Journal of Traditional Chinese Medicine ›› 2023, Vol. 43 ›› Issue (6): 1176-1189.DOI: 10.19852/j.cnki.jtcm.20231018.004
• Research Articles • Previous Articles Next Articles
Integrated 'omics analysis for the gut microbiota response to moxibustion in a rat model of chronic fatigue syndrome
LI Chaoran1, YANG Yan2, FENG Chuwen3, LI Heng7, QU Yuanyuan5, WANG Yulin6, WANG Delong6, WANG Qingyong5, GUO Jing5, SHI Tianyu5, SUN Xiaowei4, WANG Xue8, HOU Yunlong9, SUN Zhongren6( ), YANG Tiansong10(
), YANG Tiansong10( )
)
- 1 Department of Acupuncture, Zhejiang Chinese Medical University, Hangzhou 310000, China
2 Department of Chinese Medical Literature, College of Basic Medicine, Heilongjiang University of Chinese medicine, Harbin 150040, China
3 Department of Rehabilitation, the First Affiliated Hospital of Heilongjiang University of Traditional Chinese Medicine, Harbin 150040, China
4 Department of Acupuncture, the First Affiliated Hospital of Heilongjiang University of Traditional Chinese Medicine, Harbin 150040, China
5 Graduate School, the Second Affiliated Hospital of Heilongjiang University of Traditional Chinese Medicine, Harbin 150040, China
6 Department of Acupuncture, the Second Affiliated Hospital of Heilongjiang University of Traditional Chinese Medicine, Harbin 150040, China
7 Shanghai Applied Protein Technology Co., Ltd., Shanghai 200233, China
8 Department of Acupuncture, Chongqing Changshou District People's Hospital, Chongqing 401220, China
9 College of integrated Chinese and Western Medicine, Hebei University of Chinese Medicine, and National Key Laboratory of Collateral Disease Research and Innovative Chinese Medicine, Hebei 050000, China
10 Department of Rehabilitation, the First Affiliated Hospital of Heilongjiang University of Traditional Chinese Medicine, and Traditional Chinese Medicine Informatics Key Laboratory of Heilongjiang Province, Harbin 150040, China
-
Received:2023-05-22Accepted:2023-08-08Online:2023-10-25Published:2023-10-18 -
Contact:Prof. SUN Zhongren, Department of Acupuncture, Heilongjiang, University of Chinese Medicine, Harbin 150040, China. sunzhong_ren@163.com; Prof. YANG Tiansong, Department of Rehabilitation, the First Affiliated Hospital of Heilongjiang University of Traditional Chinese Medicine, and Traditional Chinese Medicine Informatics Key Laboratory of Heilongjiang Province, Harbin 150040, China. yangtiansong2006@163.com. Telephone: +86-15557137527 -
Supported by:Based on TGF-β/Smad Signaling Pathway to Studying the Role and Mechanism of NF-κB in the Treatment of Chronic Fatigue Syndrome with Electroacupuncture(81704170);Mechanism Study on Electroacupuncture Regulating MDM2 Ubiquitination PSD-95 Level and Remodeling Synaptic Structure of Hippocampal Neurons to Improve Cognitive Dysfunction in CFS(81873378);Study on the Mechanism of Electroacupuncture Adjusting Gut Microbiota to Improve Oxidative Inflammatory Response in the Treatment of Chronic Fatigue Syndrome(LH2020H092);Experimental Study on Electroacupuncture Improving Protein Expression Differences and Target Effects in Hippocampus and Hypothalamus of Rats with Chronic Fatigue Syndrome(LBH-Q18117);Study on the Effect and Mechanism of Moxibustion on Gut Microbiota in Rats with Chronic Fatigue Syndrome(ZHY2020-79);The Mechanism of TGF-β/ Smad Mediated NF- κB Inflammatory Pathway in the Treatment of Chronic Fatigue Syndrome by Electroacupuncture(ZHY16-003);The Mechanism of Electroacupuncture Improving Hippocampal Synaptic Plasticity in CFS Cognitive Dysfunction Rats(ZHY2022-136);Study on the Mechanism of Moxibustion Regulating the Gut Microbiota of Chronic Fatigue Syndrome Targeting the 5-HT Signal System(2021RCZXZK02);Study on the Mechanism of Moxibustion Repairing the Barrier Function-Inflammatory Response of Chronic Fatigue Syndrome by the Gut-Brain Axis(ZS21ZA08);Based on Gut Microbiota and Metabolism to Study the Mechanism in the Treatment of Chronic Fatigue Syndrome by Moxibustion(2019BS03)
Cite this article
LI Chaoran, YANG Yan, FENG Chuwen, LI Heng, QU Yuanyuan, WANG Yulin, WANG Delong, WANG Qingyong, GUO Jing, SHI Tianyu, SUN Xiaowei, WANG Xue, HOU Yunlong, SUN Zhongren, YANG Tiansong. Integrated 'omics analysis for the gut microbiota response to moxibustion in a rat model of chronic fatigue syndrome[J]. Journal of Traditional Chinese Medicine, 2023, 43(6): 1176-1189.
share this article

Figure 1 Experimental design A: flow chart of the experiment; B: modeling method: MCS; C: illustration of moxibustion. C1: Guanyuan (CV4) and Shenque (CV8) for the MoxA group; C2: Zusanli (ST36) for the MoxB group. RR: routine rearing; MCS: multivariate compound stress; MAT: moxibustion A treatment; MBT: moxibustion B treatment.
| Score | Spiritual statue | Skin and hair | Color of ear and tail | Feces |
|---|---|---|---|---|
| 0 | Active | Skin and fat were closely connected, pliable, and elastic; hair bright and smooth | Rosy with brightness | Dry feces, with shape |
| 1 | Slightly slow reaction, independent movement decreased | A bit flaccid skin, soft, pulling force decreased; yellow hair without brightness | Slight red, lacking of brightness | Sticky, soft feces, with shape |
| 2 | Listlessness, limb crunched with slow movement | Flaccid skin, little fat, easy to catch; hair dry or tangled, without brightness | Slight white, lacking of brightness | Shapeless, but not watery |
| 3 | Spiritual languor, disappearance of aggressive and adversarial behavior | Flaccid skin, no fat under the skin, and bony; dry and sparse hair of the whole body | Pale of green | Watery, green, sticky, malodorous |
Table 1 Semiquantitative score of the rats’ biological characteristics
| Score | Spiritual statue | Skin and hair | Color of ear and tail | Feces |
|---|---|---|---|---|
| 0 | Active | Skin and fat were closely connected, pliable, and elastic; hair bright and smooth | Rosy with brightness | Dry feces, with shape |
| 1 | Slightly slow reaction, independent movement decreased | A bit flaccid skin, soft, pulling force decreased; yellow hair without brightness | Slight red, lacking of brightness | Sticky, soft feces, with shape |
| 2 | Listlessness, limb crunched with slow movement | Flaccid skin, little fat, easy to catch; hair dry or tangled, without brightness | Slight white, lacking of brightness | Shapeless, but not watery |
| 3 | Spiritual languor, disappearance of aggressive and adversarial behavior | Flaccid skin, no fat under the skin, and bony; dry and sparse hair of the whole body | Pale of green | Watery, green, sticky, malodorous |
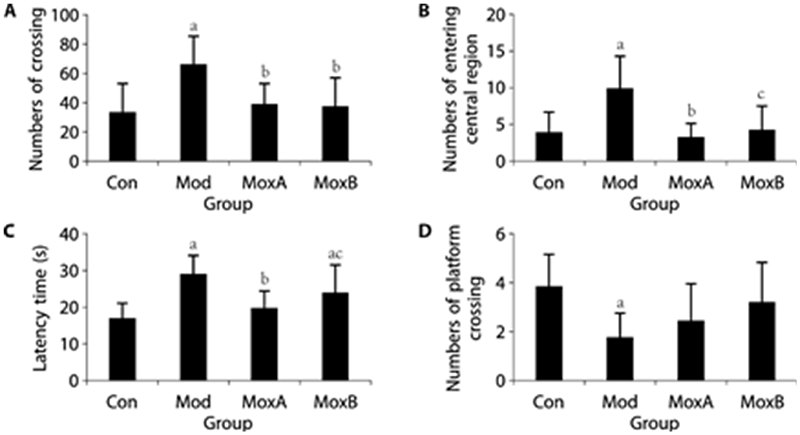
Figure 2 Behavioral indicators of rats in different groups A: numbers of crossing; B: numbers of entering the central region. A and B for OFT; C: latency time; D: numbers of platform crossing. C and D for MWMT. Data are presented as mean ± standard deviation, then analyzed using one-way analysis of variance followed by the Least-Significant Difference post hoc test for A, C, and Kruskal-Wallis sum-rank test for B, D. n = 12 per group. Compared with the Con group, aP < 0.01; compared with the Mod group, bP < 0.01; cP < 0.05. OFT: open-field test; MWMT: Morris-water-maze test. Con: Control group; Mod: Model group (multiple chronic stress for 35 d); MoxA: MoxibustionA group [moxibustion Shenque (CV8) and Guanyuan (CV4), 10 min/d, 28 d]; MoxB: MoxibustionB group [CFS model with moxibustion Zusanli (ST36), 10 min/d, 28 d].

Figure 3 Gut microbiota analysis A: alpha diversity; B: beta diversity (PCA plot); C: gut microbiota comparison at phylum levels; D: gut microbiota comparison at genus levels; E: cladogram of LEfSe analysis. N = 8 per group. PCA: principal component analysis; LSD: linear discriminant analysis effect size. Con: Control group; Mod: Model group (multiple chronic stress for 35 d); MoxA: MoxibustionA group [moxibustion Shenque (CV8) and Guanyuan (CV4), 10 min/d, 28 d]; MoxB: MoxibustionB group [CFS model with moxibustion Zusanli (ST36), 10 min/d, 28 d].
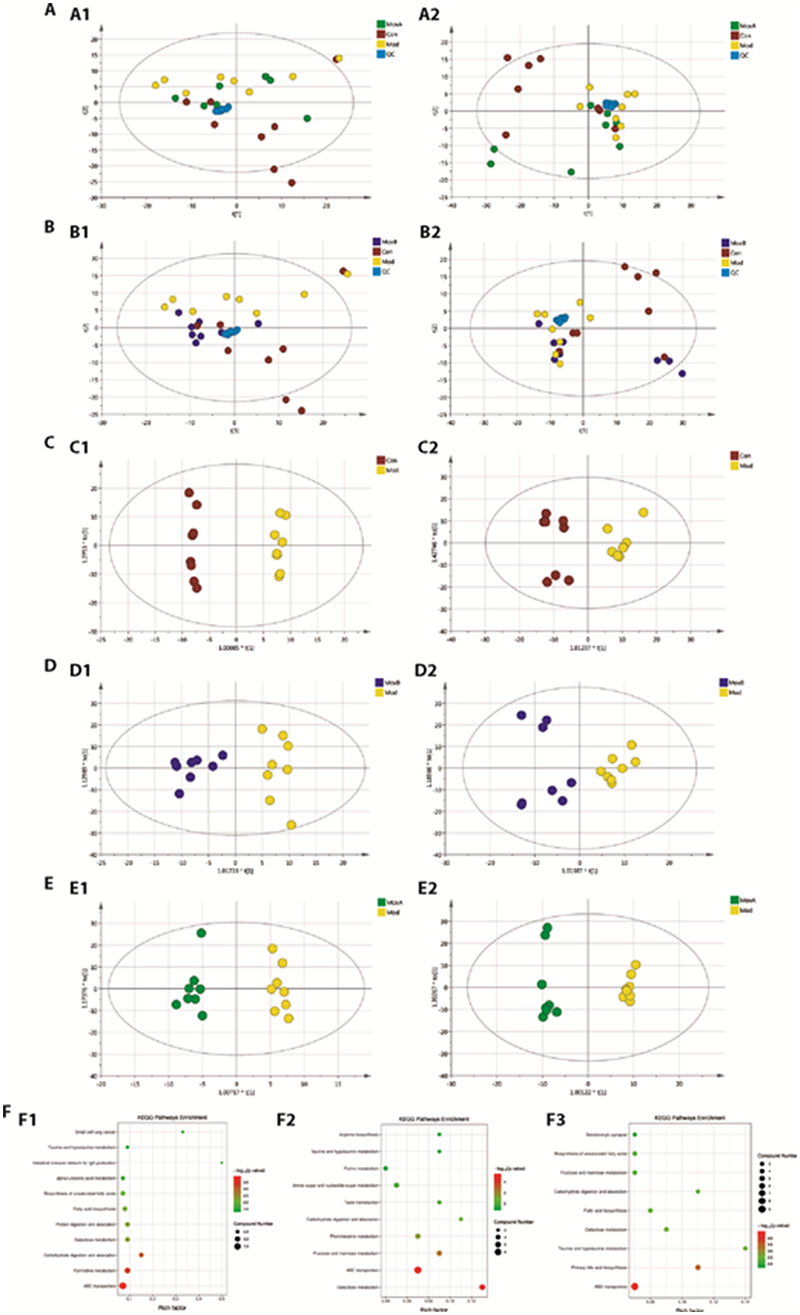
Figure 4 Metabolomics analysis A: PCA analysis in positive (A1) and negative (A2) ion modes in Con vs Mod vs MoxA group; B: PCA analysis in positive (B1) and negative (B2) ion modes in Con vs Mod vs MoxB group; C: OPLS-DA score plots among Con vs Mod group in positive (C1) and negative (C2) ion modes; D: OPLS-DA score plots among Mod vs MoxA group in positive (D1) and negative (D2) ion modes; E: OPLS-DA score plots among Mod vs MoxB group in positive (E1) and negative (E2) ion modes; F: Enriched KEGG pathways among Con vs Mod group (F1), Mod vs MoxA group (F2), and Mod vs MoxB group (F3). n = 8 per group. PCA: Principal Component Analysis; OPLS-DA: Orthogonal Partial Least Squares Discriminant Analysis; KEGG: Kyoto Encyclopedia of Genes and Genomes. Con: Control group; Mod: Model group (multiple chronic stress for 35 d); MoxA: MoxibustionA group [moxibustion Shenque (CV8) and Guanyuan (CV4), 10 min/d, 28 d]; MoxB: MoxibustionB group (CFS model with moxibustion Zusanli (ST36), 10 min/d, 28 d).
| Metabolite | M/Z | RT (s) | P value | M/C | A/M | B/M |
|---|---|---|---|---|---|---|
| Alpha-D-Glucose | 179.0573 | 581.66 | ≦0.01 | ↓ | ↑ | ↑ |
| D-Lactose | 341.1099 | 627.54 | ≦0.01 | ↓ | ↑ | ↑ |
| D-galacturonic acid | 193.0364 | 746.92 | ≦0.05 | ↓ | ↑ | ↑ |
| N-Acetyl-D-glucosamine | 202.0727 | 491.05 | ≦0.05 | ↓ | ↑ | ↑ |
| L-Arabinose | 149.0463 | 251.25 | ≦0.05 | ↓ | ↑ | ↑ |
| D-Allose | 239.0785 | 566.66 | ≦0.01 | ↓ | ↑ | ↑ |
| D-Mannose | 161.0467 | 781.22 | ≦0.01 | ↓ | ↑ | ↑ |
| Maltotriose | 487.1647 | 900.38 | ≦0.01 | ↓ | ↑ | ↑ |
| D-Maltose | 341.1098 | 748.53 | ≦0.01 | ↓ | ↑ | ↑ |
| L-Fucose | 163.0619 | 296.94 | ≦0.05 | ↓ | ↑ | ↑ |
| N,N'-Diacetylchitobiose | 425.1757 | 673.55 | ≦0.01 | ↓ | ↑ | ↑ |
| D-Tagatose | 161.0464 | 566.79 | ≦0.01 | ↓ | ↑ | ↑ |
| N-Acetylmannosamine | 280.1048 | 531.22 | ≦0.01 | ↓ | ↑ | ↑ |
| alpha-Linolenic acid | 279.2309 | 172.00 | ≦0.01 | ↓ | ↑ | ↓ |
| Eicosapentaenoic acid | 303.2309 | 92.16 | ≦0.01 | ↓ | ↑ | ↓ |
| D-Aspartic acid | 132.0310 | 775.96 | ≦0.01 | ↓ | ↑ | ↑ |
| Pyridoxal (Vitamin B6) | 166.0514 | 86.87 | ≦0.05 | ↓ | ↑ | ↑ |
| L-Ascorbic acid | 235.0471 | 628.60 | ≦0.01 | ↓ | ↑ | ↑ |
| Taurocholate | 533.3239 | 334.42 | ≦0.01 | ↓ | ↑ | ↑ |
| Hypoxanthine | 135.0320 | 348.16 | ≦0.01 | ↓ | ↑ | ↑ |
| Xanthine | 153.0397 | 329.69 | ≦0.01 | ↓ | ↑ | ↑ |
| Uridine | 303.0841 | 299.38 | ≦0.05 | ↓ | ↑ | ↑ |
| Cytosine | 112.0497 | 463.52 | ≦0.05 | ↓ | ↑ | ↑ |
| L-Pyroglutamic acid | 147.0755 | 717.28 | ≦0.05 | ↓ | ↑ | ↑ |
| L-Malic acid | 133.0152 | 790.60 | ≦0.05 | ↑ | ↑ | ↓ |
| Cholic acid | 426.3201 | 468.25 | ≦0.01 | ↓ | ↑ | ↓ |
| L-Alanine | 90.0542 | 663.82 | ≦0.01 | ↓ | ↑ | ↑ |
| L-Methionine | 148.0446 | 527.96 | ≦0.05 | ↓ | ↑ | ↑ |
| D-Proline | 116.0698 | 598.44 | ≦0.01 | ↓ | ↑ | ↑ |
| L-Serine | 104.0359 | 712.24 | ≦0.01 | ↓ | ↑ | ↑ |
| L-Threonine | 120.0646 | 679.34 | ≦0.05 | ↓ | ↑ | ↑ |
| L-Asparagine | 153.0312 | 456.38 | ≦0.05 | ↓ | ↑ | ↑ |
| Serotonin | 177.1013 | 260.55 | ≦0.01 | ↓ | ↑ | ↑ |
Table 2 Potential fecal biomarkers and their variation trends in CFS rats after moxibustion treatment
| Metabolite | M/Z | RT (s) | P value | M/C | A/M | B/M |
|---|---|---|---|---|---|---|
| Alpha-D-Glucose | 179.0573 | 581.66 | ≦0.01 | ↓ | ↑ | ↑ |
| D-Lactose | 341.1099 | 627.54 | ≦0.01 | ↓ | ↑ | ↑ |
| D-galacturonic acid | 193.0364 | 746.92 | ≦0.05 | ↓ | ↑ | ↑ |
| N-Acetyl-D-glucosamine | 202.0727 | 491.05 | ≦0.05 | ↓ | ↑ | ↑ |
| L-Arabinose | 149.0463 | 251.25 | ≦0.05 | ↓ | ↑ | ↑ |
| D-Allose | 239.0785 | 566.66 | ≦0.01 | ↓ | ↑ | ↑ |
| D-Mannose | 161.0467 | 781.22 | ≦0.01 | ↓ | ↑ | ↑ |
| Maltotriose | 487.1647 | 900.38 | ≦0.01 | ↓ | ↑ | ↑ |
| D-Maltose | 341.1098 | 748.53 | ≦0.01 | ↓ | ↑ | ↑ |
| L-Fucose | 163.0619 | 296.94 | ≦0.05 | ↓ | ↑ | ↑ |
| N,N'-Diacetylchitobiose | 425.1757 | 673.55 | ≦0.01 | ↓ | ↑ | ↑ |
| D-Tagatose | 161.0464 | 566.79 | ≦0.01 | ↓ | ↑ | ↑ |
| N-Acetylmannosamine | 280.1048 | 531.22 | ≦0.01 | ↓ | ↑ | ↑ |
| alpha-Linolenic acid | 279.2309 | 172.00 | ≦0.01 | ↓ | ↑ | ↓ |
| Eicosapentaenoic acid | 303.2309 | 92.16 | ≦0.01 | ↓ | ↑ | ↓ |
| D-Aspartic acid | 132.0310 | 775.96 | ≦0.01 | ↓ | ↑ | ↑ |
| Pyridoxal (Vitamin B6) | 166.0514 | 86.87 | ≦0.05 | ↓ | ↑ | ↑ |
| L-Ascorbic acid | 235.0471 | 628.60 | ≦0.01 | ↓ | ↑ | ↑ |
| Taurocholate | 533.3239 | 334.42 | ≦0.01 | ↓ | ↑ | ↑ |
| Hypoxanthine | 135.0320 | 348.16 | ≦0.01 | ↓ | ↑ | ↑ |
| Xanthine | 153.0397 | 329.69 | ≦0.01 | ↓ | ↑ | ↑ |
| Uridine | 303.0841 | 299.38 | ≦0.05 | ↓ | ↑ | ↑ |
| Cytosine | 112.0497 | 463.52 | ≦0.05 | ↓ | ↑ | ↑ |
| L-Pyroglutamic acid | 147.0755 | 717.28 | ≦0.05 | ↓ | ↑ | ↑ |
| L-Malic acid | 133.0152 | 790.60 | ≦0.05 | ↑ | ↑ | ↓ |
| Cholic acid | 426.3201 | 468.25 | ≦0.01 | ↓ | ↑ | ↓ |
| L-Alanine | 90.0542 | 663.82 | ≦0.01 | ↓ | ↑ | ↑ |
| L-Methionine | 148.0446 | 527.96 | ≦0.05 | ↓ | ↑ | ↑ |
| D-Proline | 116.0698 | 598.44 | ≦0.01 | ↓ | ↑ | ↑ |
| L-Serine | 104.0359 | 712.24 | ≦0.01 | ↓ | ↑ | ↑ |
| L-Threonine | 120.0646 | 679.34 | ≦0.05 | ↓ | ↑ | ↑ |
| L-Asparagine | 153.0312 | 456.38 | ≦0.05 | ↓ | ↑ | ↑ |
| Serotonin | 177.1013 | 260.55 | ≦0.01 | ↓ | ↑ | ↑ |
| 1. | Ellen WC. Beyond myalgic encephalomyelitis/chronic fatigue syndrome: an IOM report on redefining an illness. JAMA 2015; 313: 1101-2. |
| 2. |
Lim EJ, Son CG. Prevalence of chronic fatigue syndrome (CFS) in Korea and Japan: a Meta-analysis. J Clin Med 2021; 10: 3204-10.
DOI URL |
| 3. | Deumer US, Varesi A, Floris V, et al. Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS): an overview. J Clin Med 2021; 10: 4786-807. |
| 4. | Missailidis D, Annesley SJ, Fisher PR. Pathological mechanisms underlying myalgic encephalomyelitis/chronic fatigue syndrome. Diagnostics (Basel) 2019; 9: 80-99. |
| 5. |
Liu L, Huh JR, Shah K. Microbiota and the gut-brain-axis: Implications for new therapeutic design in the CNS. EBioMedicine 2022; 77: 103908-18.
DOI URL |
| 6. |
Maqsood R, Stone TW. The gut-brain axis, BDNF, NMDA and CNS disorders. Neurochem Res 2016; 41: 2819-35.
PMID |
| 7. |
König RS, Albrich WC, Kahlert CR, et al. The gut microbiome in myalgic encephalomyelitis (ME)/chronic fatigue syndrome (CFS). Front Immunol 2022; 12: 628741-64.
DOI URL |
| 8. | Varesi A, Deumer US, Ananth S, Ricevuti G. The emerging role of gut microbiota in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS): current evidence and potential therapeutic applications. J Clin Med 2021; 10: 5077-93. |
| 9. | Frémont M, Coomans D, Massart S, Meirleir KD. High-throughput 16S rRNA gene sequencing reveals alterations of intestinal microbiota in myalgic encephalomyelitis/chronic fatigue syndrome patients. Anaerobe 2013; 22: 50-6. |
| 10. |
Navaneetharaja N, Griffiths V, Wileman T, Carding SR. A role for the intestinal microbiota and virome in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS)? J Clin Med 2016; 5: 55-76.
DOI URL |
| 11. |
Giloteaux L, Goodrich JK, Walters WA, Levine SM, Ley RE, Hanson MR. Reduced diversity and altered composition of the gut microbiome in individuals with myalgic encephalomyelitis/chronic fatigue syndrome. Microbiome 2016; 4: 30-40.
DOI PMID |
| 12. | You JY, Ye J, Li HY, Ye WG, Hong ES. Moxibustion for chronic fatigue syndrome: a systematic review and Meta-analysis. Evid Based Complement Alternat Med 2021; 2021: 6418217-27. |
| 13. |
Vadim O, Clair RM, Emeran AM. The gut-brain axis and the microbiome: mechanisms and clinical implications. Clin Gastroenterol Hepatol 2019; 17: 322-32.
DOI URL |
| 14. |
Fang Y, Yue BW, Ma HB, Yuan YP. Acupuncture and moxibustion for chronic fatigue syndrome: a systematic review and network Meta-analysis. Medicine (Baltimore) 2022; 101: e29310-19.
DOI URL |
| 15. | Yin ZH, Wang LJ, Cheng Y, et al. Acupuncture for chronic fatigue syndrome: an overview of systematic reviews. Zhong Guo Jie He Yi Xue 2021; 27: 940-6. |
| 16. | CHEN SS, LIU R, WU B, et al. Acupuncture on back-Shu points of five Zang for chronic fatigue syndrome randomized control trial. Shi Jie Zhen Ji 2018; 28: 237-41+309-310. |
| 17. |
Shu Q, Wang H, Litscher D, et al. Acupuncture and moxibustion have different effects on fatigue by regulating the autonomic nervous system: a pilot controlled clinical trial. Sci Rep 2016; 6: 37846-56.
DOI PMID |
| 18. | Lin YF, Jin XQ, Zhu JF, et al. Ginger-separated moxibustion for chronic fatigue syndrome and its effect on intestinal flora. Zhong Guo Zhen Jiu 2021; 41: 269-74. |
| 19. | Li CR, Sun ZR, Wang YL, et al. Mechanism of acupuncture and moxibustion in treatment of chronic fatigue syndrome from perspective of intestinal flora. Zhong Guo Zhen Jiu 2022; 42: 956-60. |
| 20. | Ma TT, Wu J, Yang LJ, et al. Ginger-indirect moxibustion plus acupuncture versus acupuncture alone for chronic fatigue syndrome: a randomized controlled trial. J Tradit Chin Med 2022; 42: 242-9. |
| 21. | Xu XS, Ma W, Xiong LJ, et al. Effect of herbal cake-separated moxibustion on behavioral stress reactions and blood lactic acid level and muscular AMPK/PGC-1asignaling in rats with chronic fatigue syndrome. Acupuncture Research 2022; 47: 878-84. |
| 22. | Shao CZ, Ren YM, Wang ZN, Kang CZ, Jiang HK, Chi AP. Detection of urine metabolites in a rat model of chronic fatigue syndrome before and after exercise. Biomed Res Int 2017; 2017: 8182020-31. |
| 23. |
Shao CZ, Song J, Zhao SG, Jiang HK, Wang BP, Chi AP. Therapeutic effect and metabolic mechanism of a selenium-polysaccharide from Ziyang green tea on chronic fatigue syndrome. Polymers (Basel) 2018; 10: 1269-86.
DOI URL |
| 24. |
Chi AP, Shen ZM, Zhu WF, Sun YL, Kang YJ, Guo F. Characterization of a protein-bound polysaccharide from Herba Epimedii and its metabolic mechanism in chronic fatigue syndrome. J Ethnopharmacol 2017; 203: 241-51.
DOI PMID |
| 25. |
Chi AP, Zhang Y, Kang YJ, Shen ZM. Metabolic mechanism of a polysaccharide from Schisandra chinensis to relieve chronic fatigue syndrome. Int J Biol Macromol 2016; 93: 322-32.
DOI PMID |
| 26. | Yuan GQ, Jia ZH, Yang HT, et al. Comfortable lifestyle-induced imbalance of neuro-endocrine-immunity network: a possible mechanism of vascular endothelial dysfunction. Zhong Guo Jie He Yi Xue 2010; 16: 54-60. |
| 27. | Liang FR, Wang H. Science of acupuncture and moxibustion. 5th ed. Beijing: China Press of Traditional Chinese Medicine, 2021: 297-8. |
| 28. | Xu XS, Ma W, Xiong LJ, Zhai CT, Li W, Tian YF. Effect of herbal cake-separated moxibustion on behavioral stress reactions and blood lactic acid level and muscular AMPK/PGC-1α signaling in rats with chronic fatigue syndrome. Zhen Ci Yan Jiu 2022; 47: 878-84. |
| 29. | Liu CZ, Lei B. Effect of moxibustion at Shenque point on exercise capacity of rats with chronic fatigue syndrome. You Jiang Yi Xue 2022; 50: 653-6. |
| 30. |
Nagy-Szakal D, Williams BL, Mishra N, et al. Fecal metagenomic profiles in subgroups of patients with myalgic encephalomyelitis/chronic fatigue syndrome. Microbiome 2017; 5: 44-60.
DOI PMID |
| 31. |
Nagy-Szakal D, Barupal DK, Lee B, et al. Insights into myalgic encephalomyelitis/chronic fatigue syndrome phenotypes through comprehensive metabolomics. Sci Rep 2018; 8: 10056-66.
DOI PMID |
| 32. |
Kesika P, Suganthy N, Sivamaruthi BS, et al. Role of gut-brain axis, gut microbial composition, and probiotic intervention in Alzheimer's disease. Life Sci 2021; 264: 118627-37.
DOI URL |
| 33. |
Dogra N, Mani RJ, Katare DP. The gut-brain axis: two ways signaling in Parkinson's disease. Cell Mol Neurobiol 2022; 42: 315-32.
DOI |
| 34. |
Morris G, Anderson G, Galecki P, Berk M, Maes M. A narrative review on the similarities and dissimilarities between myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) and sickness behavior. BMC Med 2013; 11: 64-82.
DOI PMID |
| 35. |
Gucht VD, Garcia FK, Engelsman MD, Maes S. Differences in physical and psychosocial characteristics between CFS and fatigued non-CFS Patients, a case-control study. Int J Behav Med 2016; 23: 589-94.
DOI PMID |
| 36. |
Heins MJ, Knoop H, Bleijenberg G. The role of the therapeutic relationship in cognitive behaviour therapy for chronic fatigue syndrome. Behav Res Ther 2013; 51: 368-76.
DOI PMID |
| 37. | Dougherty JP, Springer DA, Cullen MJ, Gershengorn MC. Evaluation of the effects of chemotherapy-induced fatigue and pharmacological interventions in multiple mouse behavioral assays. Behav Brain Res 2019; 360: 255-61. |
| 38. | Zombeck JA, Fey EG, Lyng GD, Sonis ST. A clinically translatable mouse model for chemotherapy-related fatigue. Comp Med 2013; 63: 491-7. |
| 39. |
Giloteaux L, Hanson MR, Keller BA. A pair of identical twins discordant for myalgic encephalomyelitis/chronic fatigue syndrome differ in physiological parameters and gut microbiome composition. Am J Case Rep 2016; 17: 720-9.
DOI URL |
| 40. | Yang H, Cai R, Kong ZY, et al. Teasaponin ameliorates murine colitis by regulating gut microbiota and suppressing the immune system response. Front Med (Lausanne) 2020; 7: 584369-86. |
| 41. |
Ye J, Zhao Y, Chen XM, et al. Pu-erh tea ameliorates obesity and modulates gut microbiota in high fat diet fed mice. Food Res Int 2021; 144: 110360-72.
DOI URL |
| 42. |
Liang S, Wu XL, Hu X, Wang T, Jin F. Recognizing depression from the microbiota⁻gut⁻brain axis. Int J Mol Sci 2018; 19: 1592-607.
DOI URL |
| 43. |
Shukla SK, Cook D, Meyer J, et al. Changes in gut and plasma microbiome following exercise challenge in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS). PLoS One 2015; 10: e0145453-67.
DOI URL |
| 44. |
Sheedy JR, Wettenhall REH, Scanlon D, et al. Increased d-lactic acid intestinal bacteria in patients with chronic fatigue syndrome. In Vivo 2009; 23: 621-8.
PMID |
| 45. |
Singh PK, Chopra K, Kuhad A, Kaur IP. Role of Lactobacillus acidophilus loaded floating beads in chronic fatigue syndrome: behavioral and biochemical evidences. Neurogastroenterol Motil 2012; 24: 366-e170.
DOI URL |
| 46. |
Sullivan A, Nord CE, Evengård B. Effect of supplement with lactic-acid producing bacteria on fatigue and physical activity in patients with chronic fatigue syndrome. Nutr J 2009; 8: 4-9.
DOI PMID |
| 47. |
Rao AV, Alison C, Bested AC, et al. A randomized, double-blind, placebo-controlled pilot study of a probiotic in emotional symptoms of chronic fatigue syndrome. Gut Pathog 2009; 1: 6-11.
DOI PMID |
| 48. |
Armstrong CW, McGregor NR, Lewis DP, Butt HL, Gooley PR. The association of fecal microbiota and fecal, blood serum and urine metabolites in myalgic encephalomyelitis/chronic fatigue syndrome. Metabolomics 2017; 13: 8-20.
DOI URL |
| 49. |
Larsen JM. The immune response to Prevotella bacteria in chronic inflammatory disease. Immunology 2017; 151: 363-74.
DOI PMID |
| 50. |
Castillo NA, Perdigón G, Leblanc AM. Oral administration of a probiotic Lactobacillus modulates cytokine production and TLR expression improving the immune response against Salmonella enterica serovar Typhimurium infection in mice. BMC Microbiol 2011; 11: 177-88.
DOI PMID |
| 51. |
Pellegrini C, Antonioli L, Colucci R, Blandizzi C, Fornai M. Interplay among gut microbiota, intestinal mucosal barrier and enteric neuro-immune system: a common path to neurodegenerative diseases? Acta Neuropathol 2018; 136: 345-61.
DOI PMID |
| 52. |
Cirstea MS, Yu AC, Golz E. Microbiota composition and metabolism are associated with gut function in Parkinson's disease. Mov Disord 2020; 35: 1208-17.
DOI URL |
| 53. |
Li ZM, Lai JB, Zhang PF, et al. Multi-omics analyses of serum metabolome, gut microbiome and brain function reveal dysregulated microbiota-gut-brain axis in bipolar depression. Mol Psychiatry 2022; 27: 4123-35.
DOI |
| 54. |
Shi JF, Qiu HY, Xu Q, et al. Integrated multi-omics analyses reveal effects of empagliflozin on intestinal homeostasis in high-fat-diet mice. iScience 2022; 26: 105816-31.
DOI URL |
| 55. |
Gao YH, Liu XL, Pan MY, et al. Integrated untargeted fecal metabolomics and gut microbiota strategy for screening potential biomarkers associated with schizophrenia. J Psychiatr Res 2022; 156: 628-38.
DOI PMID |
| 56. |
Fu YP, Li CY, Peng X, Wangensteen H, Inngjerdingen KT, Zou YF. Pectic polysaccharides from Aconitum carmichaelii leaves protects against DSS-induced ulcerative colitis in mice through modulations of metabolism and microbiota composition. Biomed Pharmacother 2022; 155: 113767-78.
DOI URL |
| 57. |
Iribarren C, Magnusson MK, Vigsnæs LK, et al. The Effects of human milk oligosaccharides on gut microbiota, metabolite profiles and host mucosal response in patients with irritable bowel syndrome. Nutrients 2021; 13: 3836-54.
DOI URL |
| 58. |
Guenther S, Loebel M, Mooslechner AA, et al. Frequent IgG subclass and mannose binding lectin deficiency in patients with chronic fatigue syndrome. Hum Immunol 2015; 76: 729-35.
DOI PMID |
| 59. |
Miao XY, Xiao BK, Shui SF, Yang JY, Huang RQ, Dong JX. Metabolomics analysis of serum reveals the effect of Danggui Buxue Tang on fatigued mice induced by exhausting physical exercise. J Pharm Biomed Anal 2018; 151: 301-9.
DOI PMID |
| 60. |
Canfora EE, Jocken JW, Blaak EE. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat Rev Endocrinol 2015; 11: 577-91.
DOI PMID |
| 61. |
Sharma V, Jamie Smolin J, Nayak J, et al. Mannose alters gut microbiome, prevents diet-induced obesity, and improves host metabolism. Cell Rep 2018; 24: 3087-98.
DOI PMID |
| 62. |
Stojanov S, Berlec A, Štrukelj B. The influence of probiotics on the Firmicutes/Bacteroidetes ratio in the treatment of obesity and inflammatory bowel disease. Microorganisms 2020; 8: 1715-30.
DOI URL |
| 63. |
Zhang D, Chia C, Jiao X, et al. D-mannose induces regulatory T cells and suppresses immunopathology. Nat Med 2017; 23: 1036-45.
DOI PMID |
| 64. |
Shi YB, Yin DL. A good sugar, A good sugar, d-mannose, suppresses autoimmune diabetes. Cell Biosci 2017; 7: 48-9.
DOI |
| 65. | Guo LJ, Hou YN, Song L, Zhu SY, Lin FR, Bai YX. D-Mannose enhanced immunomodulation of periodontal ligament stem cells via inhibiting IL-6 secretion. Stem Cells Int 2018; 2018: 7168231-41. |
| 66. |
Wu JQ, Gao WN, Wei JY, Yang JJ, Pu LL, Guo CJ. Quercetin alters energy metabolism in swimming mice. Appl Physiol Nutr Metab 2012; 37: 912-22.
DOI URL |
| 67. |
Chen SK, Liu YL, Wang XY, et al. Asparagine improves intestinal integrity, inhibits TLR4 and NOD signaling, and differently regulates p38 and ERK1/2 signaling in weanling piglets after LPS challenge. Innate Immun 2016; 22: 577-87.
DOI URL |
| 68. |
Zhu HL, Pi DA, Leng WB, et al. Asparagine preserves intestinal barrier function from LPS-induced injury and regulates CRF/CRFR signaling pathway. Innate Immun 2017; 23: 546-56.
DOI URL |
| 69. |
Stegink LD, Filer LJ Jr, Bell EF, Ziegler EE, Tephly TR. Effect of repeated ingestion of aspartame-sweetened beverage on plasma amino acid, blood methanol, and blood formate concentrations in normal adults. Metabolism 1989; 38: 357-63.
DOI URL |
| 70. |
Wilkinson DJ, Smeeton NJ, Watt PW. Ammonia metabolism, the brain and fatigue; revisiting the link. Prog Neurobiol 2010; 91: 200-19.
DOI PMID |
| 71. |
Coqueiro AY, Raizel R, Bonvini A. Effects of glutamine and alanine supplementation on central fatigue markers in rats submitted to resistance training. Nutrients 2018; 10: 119-33.
DOI URL |
| 72. |
Newsholme P, Bender K, Kiely A, Brennan L. Amino acid metabolism, insulin secretion and diabetes. Biochem Soc Trans 2007; 35: 1180-6.
DOI URL |
| 73. | Rodriguez AE, Ducker GS, Billingham LK, et al. Serine metabolism supports macrophage IL-1β production. Cell Metab 2019; 29: 1003-11. |
| 74. |
Gaifem J, Gonçalves LG, Dinis-Oliveira RJ. L-threonine supplementation during colitis onset delays disease recovery. Front Physiol 2018; 9: 1247-53.
DOI PMID |
| 75. |
Zhang HW, Hua R, Zhang BX, Zhang XM, Yang H, Zhou XH. Serine alleviates dextran sulfate sodium-induced colitis and regulates the gut microbiota in mice. Front Microbiol 2018; 9: 3062-71.
DOI PMID |
| 76. | Wu CH, Ko JL, Liao JM, et al. D-methionine alleviates cisplatin-induced mucositis by restoring the gut microbiota structure and improving intestinal inflammation. Ther Adv Med Oncol 2019; 11: 1758835918821021-38. |
| 77. |
Ge XL, Ding C, Zhao W, et al. Antibiotics-induced depletion of mice microbiota induces changes in host serotonin biosynthesis and intestinal motility. J Transl Med 2017; 15: 13-21.
DOI PMID |
| 78. |
Cao HL, Liu X, An YY, et al. Dysbiosis contributes to chronic constipation development via regulation of serotonin transporter in the intestine. Sci Rep 2017; 7: 10322-33.
DOI PMID |
| 79. |
Reifen R, Karlinsky A, Stark AH, Berkovich Z, Nyska A. α-Linolenic acid (ALA) is an anti-inflammatory agent in inflammatory bowel disease. J Nutr Biochem 2015; 26: 1632-40.
DOI PMID |
| 80. |
Maes M, Mihaylova I, Leunis JC. In chronic fatigue syndrome, the decreased levels of omega-3 poly-unsaturated fatty acids are related to lowered serum zinc and defects in T cell activation. Neuro Endocrinol Lett 2005; 26: 745-51.
PMID |
| 81. | Warren G, McKendrick M, Peet M. The role of essential fatty acids in chronic fatigue syndrome. A case-controlled study of red-cell membrane essential fatty acids (EFA) and a placebo-controlled treatment study with high dose of EFA. Acta Neurol Scand 1999; 99: 112-6. |
| 82. |
Lee JS, Wang RX, Alexeev EE, et al. Hypoxanthine is a checkpoint stress metabolite in colonic epithelial energy modulation and barrier function. J Biol Chem 2018; 293: 6039-51.
DOI PMID |
| 83. |
Izabella Surowiec, Gjesdal CG, Jonsson G, et al. Metabolomics study of fatigue in patients with rheumatoid arthritis naïve to biological treatment. Rheumatol Int 2016; 36: 703-11.
DOI PMID |
| 84. |
Bjørklund G, Dadar M, Pen JJ, Chirumbolo S, Aaseth J. Chronic fatigue syndrome (CFS): suggestions for a nutritional treatment in the therapeutic approach. Biomed Pharmacother 2019; 109: 1000-7.
DOI PMID |
| 85. |
Maric D, Brkic S, Tomic S, Mikic AN, Cebovic T, Turkulov V. Multivitamin mineral supplementation in patients with chronic fatigue syndrome. Med Sci Monit 2014; 20: 47-53.
DOI URL |
| 86. |
Cai J, Sun LL, Gonzalez FJ. Gut microbiota-derived bile acids in intestinal immunity, inflammation, and tumorigenesis. Cell Host Microbe 2022; 30: 289-300.
DOI PMID |
| 87. |
Guo C, Xie S, Chi Z, et al. Bile acids control inflammation and metabolic disorder through Inhibition of NLRP3Inflammasome. Immunity 2016; 45: 802-16.
DOI URL |
| 88. |
Li Q, Li M, Li FH, et al. Qiang-Gan formula extract improves non-alcoholic steatohepatitis via regulating bile acid metabolism and gut microbiota in mice. J Ethnopharmacol 2020; 258: 112896-906.
DOI URL |
| 89. |
Lin S, Yang XM, Long YR, et al. Dietary supplementation with Lactobacillus plantarum modified gut microbiota, bile acid profile and glucose homoeostasis in weaning piglets. Br J Nutr 2020; 124: 797-808.
DOI URL |
| 90. |
Guo Q, Tang Y, Li Y, et al. Perinatal high-salt diet induces gut microbiota dysbiosis, bile acid homeostasis disbalance, and NAFLD in weanling mice offspring. Nutrients 2021; 13: 2135-47.
DOI URL |
| 91. |
Hui SC, Huang L, Wang XL, et al. Capsaicin improves glucose homeostasis by enhancing glucagon-like peptide-1 secretion through the regulation of bile acid metabolism via the remodeling of the gut microbiota in male mice. FASEB J 2020; 34: 8558-73.
DOI URL |
| 92. |
Hosseinkhani F, Heinken A, Thiele I, Lindenburg PW, Harms AC, Hankemeier T. The contribution of gut bacterial metabolites in the human immune signaling pathway of non-communicable diseases. Gut Microbes 2021; 13: 1-22.
DOI PMID |
| 93. |
Brown EM, Ke XB, Hitchcock D. Bacteroides-derived sphingolipids are critical for maintaining intestinal homeostasis and symbiosis. Cell Host Microbe 2019; 25: 668-80.
DOI PMID |
| 94. | Zafar H, Saier MH Jr. Gut Bacteroides species in health and disease. Gut Microbes 2021; 13: 1-20. |
| 95. |
Vinolo MA, Rodrigues HG, Nachbar RT, Curi R. Regulation of inflammation by short chain fatty acids. Nutrients 2011; 3: 858-76.
DOI PMID |
| 96. |
Nagpal R, Wang SH, Ahmadi S, et al. Human-origin probiotic cocktail increases short-chain fatty acid production via modulation of mice and human gut microbiome. Sci Rep 2018; 8: 12649-63.
DOI |
| 97. |
Murphy EC, Mörgelin M, Cooney JC, Frick I-M. Interaction of Bacteroides fragilis and Bacteroides thetaiotaomicron with the kallikrein-kinin system. Microbiology (Reading) 2011; 157: 2094-105.
DOI PMID |
| 98. |
Wexler HM. Bacteroides: the good, the bad, and the nitty-gritty. Clin Microbiol Rev 2007; 20: 593-621.
DOI PMID |
| [1] | WANG Miao, ZHU Yan, ZHAO Hui, ZHAO Hongfang. Moxibustion enables protective effects on rheumatoid arthritis-induced myocardial injury via transforming growth factor beta1 signaling and metabolic reprogramming [J]. Journal of Traditional Chinese Medicine, 2023, 43(6): 1190-1199. |
| [2] | JIANG Wenjing, JIANG Huaying, YUAN Lihua, SA Yuanhong, XIAO Jimei, SUN Hongqi, SONG Jingyan, SUN Zhengao. Xiaoyi Yusi decoction (消异育嗣汤) improves in vitro fertilization and embryo transfer outcomes in patients with endometriosis [J]. Journal of Traditional Chinese Medicine, 2023, 43(5): 1026-1033. |
| [3] | ZHOU Jun, WANG Junhua, LI Xiaobing, WAN Chenyi, LI Fangjun, Lü Yanni, CHEN Hao, SUN Meiying. Efficacy of Heshouwu (Radix Polygoni Multiflori) on gut mircobiota in mice with autoimmune encephalomyelitis [J]. Journal of Traditional Chinese Medicine, 2023, 43(4): 676-685. |
| [4] | LIU Di, ZHANG Yingqi, YU Tianyuan, LIU Zhifeng, JIAO Yi, WANG Hourong, XU Yajing, GUAN Qian, CHEN Lulu, HU Hui. Protective mechanisms of Tuina therapy against lipopolysaccharide-induced fever in young rabbits based on untargeted metabolomics analysis [J]. Journal of Traditional Chinese Medicine, 2023, 43(4): 725-733. |
| [5] | LI Xingjie, LIU Qiqi, XIA Rui, LIU Jun, WANG Dan, SHI Jiao, KUANG Yuxing, DAI Yalan, HUANG Haoyu, TANG Wei, CHEN Shangjie. Moxibustion modulates working memory in patients with amnestic mild cognitive impairment: a functional magnetic resonance imaging study [J]. Journal of Traditional Chinese Medicine, 2023, 43(4): 801-808. |
| [6] | MA Fangfang, ZHANG Hewei, LI Bingxue, CHENG Peiyu, YU Mingwei, WANG Xiaomin. Acupuncture and moxibustion for malignant tumor patients with psychological symptoms of insomnia, anxiety and depression: a systematic review and Meta-analysis [J]. Journal of Traditional Chinese Medicine, 2023, 43(3): 441-456. |
| [7] | JIANG Yiqian, ZHOU Xibin, PU Wenyuan, ZHOU Chunxiang. Sanwu Baisan decoction (三物白散) inhibits colorectal cancer progression in mice by remodeling gut microbiota and tumorigenesis [J]. Journal of Traditional Chinese Medicine, 2023, 43(3): 466-473. |
| [8] | YANG Yang, YUAN Haining, JIA Hongxiao, NING Yanzhe, WANG Di, ZHANG Lei, YAN Kaijuan, GUO Yumeng, WANG Fei, SUN Weishuang, CHEN Pei. Therapy of replenishing Yin and regulating Yang for manic episode in bipolar disorder: study protocol for a prospective, double-blind, randomized controlled trial [J]. Journal of Traditional Chinese Medicine, 2023, 43(3): 594-601. |
| [9] | MIN Youjiang, YAO Haihua, WANG Zhiqin, LUO Kaitao, SUN Jie, YUAN Zheng, WU Huiqi, CHENG Lihong. Efficacy of suspended moxibustion stimulating Shenshu (BL23) and Guanyuan (CV4) on the amygdala-HPA axis in rats with kidney-Yang deficiency symptom pattern induced by hydrocortisone [J]. Journal of Traditional Chinese Medicine, 2023, 43(1): 113-123. |
| [10] | YAO Yao, ZHAO Zhenni, CHEN Fengqin, LENG Yufei, PANG Xiangtian, XU Xiao, SUN Zhiling. Effectiveness of moxibustion alone on lumbar disc herniation: a Meta-analysis of randomized controlled trials [J]. Journal of Traditional Chinese Medicine, 2023, 43(1): 14-26. |
| [11] | YANG Jun, XIONG Jun, XU Shaozhong, XIE Hongwu, XIANG Jie. Effect and cerebral mechanism of moxibustion at heat-sensitized Yaoyangguan (GV3) in patients with lumbar disc herniation and myofascial pain syndrome by resting-state functionality magnetic resonance imaging: protocol for an observational study [J]. Journal of Traditional Chinese Medicine, 2023, 43(1): 175-180. |
| [12] | FANG Jing, PAN Wen, WANG Xiangyun, LI Fengxing, ZHAO Ling, HUANG Zouqin, SHEN Xueyong. Efficacy of stimulating Mingmen (GV4) and Guanyuan (CV4) on kidney Yang deficiency in rat model: laser irradiation vs traditional moxibustion [J]. Journal of Traditional Chinese Medicine, 2022, 42(6): 972-979. |
| [13] | ZHOU Haiyan, ZHONG Yumei, GAO Xiuhua, WU Fei, JIA Min, YANG Xin. Efficacy of Moxa-burning heat stimulating Zusanli (ST36) and Shenshu (BL23) on expressions of macrophage migration inhibitory factor and macrophage apoptosis in rabbits with adjuvant-induced arthritis [J]. Journal of Traditional Chinese Medicine, 2022, 42(6): 980-987. |
| [14] | SUN Mengzhu, ZHANG Yujie, SONG Yafang, GUO Jing, ZHAO Tingting, WANG Yuhang, PEI Lixia, SUN Jianhua. Electroacupuncture at Tianshu (ST25) and Zusanli (ST36) alleviates stress-induced irritable bowel syndrome in mice by modulating gut microbiota and corticotropin-releasing factor [J]. Journal of Traditional Chinese Medicine, 2022, 42(5): 732-740. |
| [15] | YU Zeyue, HAO Liyu, LI Zongyuan, SUN Jianhui, CHEN Hongying, HUO Hairu, LI Xiaoqin, SHAN Zhongchao, LI Hongmei. Correlation between slow transit constipation and spleen Qi deficiency, and gut microbiota: a pilot study [J]. Journal of Traditional Chinese Medicine, 2022, 42(3): 353-363. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||

