 231.22±17.13a 119.48±8.86bc 245.94±12.18ac 182.72±15.86ac 114.41±5.69bd |
Table 1 Effect of C. paliurus polysaccharide on the glucose, glycogen, insulin, ALT, AST and ALP level in plasma of diabetic rats
| Group | n | Glucose (mmol/L) | Glycogen (mg/g) | Insulin (pg/mL) | ALT (U/L) | AST (U/L) | ALP (U/L) |
|---|---|---|---|---|---|---|---|
| Normal Diabetic control Metformin Low-dose Middle-dose High-dose | 10 10 10 10 10 10 | 4.47±0.73 27.42±5.18a 7.81±1.52bc 18.39±1.73ac 14.54±1.65ac 10.33±2.76bd | 28.51±1.39 7.66±3.87a 20.49±2.46bd 9.70±1.24a 12.42±1.38ac 19.28±3.66bd | 6.13±0.08 1.68±0.48a 4.08±0.24bd 2.04±0.14ac 3.43±0.23ad 3.82±0.63ad | 126.32±4.47 468.57±13.32a 201.63±9.75bd 440.85±15.77a 361.29±18.25ac 246.12±10.61bc | 253.24±7.27 586.95±11.35a 375.36±8.93bc 542.67±11.50a 407.31±16.92bc 330.74±6.38d | 72.64±5.14 231.22±17.13a 119.48±8.86bc 245.94±12.18ac 182.72±15.86ac 114.41±5.69bd |
| Group | n | SOD | CAT | GSH-Px | GR |
|---|---|---|---|---|---|
| Normal Diabetic control Metformin Low-dose | 10 10 10 10 | 8.79±10.72 3.67±4.08a 7.18±9.15b 4.28±5.47c | 79.68±12.23 44.29±6.18c 77.31±14.59d 52.31±6.61c | 9.15±1.58 3.48±0.57a 8.76±1.03b 5.27±0.65b | 86.07±10.25 41.25±5.72a 74.56±8.59d 57.11±6.58c |
| Middle-dose High-dose | 10 10 | 5.67±6.53cd 7.95±8.02b | 64.57±7.29cd 75.86±8.73d | 6.38±0.65cd 7.98±0.94b | 68.89±7.85cd 77.33±9.40d |
Table 2 Effect of C. paliurus polysaccharide on the SOD, CAT, GSH-Px and GR level in liver of diabetic rats (U/mg)
| Group | n | SOD | CAT | GSH-Px | GR |
|---|---|---|---|---|---|
| Normal Diabetic control Metformin Low-dose | 10 10 10 10 | 8.79±10.72 3.67±4.08a 7.18±9.15b 4.28±5.47c | 79.68±12.23 44.29±6.18c 77.31±14.59d 52.31±6.61c | 9.15±1.58 3.48±0.57a 8.76±1.03b 5.27±0.65b | 86.07±10.25 41.25±5.72a 74.56±8.59d 57.11±6.58c |
| Middle-dose High-dose | 10 10 | 5.67±6.53cd 7.95±8.02b | 64.57±7.29cd 75.86±8.73d | 6.38±0.65cd 7.98±0.94b | 68.89±7.85cd 77.33±9.40d |
| Group | n | NF-κB | TNF-α | IL-1β | IL-6 |
|---|---|---|---|---|---|
| Normal Diabetic control Metformin Low-dose | 10 10 10 10 | 0.53±0.05 3.94±0.43a 0.69±0.07b 2.52±0.29ac | 0.66±0.06 4.17±0.48a 1.23±0.14cd 3.76±0.40a | 0.47±0.05 5.93±0.63a 1.61±0.20ab 4.25±0.46ac | 1.07±0.09 14.79±1.33a 2.41±0.29cd 9.36±1.07ac |
| Middle-dose High-dose | 10 10 | 1.64±0.21ab 0.89±0.09b | 2.65±0.30ac 1.36±0.16cd | 3.01±0.34ab 1.55±0.18ab | 6.42±0.68ab 3.59±0.40cd |
Table 3 Effect of C. paliurus polysaccharide on NF-κB, TNF-α, IL-1β and IL-6 mRNA levels of liver in diabetic rats
| Group | n | NF-κB | TNF-α | IL-1β | IL-6 |
|---|---|---|---|---|---|
| Normal Diabetic control Metformin Low-dose | 10 10 10 10 | 0.53±0.05 3.94±0.43a 0.69±0.07b 2.52±0.29ac | 0.66±0.06 4.17±0.48a 1.23±0.14cd 3.76±0.40a | 0.47±0.05 5.93±0.63a 1.61±0.20ab 4.25±0.46ac | 1.07±0.09 14.79±1.33a 2.41±0.29cd 9.36±1.07ac |
| Middle-dose High-dose | 10 10 | 1.64±0.21ab 0.89±0.09b | 2.65±0.30ac 1.36±0.16cd | 3.01±0.34ab 1.55±0.18ab | 6.42±0.68ab 3.59±0.40cd |
| Group | n | NF-κB | TNF-α | IL-1β | IL-6 |
|---|---|---|---|---|---|
| Normal Diabetic control Metformin Low-dose | 10 10 10 10 | 189.2±27.5 361.5±31.2a 203.9±23.4b 324.3±24.6c | 24.6±2.2 56.0±3.4a 28.2±3.2b 49.5±5.7a | 18.7±2.3 34.6±4.1c 21.6±2.6b 27.1±3.6bc | 28.9±2.4 52.4±6.4c 32.7±3.7b 48.2±4.9c |
| Middle-dose High-dose | 10 10 | 267.2±32.7bc 219.4±6.4b | 32.1±3.5bc 27.4±3.2d | 23.8±2.4bc 21.7±3.5b | 42.9±5.1bc 33.3±3.6b |
Table 4 Effect of C. paliurus polysaccharide on NF-κB, TNF-α, IL-1β and IL-6 level in liver of diabetic rats (ng/L)
| Group | n | NF-κB | TNF-α | IL-1β | IL-6 |
|---|---|---|---|---|---|
| Normal Diabetic control Metformin Low-dose | 10 10 10 10 | 189.2±27.5 361.5±31.2a 203.9±23.4b 324.3±24.6c | 24.6±2.2 56.0±3.4a 28.2±3.2b 49.5±5.7a | 18.7±2.3 34.6±4.1c 21.6±2.6b 27.1±3.6bc | 28.9±2.4 52.4±6.4c 32.7±3.7b 48.2±4.9c |
| Middle-dose High-dose | 10 10 | 267.2±32.7bc 219.4±6.4b | 32.1±3.5bc 27.4±3.2d | 23.8±2.4bc 21.7±3.5b | 42.9±5.1bc 33.3±3.6b |
| 1. | Khondkaryan L, Margaryan S, Poghosyan D, Manukyan G. Impaired inflammatory response to LPS in type 2 diabetes mellitus. Int J Inflam 2018; 2018: 2157434. |
| 2. | Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract 2014; 103: 137-49. |
| 3. | Amos AF, McCarty DJ, Zimmet P. The rising global burden of diabetes and its complications: estimates and projections to the year 2010. Diabet Med 1997; 5: S1-85. |
| 4. |
Ashcroft FM, Rorsman P. Diabetes mellitus and the β cell: the last ten years. Cell 2012; 148: 1160-71.
DOI PMID |
| 5. | Wróbel MP, Marek B, Kajdaniuk D, Rokicka D, Szymborska-Kajanek A, Strojek K. Metformin-a new old drug. Endokrynol Pol 2017; 68: 482-96. |
| 6. |
Yin W, Li B, Li X, et al. Anti-inflammatory effects of grape seed procyanidin B2 on a diabetic pancreas. Food Funct 2015; 6: 3065-71.
DOI PMID |
| 7. |
Poprac P, Jomova K, Simunkova M, Kollar V, Rhodes CJ, Valko M. Targeting free radicals in oxidative stress-related human diseases. Trends Pharmacol Sci 2017; 38: 592-607.
DOI PMID |
| 8. |
Klein RD, Borges VD, Rosa CE, et al. Effects of increasing temperature on antioxidant defense system and oxidative stress parameters in the Antarctic fish Notothenia coriiceps and Notothenia rossii. J Therm Biol 2017; 68: 110-8.
DOI PMID |
| 9. | Cichoż-Lach H, Michalak A. Oxidative stress as a crucial factor in liver diseases. World J Gastroenterol 2014; 20: 8082-91. |
| 10. |
Elosta A, Ghous T, Ahmed N. Natural products as anti-glycation agents: possible therapeutic potential for diabetic complications. Curr Diabetes Rev 2012; 8: 92-108.
DOI PMID |
| 11. |
Chandirasegaran G, Elanchezhiyan C, Ghosh K. Effects of Berberine chloride on the liver of streptozotocin-induced diabetes in albino Wistar rats. Biomed Pharmacother 2018; 99: 227-36.
DOI PMID |
| 12. | Rashid K, Sil PC. Curcumin enhances recovery of pancreatic islets from cellular stress induced inflammation and apoptosis in diabetic rats. Toxicol Appl Pharmacol 2015; 282: 297-310. |
| 13. | Choudhury S, Ghosh S, Gupta P, Mukherjee S, Chattopadhyay S. Inflammationinduced ROS generation causes pancreatic cell death through modulation of Nrf2-NF-kB and SAPK/JNK pathway. Free Radic Res 2015; 49: 1371-83. |
| 14. | Yoshitomi H, Tsuru R, Li L, et al. Cyclocarya paliurus extract activates insulin signaling via Sirtuin1 in C2C12 myotubes and decreases blood glucose level in mice with impaired insulin secretion. PLoS One 2017; 12: e0183988. |
| 15. | Xiao HT, Wen B, Ning ZW, et al. Cyclocarya paliurus tea leaves enhances pancreatic β cell preservation through inhibition of apoptosis. Sci Rep 2017; 7: 9155. |
| 16. | Yu Y, Shen M, Wang Z, Wang Y, Xie M, Xie J. Sulfated polysaccharide from Cyclocarya paliurus enhances the immunomodulatory activity of macrophages. Carbohydr Polym 2017; 174: 669-76. |
| 17. |
Lin Z, Wu ZF, Jiang CH, et al. The chloroform extract of Cyclocarya paliurus attenuates high-fat diet induced non-alcoholic hepatic steatosis in Sprague Dawley rats. Phytomedicine 2016; 23: 1475-83.
DOI PMID |
| 18. |
Wang Q, Jiang C, Fang S, et al. Antihyperglycemic, antihyperlipidemic and antioxidant effects of ethanol and aqueous extracts of Cyclocarya paliurus leaves in type 2 diabetic rats. J Ethnopharmacol 2013; 150: 1119-27.
DOI PMID |
| 19. | Liu Y, Cao Y, Fang S, et al. Antidiabetic effect of Cyclocarya paliurus leaves depends on the contents of antihyperglycemic flavonoids and antihyperlipidemic triterpenoids. Molecules 2018; 23: 1042. |
| 20. | Xie JH, Xie MY, Shen MY, Nie SP, Li C, Wang YX. Optimisation of microwave-assisted extraction of polysaccharide from Cyclocarya paliurus (Batal.) Iljinskaja using response surface methodology. J Sci Food Agric 2010; 90: 1353-60. |
| 21. | Xu X, Yin Z, Chen J, Wang X, Peng D, Shangguan X. De novo transcriptome assembly and annotation of the leaves and callus of Cyclocarya paliurus (Bata1) Iljinskaja. PLoS One 2016; 11: e0160279. |
| 22. | Zhao MG, Sheng XP, Huang YP, et al. Triterpenic acids-enriched fraction from Cyclocarya paliurus attenuates non-alcoholic fatty liver disease via improving oxidative stress and mitochondrial dysfunction. Biomed Pharmacother 2018; 104: 229-39. |
| 23. |
Gokce G, Haznedaroglu MZ. Evaluation of antidiabetic, antioxidant and vasoprotective effects of Posidonia oceanica extract. J Ethnopharmacol 2008; 115: 122-30.
PMID |
| 24. | Nasirian F, Dadkhah M, Moradi-Kor N, Obeidavi Z. Effects of Spirulina platensis microalgae on antioxidant and anti-inflammatory factors in diabetic rats. Diabetes Metab Syndr Obes 2018; 11: 375-80. |
| 25. |
Xia X, Zhang Q, Liu R, et al. Effects of 20-hydroxyecdysone on improving memory deficits in streptozotocin-induced type 1 diabetes mellitus in rat. Eur J Pharmacol 2014; 740: 45-52.
DOI PMID |
| 26. | Eiland L, McLarney M, Thangavelu T, Drincic A. App-based insulin calculators: current and future state. Curr Diab Rep 2018; 18: 123. |
| 27. |
Bełtowski J, Wójcicka G, Jamroz-Wiśniewska A. Hydrogen sulfide in the regulation of insulin secretion and insulin sensitivity: implications for the pathogenesis and treatment of diabetes mellitus. Biochem Pharmacol 2018; 149: 60-76.
DOI PMID |
| 28. | Kim YJ, Lee NY, Lee KA, Park TS, Jin HY. Influence of glucose fluctuation on peripheral nerve damage in streptozotocin-induced diabetic rats. Diabetes Metab J 2022; 46: 117-28. |
| 29. | Xia X, Yan J, Shen Y, et al. Berberine improves glucose metabolism in diabetic rats by inhibition of hepatic gluconeogenesis. PLoS One 2011; 6: e16556. |
| 30. | Mills EP, Brown KPD, Smith JD, Vang PW, Trotta K. Treating nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus: a review of efficacy and safety. Ther Adv Endocrinol Metab 2018; 9: 15-28. |
| 31. |
Ghosh S, Chowdhury S, Sarkar P, Sil PC. Ameliorative role of ferulic acid against diabetes associated oxidative stress induced spleen damage. Food Chem Toxicol 2018; 118: 272-86.
DOI PMID |
| 32. | Cheng C, Yi J, Wang R, Cheng L, Wang Z, Lu W. Protection of spleen tissue of γ-ray irradiated mice against immunosuppressive and oxidative effects of radiation by adenosine 5'-monophosphate. Int J Mol Sci 2018; 19: E1273. |
| 33. |
Hu B, Oki Y. Novel immunotherapy options for extranodal NK/T-cell lymphoma. Front Oncol 2018; 8: 139.
DOI PMID |
| 34. |
Bartekova M, Radosinska J, Jelemensky M, Dhalla NS. Role of cytokines and inflammation in heart function during health and disease. Heart Fail Rev 2018; 23: 733-58.
DOI PMID |
| 35. |
Sierra-Mondragon E, Molina-Jijon E, Namorado-Tonix C, Rodríguez-Muñoz R, Pedraza-Chaverri J, Reyes JL. All-trans retinoic acid ameliorates inflammatory response mediated by TLR4/NF-κB during initiation of diabetic nephropathy. J Nutr Biochem 2018; 60: 47-60.
DOI PMID |
| 36. | Khurana A, Tekula S, Godugu C. Nanoceria suppresses multiple low doses of streptozotocin-induced Type 1 diabetes by inhibition of Nrf2/NF-κB pathway and reduction of apoptosis. Nanomedicine (Lond) 2018; 13: 1905-22. |
| 37. | Mohamed ME, Medhat A, Khaled SA, Koichiro U, Mitsuhiro E, Ahmed N. Antioxidative capacity of liver- and adipose-derived mesenchymal stem cell-conditioned media and their Aapplicability in treatment of type 2 diabetic rats. Oxid Med Cell Longev 2021; 2021: 8833467. |
| 38. |
Hirotani Y, Ikeda K, Myotoku M. Effects of the herbal medicine Hachimi-jio-gan (Ba-Wei-Di-Huang-Wan) on insulin secretion and glucose tolerance in type 2 diabetic Goto-Kakizaki rats. Drug Discov Ther 2010; 4: 129-34.
PMID |
| 39. |
Yang Y, Wang Y, Kong Y, et al. Carnosine prevents type 2 diabetes-induced osteoarthritis through the ROS/NF-κB pathway. Front Pharmacol 2018; 9: 598.
DOI PMID |
| [1] | SUN Chuanbo, XU Guangpei, JIANG Ping, HUANG Shipping, CHEN Cunwu, HE Yanfei. Protective effect of Zhizi Huangqi Shanzha formula (栀子黄芪山楂方) on aflatoxin poisoning in mice and its effect on intestinal flora [J]. Journal of Traditional Chinese Medicine, 2024, 44(5): 926-933. |
| [2] | WANG Chao, WU Qiong, LI Ping, WANG Zhigang, LOU Xusheng, LI Yuanyuan, ZHANG Lin. Effect of Traditional Chinese Medicine combined with Western Medicine on blood lipid levels and inflammatory factors in patients with angina pectoris in coronary heart disease identified as intermingled phlegm and blood stasis syndrome: a network Meta-analysis [J]. Journal of Traditional Chinese Medicine, 2023, 43(4): 640-649. |
| [3] | Esma Anissa Trad Khodja, Abd El Hamid Khabtane, Rabah Arhab, Djamila Benouchenne, Mohamed Sabri Bensaad, Chawki Bensouici, Ramazan Erenler. In vitro assessment of antioxidant, neuroprotective, anti-urease and anti-tyrosinase capacities of Tamarix africana leaves extracts [J]. Journal of Traditional Chinese Medicine, 2023, 43(2): 252-264. |
| [4] | Naser Mirazi, Sheida Hesami, Alireza Nourian, Abdolkarim Hosseini. Protective efficacy of dark chocolate in letrozole-induced ovary toxicity model rats: hormonal, biochemical, and histopathological investigation [J]. Journal of Traditional Chinese Medicine, 2022, 42(5): 741-748. |
| [5] | Kamal Dawood, Roohullah, Rabbi Fazle, Naz Attiqa, Bilal Muhammad. In-vitro and in-vivo pharmacological screening of Iris albicans [J]. Journal of Traditional Chinese Medicine, 2022, 42(1): 9-16. |
| [6] | Elham A.Abd-Allah, Nouf S.Al-Abbas, Mona M.Atia, Fawzia Alzahrani, El-Mokhtar M.Ahmed, Soad S.Ali, Soad K.Al Jaouni. Can Fig and Olive Ameliorate the toxicity Induced by 2-nitropropane in some organs of mice? role of inflammatory versus anti-inflammatory genes [J]. Journal of Traditional Chinese Medicine, 2021, 41(6): 891-899. |
| [7] | XIA Xichao, LI Bin, QIU Ju, TIAN Gang, CHEN Changdong, LA Ming, ZHANG Ke, QI Jinxu, LI Yanyan, GAO Huashan, SHAO Xiangyang, SU Congying, WANG Mengqi, OUYANG Jingfeng. Antioxidative and immunological effects of Cyclocarya paliurus polysaccharides on the spleen injury of diabetic rats [J]. Journal of Traditional Chinese Medicine, 2021, 41(5): 739-746. |
| [8] | TANG Chengfang, GAO Yang, Gulibairemu Yusuyin, MAO Yan, LI Yujun, WANG Yandong, GU Zhengyi. Anti-cataract effects of Dajizhi(Euphorbium) eye drops on selenite-induced cataracts in rats [J]. Journal of Traditional Chinese Medicine, 2021, 41(5): 747-752. |
| [9] | Mohsen Akbaribazm, Fatemeh Khazaei, Leila Naseri, Mona Pazhouhi, Mohammad Zamanian, Mozafar Khazaei. Pharmacological and therapeutic properties of the Red Clover(Trifolium pratense L.): an overview of the new findings [J]. Journal of Traditional Chinese Medicine, 2021, 41(4): 642-649. |
| [10] | ?nder Aybast?er;. Efficacy of methanol-water extract of Inula helenium root against oxidative DNA damage [J]. Journal of Traditional Chinese Medicine, 2021, 41(2): 293-300. |
| [11] | KONG Deyan, LUO Jiefeng, SHI Shengliang, HUANG Zhenhua. Efficacy of tanshinone ⅡA and mesenchymal stem cell treatment of learning and memory impairment in a rat model of vascular dementia [J]. Journal of Traditional Chinese Medicine, 2021, 41(1): 133-139. |
| [12] | Zhang Yandong, Biao Yaning, Chu Xinqiao, Hao Lei, Shi Cheng, Liu Yu, Zhang Yixin, Wang Xi. Protective effect of Chushizi(Fructus Broussonetiae) on acetaminophen-induced rat hepatitis by inhibiting the Toll-like receptor 3/c-Jun N-terminal kinase/c-jun/c-fos/janus protein tyrosine kinase/activators of transcription 3 pathway [J]. Journal of Traditional Chinese Medicine, 2020, 40(6): 965-973. |
| [13] | Hou Jiguang, Fang Fang, Kang Shunai, Wang Zhicheng, Yang Yanming. Curcumin from Jianghuang (Rhizoma Curcumae Longae) protects against exposure to ultraviolet B by antioxidation and attenuating mitochondrion-dependent apoptosis [J]. Journal of Traditional Chinese Medicine, 2020, 40(5): 782-791. |
| [14] | Emel Akta?, Hilal Yildiran. Antioxidant and ntiinflammatory efficacy of curcumin on lung tissue in rats with sepsis [J]. Journal of Traditional Chinese Medicine, 2020, 40(5): 820-826. |
| [15] | Seval Yilmaz, Emre Kaya, Erhan Yilmaz, Ahmet Kavakli, Suleyman Gurbuz, Mustafa Ozkaraca. Effect of acupuncture therapy on fracture healing in rats with femur fractures [J]. Journal of Traditional Chinese Medicine, 2020, 40(2): 275-283. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||

