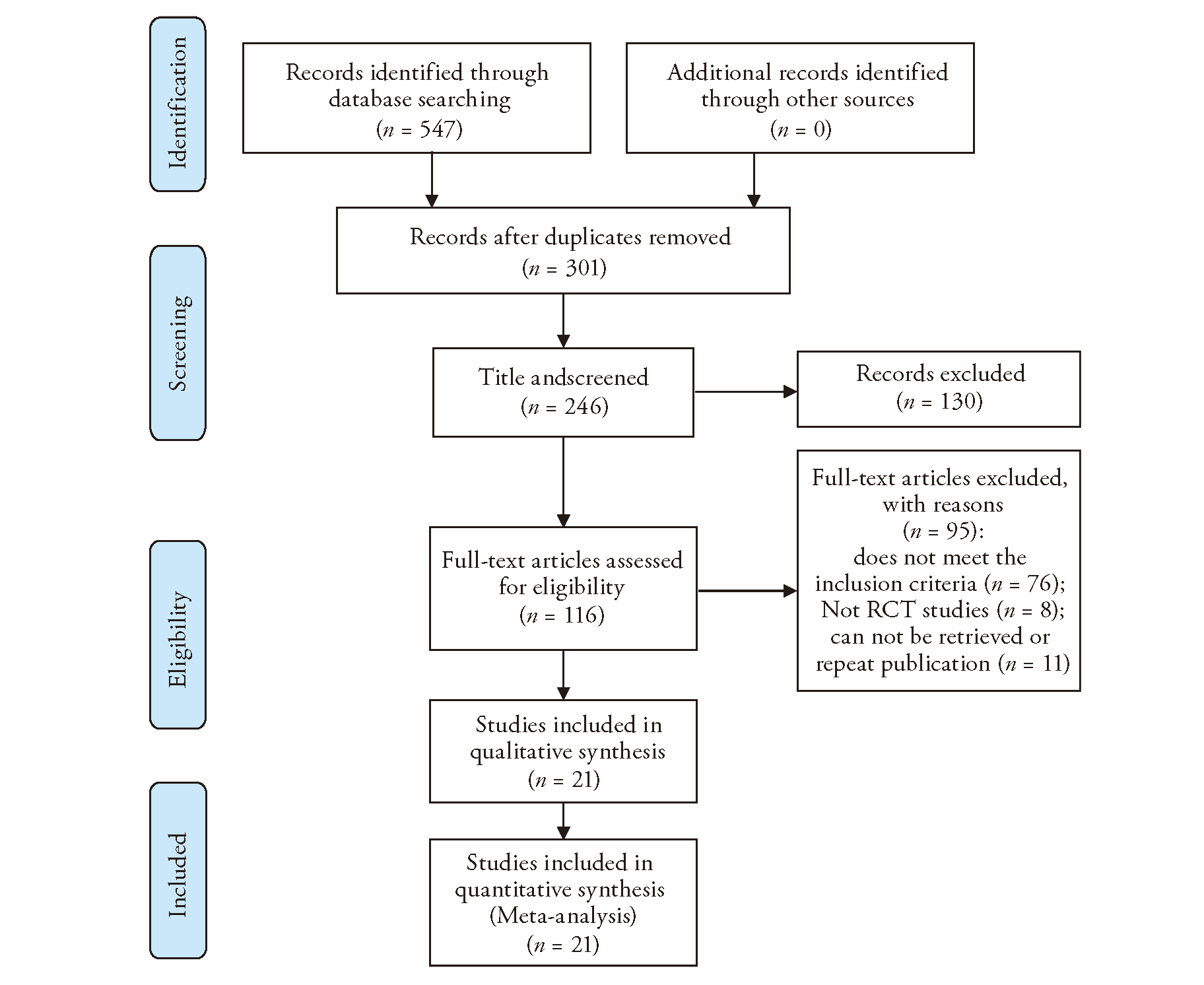
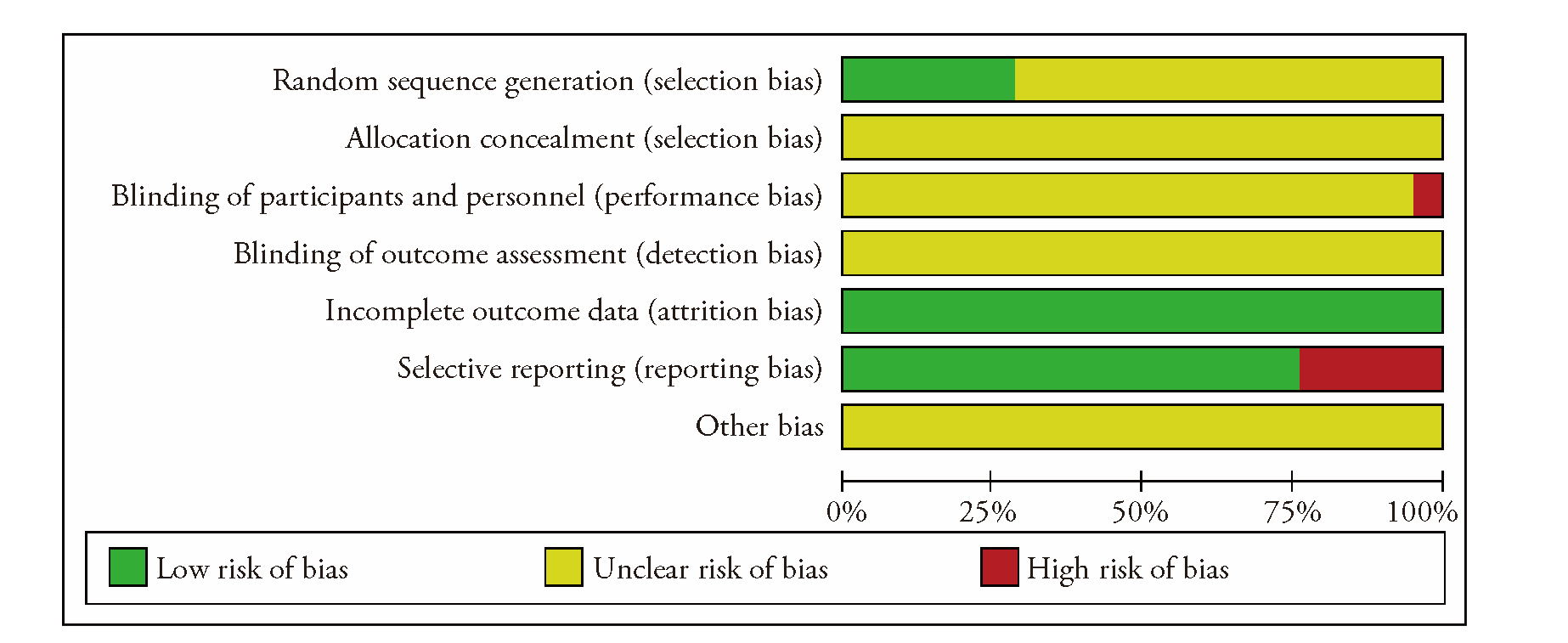
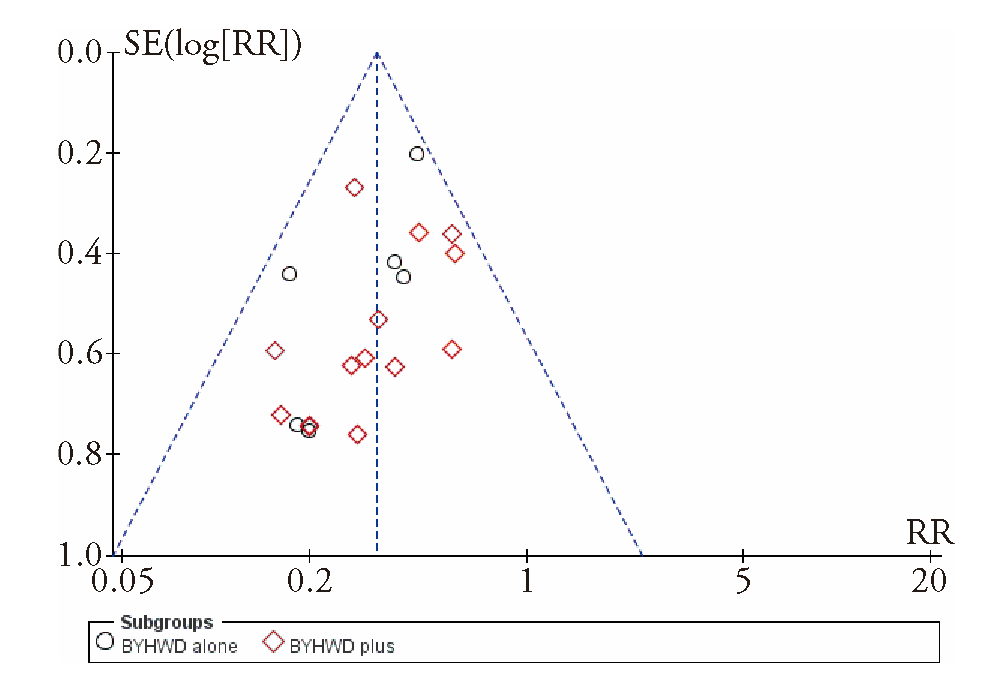
Journal of Traditional Chinese Medicine ›› 2023, Vol. 43 ›› Issue (5): 841-850.DOI: 10.19852/j.cnki.jtcm.20230802.002
• Meta Analysis • Previous Articles Next Articles
Efficacy and safety of Buyang Huanwu decoction (补阳还五汤) for diabetic peripheral neuropathy: a systematic review and Meta-analysis
ZHANG Meizhen1, HAO Xiaohui2,3, TANG Yiting1, CHEN Yupeng1, HE Puyu1, ZHAO Liming1, PANG Bing1( ), NI Qing1(
), NI Qing1( )
)
- 1 Department of Endocrinology, Guang’anmen Hospital of China Academy of Chinese Medical Sciences, Beijing 100053, China
2 Graduate School of Beijing University of Chinese Medicine, Beijing 100029, China
3 Department of Endocrinology, Guang’anmen Hospital of China Academy of Chinese Medical Sciences, Beijing 100053, China
-
Received:2022-09-26Accepted:2022-12-11Online:2023-10-15Published:2023-08-02 -
Contact:Prof. NI Qing, Department of Endocrinology, Guang’anmen Hospital of China Academy of Chinese Medical Sciences, Beijing 100053, China.niqing669@163.com ; PANG Bing, Department of Endocrinology, Guang’anmen Hospital of China Academy of Chinese Medical Sciences, Beijing 100053, China,307636788@qq.com . Telephone: +86-18810535932 -
Supported by:Natural Science Foundation-funded Project, Optimization of Distribution of Anti-Diabetes Traditional Chinese Medicine based on Glucose Metabolism Signal Pathway and Uniform Design(7182143)
Cite this article
ZHANG Meizhen, HAO Xiaohui, TANG Yiting, CHEN Yupeng, HE Puyu, ZHAO Liming, PANG Bing, NI Qing. Efficacy and safety of Buyang Huanwu decoction (补阳还五汤) for diabetic peripheral neuropathy: a systematic review and Meta-analysis[J]. Journal of Traditional Chinese Medicine, 2023, 43(5): 841-850.
share this article
| Source | Sample | Sex | Age (years) | Intervention | Treatment | DN | Outcome | ||
|---|---|---|---|---|---|---|---|---|---|
| (T/C) | (male | period | duration | measure | |||||
| /female) | Treatment group | Control group | (weeks) | (years) | |||||
| Ning RZ et al 2020 | 30/30 | T:13/17 | T:62.53±3.67 | Modified BYHWD (150 mL, bid)+ Mecobalamine (500 μg tid, po)+BT | Mecobalamine (500 μg tid, po)+BT | 12 | T:1.83 ± 1.78 | CER | |
| C:16/14 | C:61.43±4.29 | C: 2.2 ± 1.17 | |||||||
| Han Y 2005 | 30/20 | Not reported | Not reported | Modified BYHWD (bid)+BT | Mecobalamin (500 μg, tid, po)+BT | 14 | Not reported | NCV+Hemorheology | |
| Gong LZ et al 2017 | 60/60 | Not reported | Not reported | Modified BYHWD (200 mL, bid) | Mecobalamin (1 mg,qd, iv)+BT | 8 | Not reported | NCV | |
| +BT | |||||||||
| Wu Y, Ma D 2015 | 60/60 | T:27/33 | T:45-75 | Modified BYHWD+ BT | Mecobalamin (0.5-1 mg, qd, iv)+BT | 12 | T:0.25-3 | CER+NCV | |
| C: 32/28 | C: 48-74 | Mecobalamin (0.5-1 mg, qd, iv) | C: 0.42-3 | ||||||
| Sun SZ, Wang YZ 2008 | 42/42 | T:23/19 | T:44-73 | BYHWD (250 mL, bid)+Mecobalamin | Mecobalamin (0.5 mL, qd, im)+BT | 12 | T:0.8-13.5 | CER+NCV+ Hemorheology | |
| C: 22/20 | C: 45-72 | (0.5 mL, qd, im)+BT | C: 0.6-13.6 | ||||||
| Zhang T 2008 | 60/60 | T:34/26 | T:54.6±5.9 | Modified BYHWD (bid)+BT | Mecobalamin (500 μg, qd, im)+BT | 12 | T:0.5-3 | CER+NCV | |
| C: 32/28 | C: 54.8±5.8 | C: 0.5-3 | |||||||
| Sun BX, Li LQ 2017 | 47/47 | T:24/23 | T:59.64±10.51 | Modified BYHWD (bid)+BT | Mecobalamin (0.5 mg, qd, im)+BT | 12 | T:6.87±3.41 | CER | |
| C: 22/25 | C: 59.35±10.33 | C: 7.05±3.81 | |||||||
| Yang YQ, Xing DZ 2019 | 38/38 | T:19/19 | T:67.91±2.18 | Modified BYHWD (250 mL, bid) + Mecobalamin (0.5-1 mg, qd,im) | Mecobalamin (0.5-1 mg, qd, im) | 12 | Not reported | CER | |
| C: 18/20 | C: 68.59±2.12 | ||||||||
| Liu B 2018 | 41/41 | T:22/19 | T:59.85±2.82 | Modified BYHWD (200 mL, bid)+ Mecobalamine (0,5 mg, tid, po) + BT | Mecobalamine (0.5 mg, tid, po)+BT | 8 | Not reported | CER+NCV | |
| C: 21/20 | C: 59.35±2.76 | ||||||||
| Pan XJ 2017 | 30/30 | T:13/17 | T:50.74±9.25 | Modified BYHWD (150 mL, bid) + | Mecobalamine (500 μg, tid, po)+BT | 12 | T:8.45±1.26 | CER+NCV+Hemorheology | |
| C: 16/14 | C: 49.62±9.83 | Meco-balamine (500 μg, tid, po) + BT | C: 8.72±1.35 | ||||||
| Li YF 2009 | 36/36 | T:20/16 | T:42-69 | Modified BYHWD (bid) + BT | Mecobalamine (500 μg, tid, po)+BT | 8 | Not reported | CER+NCV+Hemorheology | |
| C: 16/20 | C: 41- | ||||||||
| Peng H 2009 | 30/30 | T:18/12 | T:52.7±11.5 | Modified BYHWD (200 mL, bid) +Mecobalamine (0.5 mg, tid, po)+BT | Mecobalamine (0.5 mg, tid, po)+BT | 12 | T:6.52±1.21 | CER+ NCV+Hemorheology | |
| C: 14/16 | C: 50.8±12.4 | C: 6.46±1.12 | |||||||
| Luo XY 2007 | 40/40 | T:22/18 | T:66.68±9.82 | BYHWD (100 mL, bid)+Mecobalamine ( | Mecobalamine (500 μg, tid, po)+BT | 8 | Not reported | CER +NCV+Hemorheology | |
| C: 21/19 | C: 66.9±10.53 | 500 μg, tid, po)+BT | |||||||
| Wang SX et al 2005 | 130/78 | T:78/52 | T:56.6±6.3 | Modified BYHWD (bid)+BT | Mecobalamine (500 μg, tid, po)+BT | 8 | T:2.3±1.2 | CER+ NCV+Hemorheology | |
| C: 45/33 | C: 55.8±6.5 | C: 2.3±1.5 | |||||||
| Liu CH et al 2003 | 42/26 | T:22/10 | T:55.6±4.5 | Modified BYHWD (100 mL, bid)+BT | Blank+BT | 8 | Not reported | CER+ NCV+Hemorheology | |
| C: 14/12 | C: 57.8± 3.4 | ||||||||
| CuiY, Pan MX 2004 | 100/92 | T:58/42 | T:39-73 | Modified BYHWD (200 mL, bid)+ Meco- | Mecobalamine (500 μg, qod, im) +BT | 8 | T:0.25-10 | CER+ NCV+Hemorheology | |
| C: 52/40 | C: 37-70 | balamine (500 μg, qod, im) +BT | C: 0.25-11 | ||||||
| Luo XY 2007 | 40/40 | T:22/18 | T:66.68±9.82 | BYHWD (100 mL, bid)+Mecobalamine | Mecobalamine (500 μg, tid, po)+BT | 8 | Not reported | CER +NCV+Hemorheology | |
| C: 21/19 | C: 66.9±10.53 | 500 μg, tid, po)+BT | |||||||
| Zhuang HZ 2011 | 30/30 | T:14/16 | T:54.1±3.7 | Modified BYHWD (bid)+Mecobalamine | Mecobalamine (500 μg, qd, im)+BT | 8 | T:4.8±2.2 | CER | |
| C:16/14 | C:55.1±3.9 | (500 μg,qd, im) +BT | C:5.1±3.3 | ||||||
| Liu JG 2015 | 54/54 | T:28/26 | T:62.07±6.93 | Modified BYHWD (200 mL, bid)+Mecob- | Mecobalamine (0.5 mg, tid, po)+BT | 12 | T:5.45±2.81 | CER | |
| C:29/25 | C:61.58±6.52 | alamine (0.5 mg, tid, po) +BT | C:5.16±2.70 | ||||||
| Ji TC 2014 | 32/31 | T:18/14 | T:40-73 | Modified BYHWD (bid)+ Mecobalamine (500 μg, tid)+BT | Mecobalamine (500 μg, tid)+BT | 6 | T:1-15 | CER | |
| C:16/15 | C:41-74 | C:1-14 | |||||||
| Jiao FE et al 2013 | 56/52 | T:30/26 | T:40-72 | Modified BYHWD (bid)+ Mecobalamine (500 μg, tid)+BT | Mecobalamine (500 μg, tid,)+BT | 8 | T:0.33-8.5 | CER | |
| C:31/21 | C:42-70 | C:0.25-8 | |||||||
| Jiang ZS et al 2005 | 30/30 | T:18/12 | T:48.6±9 | Modified BYHWD (200 mL, bid) + Mecobalamine (first four weeks, im; | Mecobalamine (first four weeks, im; last four weeks, po)+BT | 8 | T:13.6± 8.3a | CER+NCV | |
| C:16/14 | C:50±10.8 | last four weeks, po)+BT | C:13± 9.8a | ||||||
Table 1 Characteristics of the trials included in the Meta-analysis
| Source | Sample | Sex | Age (years) | Intervention | Treatment | DN | Outcome | ||
|---|---|---|---|---|---|---|---|---|---|
| (T/C) | (male | period | duration | measure | |||||
| /female) | Treatment group | Control group | (weeks) | (years) | |||||
| Ning RZ et al 2020 | 30/30 | T:13/17 | T:62.53±3.67 | Modified BYHWD (150 mL, bid)+ Mecobalamine (500 μg tid, po)+BT | Mecobalamine (500 μg tid, po)+BT | 12 | T:1.83 ± 1.78 | CER | |
| C:16/14 | C:61.43±4.29 | C: 2.2 ± 1.17 | |||||||
| Han Y 2005 | 30/20 | Not reported | Not reported | Modified BYHWD (bid)+BT | Mecobalamin (500 μg, tid, po)+BT | 14 | Not reported | NCV+Hemorheology | |
| Gong LZ et al 2017 | 60/60 | Not reported | Not reported | Modified BYHWD (200 mL, bid) | Mecobalamin (1 mg,qd, iv)+BT | 8 | Not reported | NCV | |
| +BT | |||||||||
| Wu Y, Ma D 2015 | 60/60 | T:27/33 | T:45-75 | Modified BYHWD+ BT | Mecobalamin (0.5-1 mg, qd, iv)+BT | 12 | T:0.25-3 | CER+NCV | |
| C: 32/28 | C: 48-74 | Mecobalamin (0.5-1 mg, qd, iv) | C: 0.42-3 | ||||||
| Sun SZ, Wang YZ 2008 | 42/42 | T:23/19 | T:44-73 | BYHWD (250 mL, bid)+Mecobalamin | Mecobalamin (0.5 mL, qd, im)+BT | 12 | T:0.8-13.5 | CER+NCV+ Hemorheology | |
| C: 22/20 | C: 45-72 | (0.5 mL, qd, im)+BT | C: 0.6-13.6 | ||||||
| Zhang T 2008 | 60/60 | T:34/26 | T:54.6±5.9 | Modified BYHWD (bid)+BT | Mecobalamin (500 μg, qd, im)+BT | 12 | T:0.5-3 | CER+NCV | |
| C: 32/28 | C: 54.8±5.8 | C: 0.5-3 | |||||||
| Sun BX, Li LQ 2017 | 47/47 | T:24/23 | T:59.64±10.51 | Modified BYHWD (bid)+BT | Mecobalamin (0.5 mg, qd, im)+BT | 12 | T:6.87±3.41 | CER | |
| C: 22/25 | C: 59.35±10.33 | C: 7.05±3.81 | |||||||
| Yang YQ, Xing DZ 2019 | 38/38 | T:19/19 | T:67.91±2.18 | Modified BYHWD (250 mL, bid) + Mecobalamin (0.5-1 mg, qd,im) | Mecobalamin (0.5-1 mg, qd, im) | 12 | Not reported | CER | |
| C: 18/20 | C: 68.59±2.12 | ||||||||
| Liu B 2018 | 41/41 | T:22/19 | T:59.85±2.82 | Modified BYHWD (200 mL, bid)+ Mecobalamine (0,5 mg, tid, po) + BT | Mecobalamine (0.5 mg, tid, po)+BT | 8 | Not reported | CER+NCV | |
| C: 21/20 | C: 59.35±2.76 | ||||||||
| Pan XJ 2017 | 30/30 | T:13/17 | T:50.74±9.25 | Modified BYHWD (150 mL, bid) + | Mecobalamine (500 μg, tid, po)+BT | 12 | T:8.45±1.26 | CER+NCV+Hemorheology | |
| C: 16/14 | C: 49.62±9.83 | Meco-balamine (500 μg, tid, po) + BT | C: 8.72±1.35 | ||||||
| Li YF 2009 | 36/36 | T:20/16 | T:42-69 | Modified BYHWD (bid) + BT | Mecobalamine (500 μg, tid, po)+BT | 8 | Not reported | CER+NCV+Hemorheology | |
| C: 16/20 | C: 41- | ||||||||
| Peng H 2009 | 30/30 | T:18/12 | T:52.7±11.5 | Modified BYHWD (200 mL, bid) +Mecobalamine (0.5 mg, tid, po)+BT | Mecobalamine (0.5 mg, tid, po)+BT | 12 | T:6.52±1.21 | CER+ NCV+Hemorheology | |
| C: 14/16 | C: 50.8±12.4 | C: 6.46±1.12 | |||||||
| Luo XY 2007 | 40/40 | T:22/18 | T:66.68±9.82 | BYHWD (100 mL, bid)+Mecobalamine ( | Mecobalamine (500 μg, tid, po)+BT | 8 | Not reported | CER +NCV+Hemorheology | |
| C: 21/19 | C: 66.9±10.53 | 500 μg, tid, po)+BT | |||||||
| Wang SX et al 2005 | 130/78 | T:78/52 | T:56.6±6.3 | Modified BYHWD (bid)+BT | Mecobalamine (500 μg, tid, po)+BT | 8 | T:2.3±1.2 | CER+ NCV+Hemorheology | |
| C: 45/33 | C: 55.8±6.5 | C: 2.3±1.5 | |||||||
| Liu CH et al 2003 | 42/26 | T:22/10 | T:55.6±4.5 | Modified BYHWD (100 mL, bid)+BT | Blank+BT | 8 | Not reported | CER+ NCV+Hemorheology | |
| C: 14/12 | C: 57.8± 3.4 | ||||||||
| CuiY, Pan MX 2004 | 100/92 | T:58/42 | T:39-73 | Modified BYHWD (200 mL, bid)+ Meco- | Mecobalamine (500 μg, qod, im) +BT | 8 | T:0.25-10 | CER+ NCV+Hemorheology | |
| C: 52/40 | C: 37-70 | balamine (500 μg, qod, im) +BT | C: 0.25-11 | ||||||
| Luo XY 2007 | 40/40 | T:22/18 | T:66.68±9.82 | BYHWD (100 mL, bid)+Mecobalamine | Mecobalamine (500 μg, tid, po)+BT | 8 | Not reported | CER +NCV+Hemorheology | |
| C: 21/19 | C: 66.9±10.53 | 500 μg, tid, po)+BT | |||||||
| Zhuang HZ 2011 | 30/30 | T:14/16 | T:54.1±3.7 | Modified BYHWD (bid)+Mecobalamine | Mecobalamine (500 μg, qd, im)+BT | 8 | T:4.8±2.2 | CER | |
| C:16/14 | C:55.1±3.9 | (500 μg,qd, im) +BT | C:5.1±3.3 | ||||||
| Liu JG 2015 | 54/54 | T:28/26 | T:62.07±6.93 | Modified BYHWD (200 mL, bid)+Mecob- | Mecobalamine (0.5 mg, tid, po)+BT | 12 | T:5.45±2.81 | CER | |
| C:29/25 | C:61.58±6.52 | alamine (0.5 mg, tid, po) +BT | C:5.16±2.70 | ||||||
| Ji TC 2014 | 32/31 | T:18/14 | T:40-73 | Modified BYHWD (bid)+ Mecobalamine (500 μg, tid)+BT | Mecobalamine (500 μg, tid)+BT | 6 | T:1-15 | CER | |
| C:16/15 | C:41-74 | C:1-14 | |||||||
| Jiao FE et al 2013 | 56/52 | T:30/26 | T:40-72 | Modified BYHWD (bid)+ Mecobalamine (500 μg, tid)+BT | Mecobalamine (500 μg, tid,)+BT | 8 | T:0.33-8.5 | CER | |
| C:31/21 | C:42-70 | C:0.25-8 | |||||||
| Jiang ZS et al 2005 | 30/30 | T:18/12 | T:48.6±9 | Modified BYHWD (200 mL, bid) + Mecobalamine (first four weeks, im; | Mecobalamine (first four weeks, im; last four weeks, po)+BT | 8 | T:13.6± 8.3a | CER+NCV | |
| C:16/14 | C:50±10.8 | last four weeks, po)+BT | C:13± 9.8a | ||||||
| 1. |
Danaei G, Finucane MM, Lu Y, et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet 2011; 378: 31-40.
DOI URL |
| 2. | Chinese Diabetes Society. Guidelines for the prevention and control of type 2 diabetes in China (2017 Edition). Zhong Guo Tang Niao Bing Za Zhi 2018; 10: 4-67. |
| 3. |
Lee CC, Perkins BA, Kayaniyil S, et al. Peripheral neuropathy and nerve dysfunction in individuals at high risk for type 2 diabetes: The PROMISE Cohort. Diabetes Care 2015; 38: 793-800.
DOI PMID |
| 4. |
Saeedi P, Petersohn I, Salpea P, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th ed. Diabetes Res Clin Pract 2019; 157: 107843.
DOI URL |
| 5. | Wang QR (Qing dynasty), Yi Lin Gai Cuo. Beijing: China Medical Science and Technology Press, 2011: 1-67. |
| 6. | Xu S, Hua WJ. Research progress of Buyang Huanwu decoction in the treatment of diabetic peripheral neuropathy. Zhong Yi Xue 2020; 9: 92-7. |
| 7. |
Pan R, Cai J, Zhan L, et al. Buyang Huanwu decoction facilitates neurorehabilitation through an improvement of synaptic plasticity in cerebral ischemic rats. BMC Complement Altern Med 2017; 17: 173.
DOI URL |
| 8. | Zheng XY. Guiding principles for clinical research of new drugs of Traditional Chinese Medicine. Beijing: China Medical Science and Technology Press, 2002: 233-7. |
| 9. | Higgins JPT, Green S. Corchrane Reviewers’ Handbook 5.2 [updated March 2013], ReviewManager (RevMan) [Computer program]. Version 5.2; 2013. |
| 10. | Ning RZ, Zhang TY, Ma J. Effect of modified Buyang Huanwu decoction on diabetic peripheral neuropathy and insulin-like growth factor. Zhong Yi Yao Xue Bao 2020; 48: 41-4. |
| 11. | Liu B. Therapeutic effect of modified Buyang Huanwu ecoction on diabetic peripheral neuropathy of Qi deficiency and blood stasis type. Tang Niao Bing Xin Shi Jie 2018; 21: 179-80. |
| 12. | Pan XJ. Therapeutic effect of Buyang Huanwu decoction on T2DM peripheral neuropathy of Qi deficiency and blood stasis type. Yunnan: Yunnan university of Chinese medicine, 2017: 1-51. |
| 13. | Li YF. Clinical study of modified Buyang Huanwu decoction in the treatment of diabetic peripheral neuropathy. Guangxi: Guangxi university of Chinese medicine, 2009: 1-55. |
| 14. | Peng H. Clinical observation on the treatment of diabetic peripheral neuropathy with modified Buyang Huanwu decoction. Hubei: Hubei University Of Chinese Medicine, 2009: 1-36. |
| 15. | Luo XY. Clinical study on Buyang Huanwu decoction in the treatment of type 2 diabetic peripheral neuropathy. Hubei: Hubei University Of Chinese Medicine, 2007: 1-42. |
| 16. | Wang SX, Chen L, Ma XJ. 130 cases of diabetic peripheral neuropathy treated with modified Buyang Huanwu decoction. Zhong Guo Zhong Yi Yao Xin Xi Za Zhi 2005; 12: 60-1. |
| 17. | Liu CH, Fan GJ, Tang XY. Clinical observation on 42 cases of diabetic peripheral neuropathy treated with Buyang Huanwu decoction. Sichuan Zhong Yi 2003; 21: 31-2. |
| 18. | Han Y. Treatment of 30 cases of type 2 diabetic peripheral neuropathy with Buyang Huanwu decoction: a control study of 20 cases treated with Mecobalamin tablets. Zhejiang Zhong Yi Za Zhi 2005; 40: 430-1. |
| 19. | Gong LZ, Li YF, Zhang JX, Zhang YP, Qiao XY, Zhang J. Clinical observation on the treatment of diabetic peripheral neuropathy with Buyang Huanwu decoction. Shi Yong Zhong Yi Yao Za Zhi 2017; 33: 1364-5. |
| 20. | Wu Y, Ma D. Clinical observation of Buyang Huanwu decoction combined with mecobalamin injection in the treatment of diabetic peripheral neuropathy. Lin Chuang He Li Yong Yao Za Zhi 2015; 8: 34-5. |
| 21. | Sun SZ, Wang YZ. Clinical observation on 42 cases of diabetic peripheral neuropathy treated by Buyang Huanwu decoction combined with mecobalamin. Jiangsu Zhong Yi Yao 2008; 40: 48-9. |
| 22. | Zhang T. Buyang Huanwu decoction in treating 60 cases of diabetic peripheral neuropathy. Tianjin Zhong Yi Yao 2008; 25: 216. |
| 23. | Sun BX, Li LQ. Clinical effect of Buyang Huanwu decoction on diabetic peripheral neuropathy. Ji Yin Zu Xue Yu Ying Yong Sheng Wu Xue 2017; 36: 3398-402. |
| 24. | Yang YQ, Xing DZ. Clinical study of modified Buyang Huanwu decoction combined with mecobalamin in the treatment of senile diabetic peripheral neuropathy. Xin Zhong Yi 2019; 51: 67-9. |
| 25. | Cui Y, Pan MX. Therapeutic effect of Buyang Huanwu decoction combined with mecobalamin on diabetic peripheral neuropathy. Hebei Zhong Yi 2004; 26: 374-5. |
| 26. | Zhuang HZ. 30 cases of diabetic peripheral neuropathy treated with integrated Traditional Chinese and Western Medicine. Zhong Guo Zhong Yi Yao Xian Dai Yuan Cheng Jiao Yu 2011, 9: 60. |
| 27. | Liu JG. Therapeutic effect of Buyang Huanwu decoction on diabetic peripheral neuropathy. Zhong Yi Lin Chuang Yan Jiu 2015, 7: 113-4. |
| 28. | Ji TC. Clinical observation on the treatment of diabetic peripheral neuropathy with Buyang Huanwu decoction. Zhong Yi Lin Chuang Yan Jiu 2014; 6: 74-5. |
| 29. | Jiao FE, Cong K, Ji XY. Therapeutic effect of Buyang Huanwu decoction on diabetic peripheral neuropathy. Liaoning Zhong Yi Za Zhi 2013; 40: 740. |
| 30. | Jiang ZS, Ren ZX, Zhang SL, Li HY. Clinical study on the treatment of diabetic peripheral neuropathy by supplementing Qi and activating Yang, activating blood and dredging collaterals. Shandong Zhong Yi Yao Da Xue Xue Bao 2005; 29: 200-2. |
| 31. | Zhao L. Clinical observation of modified Buyang Huanwu decoction in the treatment of type Ⅱ diabetes mellitus. Hubei Zhong Yi Za Zhi 2002; 24: 8-9. |
| 32. | Xu XM, Chen XJ, Chen ran, Zhang JY, Zhang YL. Effect of Buyang Huanwu decoction on whole blood specific viscosity, TXB2, PLO and SOD in rats. Shanxi Yi Ke Da Xue Xue Bao 2002; 33: 212-4. |
| 33. | Xing SL, Li ZH, Sun JH, Liu YP, Pu LH. Antioxidant effect of Buyang Huanwu decoction. Jie Pou Xue Za Zhi 2005; 28: 529-32. |
| 34. | Chen LG, Qu Y, GE HY, Hu XQ, Nie YA, He MQ. Effect of Buyang Huanwu decoction on the expression of vascular endothelial cell adhesion molecules in rats with blood stasis syndrome. Zhong Cao Yao 2005; 36: 706-9. |
| 35. | Wang QY. Effect of astragalus injection on hemorheology in rabbits with blood stasis syndrome. Zhong Yao Yao Li Xue Yu Lin Chuang 2004; 20: 19-20. |
| 36. | Jiang DF, Yin CH, Yu NG, et al. Effect of Astragalosides on hemorheology in aged rats. Ji Chu Zhong Yi Za Zhi 2002; 16: 15-6. |
| 37. | Zhuang WQ. Clinical efficacy of Jingui Shenqi pill combined with Buyang Huanwu decoction in the treatment of diabetic peripheral neuropathy. clinical efficacy of diabetic peripheral neuropathy. Tang Niao Bing Xin Shi Jie 2017; 20: 168-9. |
| 38. | Jiang JL, Zhang RL, Zhang RP. Clinical study of Buyang Huanwu decoction and Huangqi Guizhi Wuwu decoction combined with western medicine in the treatment of diabetic peripheral neuropathy. Zhong Xi Yi Jie He Xin Nao Xue Guan Bing Za Zhi 2020; 18: 840-2. |
| 39. | Li S, Liu SR. Treatment of 47 cases of diabetic peripheral neuropathy with Buyang Huanwu decoction. Guang Ming Zhong Yi 2016; 31: 674-5. |
| [1] | QIN Xiaoyu, WANG Chunai, XUE Jianjun, ZHANG Jie, LU Xiaoting, DING Shengshuang, GE Long, WANG Minzhen. Efficacy of electroacupuncture on myocardial protection and postoperative rehabilitation in patients undergoing cardiac surgery with cardiopulmonary bypass: a systematic review and Meta-analysis [J]. Journal of Traditional Chinese Medicine, 2024, 44(1): 1-15. |
| [2] | DAI Xiaoling, ZHANG Anming, LIN Hui, SHI Bei, REN Yi, WEN Hongzhu, FEI Xiaoyan, LIN Jiang. Qingchang suppositry (清肠栓) induced remission in patients with mild-to-moderate ulcerative proctitis: a multicenter, prospective, randomized, parallel-controlled clinical trial [J]. Journal of Traditional Chinese Medicine, 2024, 44(1): 156-162. |
| [3] | WANG Yichen, WU Shiyi, WANG Zhengyan, CHANG Wenling, XIE Zhihao, TANG Xing, ZHAO Songmei, ZHOU Jing, CHEN Zehong, WANG Chao, YANG Chunxia. Efficacy of Zhumian Tang formula granules (助眠汤配方颗粒) combined with eszopiclone for the treatment of poor sleep quality: a multi-center, randomized controlled, superiority trial [J]. Journal of Traditional Chinese Medicine, 2024, 44(1): 163-171. |
| [4] | YANG Yi, YE Huijun, ZHENG Huiling, JIN Lihua. Clinical observation on 90 cases of primary dysmenorrhea treated by buccal acupuncture therapy: a randomized controlled study [J]. Journal of Traditional Chinese Medicine, 2024, 44(1): 172-181. |
| [5] | DAI Zeqi, LIAO Xing, GUAN Yueyue, ZENG Zixiu, TANG Jun, HU Jing. Bloodletting puncture in the treatment of acute ischemic stroke: protocol for a mixed-method study of a multi-center randomized controlled trial and focus group [J]. Journal of Traditional Chinese Medicine, 2023, 43(6): 1259-1267. |
| [6] | XU Yani, ZHANG Yutong, HE Weile, DAI Linglin, TANG Ding, WANG Jialing, ZHANG Xufen, CHEN Qin, CHEN Lifang, WANG Zhanglian, ZHAN Mingjie. Efficiency and safety of acupuncture for women with premature ovarian insufficiency: study protocol for a randomized controlled trial [J]. Journal of Traditional Chinese Medicine, 2023, 43(6): 1268-1274. |
| [7] | XU Xiangru, ZHOU Yi, CHEN Gang, LEI Ming, ZHANG Wen, WU Xinxin, PU Yuting, CHEN Caiyu, SUN Yuting, ZHOU Shuang, FANG Bangjiang. Clinical efficacy of Buzhong Yiqi decoction (补中益气汤) in the treatment of hospital-acquired pneumonia with multi-drug resistant bacteria: a prospective, randomized, multicenter controlled trial [J]. Journal of Traditional Chinese Medicine, 2023, 43(5): 1010-1018. |
| [8] | ZHAO Ming, LUO Yimiao, WANG Huichan, CAO Yu, MA Lina, PEI Hui, LI Hao. Guilingji capsule (龟龄集胶囊) for Alzheimer's disease: secondary analysis of a randomized non-inferiority controlled trial [J]. Journal of Traditional Chinese Medicine, 2023, 43(5): 1019-1025. |
| [9] | YANG Yuqing, CHEN Yuhuan, LI Chunxiao, LING Xiao, WANG Panpan, GUO Jing, ZHANG Yingying. Effectiveness and safety of Pingxiao capsule (平消胶囊) as adjuvant therapy in treatment of breast cancer: a systematic review and Meta-analysis [J]. Journal of Traditional Chinese Medicine, 2023, 43(5): 851-859. |
| [10] | SUN Wu, ZHAO Yuwei, LIAO Liang, ZHAO Zhonghui, CHEN Shiqi, YAN Xiaoling, WANG Xueyao, CHAO Guojun, ZHOU Jian. Effectiveness and safety of Xuebijing injection for patients with coronavirus disease 2019: a systematic review and Meta-analysis [J]. Journal of Traditional Chinese Medicine, 2023, 43(4): 631-639. |
| [11] | WANG Chao, WU Qiong, LI Ping, WANG Zhigang, LOU Xusheng, LI Yuanyuan, ZHANG Lin. Effect of Traditional Chinese Medicine combined with Western Medicine on blood lipid levels and inflammatory factors in patients with angina pectoris in coronary heart disease identified as intermingled phlegm and blood stasis syndrome: a network Meta-analysis [J]. Journal of Traditional Chinese Medicine, 2023, 43(4): 640-649. |
| [12] | ZHANG Xinghe, LI Qifu, YI Rong, XING Chonghui, JIN Yuhao, MENG Jiangqiong, FENG Jialei, ZHAO Siwen, LIANG Fanrong, GUO Taipin. Effect of catgut embedding at acupoints versus non-acupoints in abdominal obesity: a randomized clinical trial [J]. Journal of Traditional Chinese Medicine, 2023, 43(4): 780-786. |
| [13] | LUO Xin, XIE Jing, HUANG Li, GAN Wenfan, CHEN Ming. Efficacy and safety of activating blood circulation and removing blood stasis of Traditional Chinese Medicine for managing renal fibrosis in patients with chronic kidney disease: a systematic review and Meta-analysis [J]. Journal of Traditional Chinese Medicine, 2023, 43(3): 429-440. |
| [14] | ZHANG Yuehong, SHAO Xianzhi, ZHAO Qianlong, ZHAN Hualong, ZHANG Jianhua, DU Sisi, CHEN Jing, LIU Yingfang, ZHOU Haiwang, CHEN Xinsheng, HONG Ying, LIAN Fengmei, TONG Xiaolin, BA Yuanming. Effectiveness of Xiangsha Liujun pills (香砂六君丸) on decreased digestive function in convalescent patients of coronavirus disease 2019: a randomized, double blind, placebo controlled clinical trial [J]. Journal of Traditional Chinese Medicine, 2023, 43(3): 552-558. |
| [15] | ZHANG Yuehong, DONG Dandan, YAN Youqin, ZHANG Hao, WANG Guangli, ZHOU Wei, LI Wei, QIU Li, LI Tingming, LIU Quan, XIA Ping, MAO Lina, YANG Danlin, YANG Lu, LIAN Fengmei, TONG Xiaolin, BA Yuanming. Effectiveness and safety of Jinshuibao capsules (金水宝胶囊) in treatment of residual cardiopulmonary symptoms in convalescent patients of coronavirus disease 2019: a pilot randomized, double-blind, placebo-controlled clinical trial [J]. Journal of Traditional Chinese Medicine, 2023, 43(1): 134-139. |
| Viewed | ||||||||||||||||||||||||||||||||||||||||||||||||||
|
Full text 680
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||
|
Abstract 493
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||