Journal of Traditional Chinese Medicine ›› 2023, Vol. 43 ›› Issue (5): 1019-1025.DOI: 10.19852/j.cnki.jtcm.20230404.006
Previous Articles Next Articles
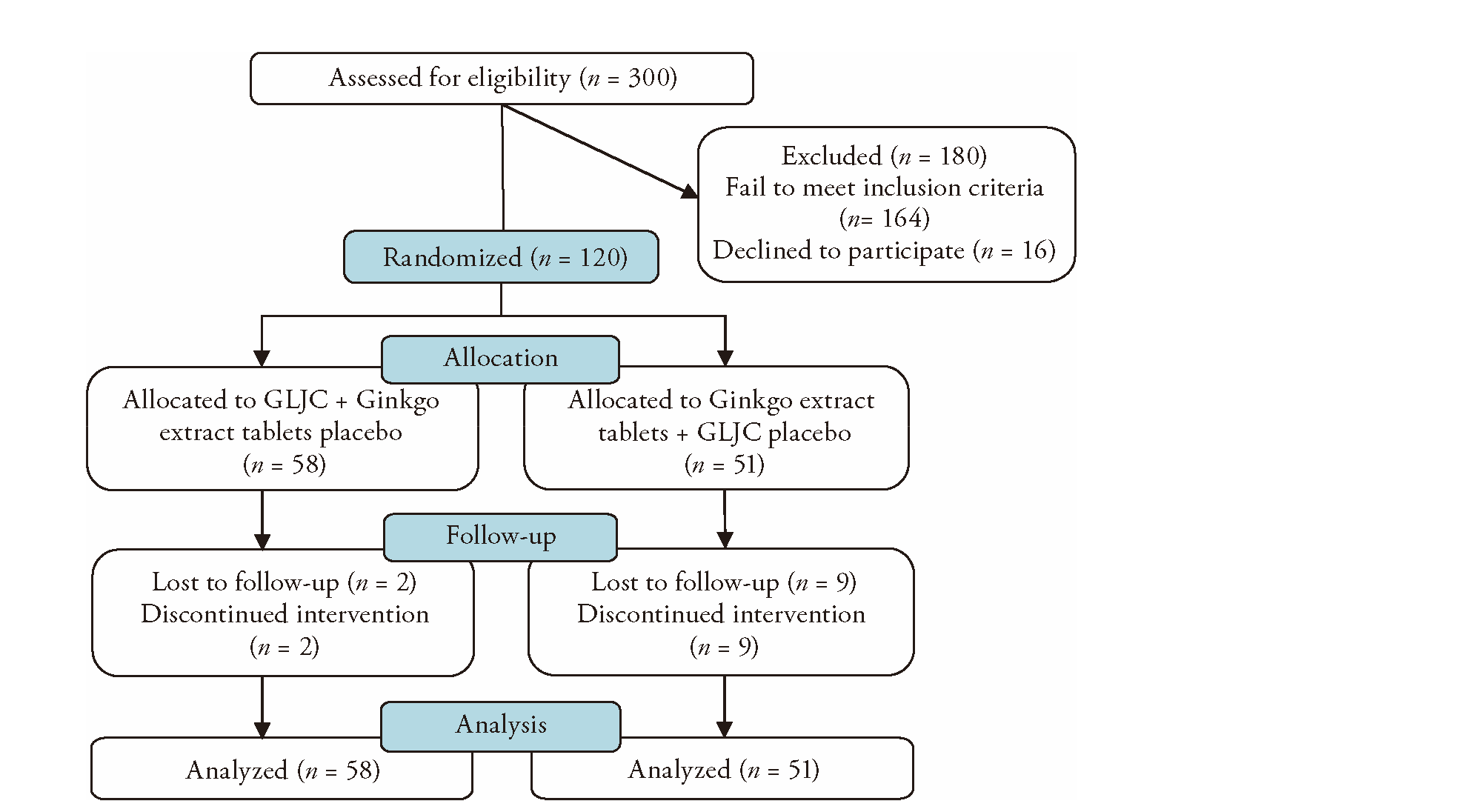
Guilingji capsule (龟龄集胶囊) for Alzheimer's disease: secondary analysis of a randomized non-inferiority controlled trial
ZHAO Ming1, LUO Yimiao2, WANG Huichan2, CAO Yu2, MA Lina2, PEI Hui2( ), LI Hao2(
), LI Hao2( )
)
- 1 Graduate School, Beijing University of Chinese Medicine, Beijing 100029, China
2 Department of Geriatrics, Xiyuan Hospital of China Academy of Chinese Medical Sciences, Beijing 100091, China
-
Received:2022-06-12Accepted:2022-09-14Online:2023-10-15Published:2023-04-04 -
Contact:Prof. PEI Hui, Department of Geriatrics, Xiyuan Hospital of China Academy of Chinese Medical Sciences, Beijing 100091, China.phxydoctor@126.com ; Prof. LI Hao, Department of Geriatrics, Xiyuan Hospital of China Academy of Chinese Medical Sciences, Beijing 100091, China.xyhplihao1965@126.com . Telephone: +86-10-62835631; 18390922812 -
Supported by:“Hundreds and Tens of Thousands” Talent Project ( Qihuang Project) Qihuang Scholars Project of Traditional Chinese Medicine Inheritance and Innovation(020450003)
Cite this article
ZHAO Ming, LUO Yimiao, WANG Huichan, CAO Yu, MA Lina, PEI Hui, LI Hao. Guilingji capsule (龟龄集胶囊) for Alzheimer's disease: secondary analysis of a randomized non-inferiority controlled trial[J]. Journal of Traditional Chinese Medicine, 2023, 43(5): 1019-1025.
share this article
| General character | GLJC group (n = 58) | Gingko group (n = 51) | P value | |
|---|---|---|---|---|
| Gender [n (%)] | ||||
| Male | 9 (31.03) | 11(42.31) | 0.386 | |
| Female | 20 (68.97) | 15 (57.69) | ||
| Age (years, | 70±6 | 70±7 | 0.754 | |
| Height (cm, | 162±7 | 163±7 | 0.725 | |
| Weight (kg, | 63±11 | 62±10 | 0.808 | |
| BMI (kg/m2, | 24±4 | 23±3 | 0.567 | |
| Blood pressure (mm Hg, | ||||
| Systolic pressure | 127±16 | 133±16 | 0.171 | |
| Diastolic pressure | 74±11 | 75±11 | 0.792 | |
| Heart rate (times/min, | 68±9 | 73±11 | 0.080 | |
| Education level [n (%)] | ||||
| Illiteracy | 1 (3.45) | 0 (0) | 0.069 | |
| Primary school | 1 (3.45) | 4 (15.38) | ||
| Secondary school | 10 (34.48) | 14 (53.85) | ||
| College degree or above | 17 (58.62) | 8 (30.77) | ||
| Hypertension [n (%)] | ||||
| Yes | 8 (27.59) | 10 (38.46) | 0.391 | |
| No | 21 (72.41) | 16 (61.54) | ||
| Coronary disease [n (%)] | ||||
| Yes | 9 (31.03) | 5 (19.23) | 0.316 | |
| No | 20 (68.97) | 21 (80.77) | ||
| Diabetes [n (%)] | ||||
| Yes | 6 (26.09) | 3 (11.54) | 0.582 | |
| No | 23 (73.91) | 23 (88.46) | ||
| Hyperlipemia [n (%)] | ||||
| Yes | 1 (3.45) | 4 (15.38) | 0.286 | |
| No | 28 (96.55) | 22 (84.62) | ||
Table 1 Clinic characteristics in the two groups at baseline
| General character | GLJC group (n = 58) | Gingko group (n = 51) | P value | |
|---|---|---|---|---|
| Gender [n (%)] | ||||
| Male | 9 (31.03) | 11(42.31) | 0.386 | |
| Female | 20 (68.97) | 15 (57.69) | ||
| Age (years, | 70±6 | 70±7 | 0.754 | |
| Height (cm, | 162±7 | 163±7 | 0.725 | |
| Weight (kg, | 63±11 | 62±10 | 0.808 | |
| BMI (kg/m2, | 24±4 | 23±3 | 0.567 | |
| Blood pressure (mm Hg, | ||||
| Systolic pressure | 127±16 | 133±16 | 0.171 | |
| Diastolic pressure | 74±11 | 75±11 | 0.792 | |
| Heart rate (times/min, | 68±9 | 73±11 | 0.080 | |
| Education level [n (%)] | ||||
| Illiteracy | 1 (3.45) | 0 (0) | 0.069 | |
| Primary school | 1 (3.45) | 4 (15.38) | ||
| Secondary school | 10 (34.48) | 14 (53.85) | ||
| College degree or above | 17 (58.62) | 8 (30.77) | ||
| Hypertension [n (%)] | ||||
| Yes | 8 (27.59) | 10 (38.46) | 0.391 | |
| No | 21 (72.41) | 16 (61.54) | ||
| Coronary disease [n (%)] | ||||
| Yes | 9 (31.03) | 5 (19.23) | 0.316 | |
| No | 20 (68.97) | 21 (80.77) | ||
| Diabetes [n (%)] | ||||
| Yes | 6 (26.09) | 3 (11.54) | 0.582 | |
| No | 23 (73.91) | 23 (88.46) | ||
| Hyperlipemia [n (%)] | ||||
| Yes | 1 (3.45) | 4 (15.38) | 0.286 | |
| No | 28 (96.55) | 22 (84.62) | ||
| Time | GLJC group (n = 58) | Gingko group (n = 51) | P value |
|---|---|---|---|
| MMSE | |||
| 0 week | 19.3 ± 3.6 | 18.3 ± 3.6 | 0.306 |
| 12 weeks | 23.1 ± 2.0a | 22.1 ± 3.6a | 0.508 |
| 24 weeks | 24.3 ± 1.6a | 23.3 ± 2.7a | 0.209 |
| ADAS-cog | |||
| 0 week | 27.6 ± 11.4 | 30.2 ± 9.2 | 0.372 |
| 12 weeks | 16.2 ± 9.4a | 18.5 ± 12.7a | 0.673 |
| 24 weeks | 13.3 ± 8.7a | 16.4 ± 12.0a | 0.423 |
| ADL | |||
| 0 week | 29.0± 8.3 | 29.1 ± 8.1 | 0.691 |
| 12 weeks | 24.1 ± 6.1a | 25.3 ± 7.6a | 0.647 |
| 24 weeks | 23.7 ± 5.4a | 24.9 ± 6.83a | 0.798 |
| CM-SS | |||
| 0 week | 20.6 ± 5.9 | 21.2 ± 7.6 | 0.726 |
| 12 weeks | 15.0 ± 4.6a | 15.4 ± 6.3a | 0.966 |
| 24 weeks | 11.9 ± 4.5a | 13.8 ± 5.8a | 0.327 |
Table 2 Outcome measures at 0, 12, and 24 weeks of treatment
| Time | GLJC group (n = 58) | Gingko group (n = 51) | P value |
|---|---|---|---|
| MMSE | |||
| 0 week | 19.3 ± 3.6 | 18.3 ± 3.6 | 0.306 |
| 12 weeks | 23.1 ± 2.0a | 22.1 ± 3.6a | 0.508 |
| 24 weeks | 24.3 ± 1.6a | 23.3 ± 2.7a | 0.209 |
| ADAS-cog | |||
| 0 week | 27.6 ± 11.4 | 30.2 ± 9.2 | 0.372 |
| 12 weeks | 16.2 ± 9.4a | 18.5 ± 12.7a | 0.673 |
| 24 weeks | 13.3 ± 8.7a | 16.4 ± 12.0a | 0.423 |
| ADL | |||
| 0 week | 29.0± 8.3 | 29.1 ± 8.1 | 0.691 |
| 12 weeks | 24.1 ± 6.1a | 25.3 ± 7.6a | 0.647 |
| 24 weeks | 23.7 ± 5.4a | 24.9 ± 6.83a | 0.798 |
| CM-SS | |||
| 0 week | 20.6 ± 5.9 | 21.2 ± 7.6 | 0.726 |
| 12 weeks | 15.0 ± 4.6a | 15.4 ± 6.3a | 0.966 |
| 24 weeks | 11.9 ± 4.5a | 13.8 ± 5.8a | 0.327 |
| Group | n | Time | Bax (ng/mL) | Bcl-2 (pg/mL) | Ach (pmol/mL) | AchE(pg/mL) |
|---|---|---|---|---|---|---|
| GLJC | 58 | 0 week | 29.10 (19.06, 45.31) | 225.45 (154.58, 452.18) | 199.46 (198.12, 335.56) | 425.78 (357.46, 515.84) |
| 24 weeks | 13.52 (8.32, 22.96)a | 305.03 (165.63, 678.05)b | 197.53 (90.89, 378.62) | 375.54 (316.05, 432.10)a | ||
| Gingko | 51 | 0 week | 24.52 (14.96, 36.86) | 333.43 (185.55, 626.96) | 195.12 (94.44, 304.58) | 379.78 (333.73, 573.37) |
| 24 weeks | 15.54 (10.44, 21.32)a | 292.95 (172.80, 524.55) | 154.07 (98.22, 279.72) | 343.06 (289.96, 428.86)a |
Table 3 Changes in serum indexes before and after treatment
| Group | n | Time | Bax (ng/mL) | Bcl-2 (pg/mL) | Ach (pmol/mL) | AchE(pg/mL) |
|---|---|---|---|---|---|---|
| GLJC | 58 | 0 week | 29.10 (19.06, 45.31) | 225.45 (154.58, 452.18) | 199.46 (198.12, 335.56) | 425.78 (357.46, 515.84) |
| 24 weeks | 13.52 (8.32, 22.96)a | 305.03 (165.63, 678.05)b | 197.53 (90.89, 378.62) | 375.54 (316.05, 432.10)a | ||
| Gingko | 51 | 0 week | 24.52 (14.96, 36.86) | 333.43 (185.55, 626.96) | 195.12 (94.44, 304.58) | 379.78 (333.73, 573.37) |
| 24 weeks | 15.54 (10.44, 21.32)a | 292.95 (172.80, 524.55) | 154.07 (98.22, 279.72) | 343.06 (289.96, 428.86)a |
| Symptom | GLJC group (n = 58) | Gingko group (n = 51) | P value | |||||
|---|---|---|---|---|---|---|---|---|
| Efficacy (n) | Nonefficacy (n) | Efficacy rate (%) | Efficacy (n) | Nonefficacy (n) | Efficacy rate (%) | |||
| Amnesia | 21 | 8 | 72.41 | 10 | 16 | 38.46 | 0.011 | |
| Hypothermia | 21 | 8 | 72.41 | 13 | 13 | 50.00 | 0.088 | |
| Soreness and weakness of waist and knees | 15 | 14 | 51.72 | 10 | 16 | 38.46 | 0.324 | |
| Tired and sleepy | 12 | 17 | 41.38 | 13 | 13 | 50.00 | 0.522 | |
| Dull expression and slow thinking | 20 | 9 | 68.97 | 11 | 15 | 42.31 | 0.034 | |
| Good shock easy fear | 13 | 16 | 44.83 | 10 | 16 | 38.46 | 0.633 | |
| Vertigo | 13 | 16 | 44.83 | 13 | 13 | 50.00 | 0.701 | |
| Tinnitus | 13 | 16 | 44.83 | 13 | 13 | 50.00 | 0.701 | |
| Auditory hallucination | 4 | 25 | 13.79 | 3 | 23 | 11.54 | 1.000 | |
| Flushed cheeks | 11 | 18 | 37.93 | 10 | 16 | 38.46 | 0.968 | |
| Aconuresis | 6 | 23 | 20.69 | 1 | 25 | 3.85 | 0.143 | |
| Fecal self-legacy | 3 | 26 | 10.34 | 2 | 24 | 7.69 | 1.000 | |
| Tongue moss | 11 | 18 | 37.93 | 8 | 18 | 30.77 | 0.577 | |
| Pulse condition | 12 | 17 | 41.38 | 10 | 16 | 38.46 | 0.825 | |
Table 4 Single symptom efficacy rate of CM-SS after 24 weeks of treatment
| Symptom | GLJC group (n = 58) | Gingko group (n = 51) | P value | |||||
|---|---|---|---|---|---|---|---|---|
| Efficacy (n) | Nonefficacy (n) | Efficacy rate (%) | Efficacy (n) | Nonefficacy (n) | Efficacy rate (%) | |||
| Amnesia | 21 | 8 | 72.41 | 10 | 16 | 38.46 | 0.011 | |
| Hypothermia | 21 | 8 | 72.41 | 13 | 13 | 50.00 | 0.088 | |
| Soreness and weakness of waist and knees | 15 | 14 | 51.72 | 10 | 16 | 38.46 | 0.324 | |
| Tired and sleepy | 12 | 17 | 41.38 | 13 | 13 | 50.00 | 0.522 | |
| Dull expression and slow thinking | 20 | 9 | 68.97 | 11 | 15 | 42.31 | 0.034 | |
| Good shock easy fear | 13 | 16 | 44.83 | 10 | 16 | 38.46 | 0.633 | |
| Vertigo | 13 | 16 | 44.83 | 13 | 13 | 50.00 | 0.701 | |
| Tinnitus | 13 | 16 | 44.83 | 13 | 13 | 50.00 | 0.701 | |
| Auditory hallucination | 4 | 25 | 13.79 | 3 | 23 | 11.54 | 1.000 | |
| Flushed cheeks | 11 | 18 | 37.93 | 10 | 16 | 38.46 | 0.968 | |
| Aconuresis | 6 | 23 | 20.69 | 1 | 25 | 3.85 | 0.143 | |
| Fecal self-legacy | 3 | 26 | 10.34 | 2 | 24 | 7.69 | 1.000 | |
| Tongue moss | 11 | 18 | 37.93 | 8 | 18 | 30.77 | 0.577 | |
| Pulse condition | 12 | 17 | 41.38 | 10 | 16 | 38.46 | 0.825 | |
| 1. | Alzheimer's Association Report. 2022 Alzheimer's disease facts and figures. Alzheimers Dement 2022; 18: 700-89. |
| 2. |
Reitz C, Mayeux R. Alzheimer disease: epidemiology, diagnostic criteria, risk factors and biomarkers. Biochem Pharmacol 2014; 88: 640-51.
DOI PMID |
| 3. |
Jia L, Du Y, Chu L, et al. Prevalence, risk factors, and management of dementia and mild cognitive impairment in adults aged 60 years or older in China: a cross-sectional study. Lancet Public Health 2020; 5: e661-71.
DOI PMID |
| 4. |
Shi J, Gao X, Zhang A, Qin X, Du G. Characterization of multiple chemical components of GuiLingJi by UHPLC-MS and 1H NMR analysis. J Pharm Anal 2022; 12: 460-9.
DOI URL |
| 5. |
Zhao SJ, Liu XJ, Tian JS, et al. Effects of Guilingji on aging rats and its underlying mechanisms. Rejuvenation Res 2020; 23: 138-49.
DOI URL |
| 6. |
Liu NY, Pei H, Liu MX, et al. Efficacy and safety of Guilingji capsules for treating mild-to-moderate cognitive impairment: study protocol for a randomized, double-blind, positive-controlled, multicenter and noninferiority trial. Chin J Integr Med 2020; 26: 577-82.
DOI |
| 7. |
Schulz KF, Altman DG, Moher D; CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Ann Intern Med 2010; 152: 726-32.
DOI PMID |
| 8. |
Jack CR Jr, Bennett DA, Blennow K, et al. NIA-AA research framework: toward a biological definition of Alzheimer's disease. Alzheimers Dement 2018; 14: 535-62.
DOI PMID |
| 9. |
McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 2011; 7: 263-9.
DOI PMID |
| 10. |
Tangalos EG, Smith GE, Ivnik RJ, et al. The mini-mental state examination in general medical practice: clinical utility and acceptance. Mayo Clin Proc 1996; 71: 829-37.
DOI PMID |
| 11. |
Fernando WM, Martins IJ, Goozee KG, et al. The role of dietary coconut for the prevention and treatment of Alzheimer's disease: potential mechanisms of action. Br J Nutr 2015; 114: 1-14.
DOI URL |
| 12. |
Breijyeh Z, Karaman R. Comprehensive review on Alzheimer's disease: causes and treatment. Molecules 2020; 25: 5789.
DOI URL |
| 13. |
Cruchaga C, Del-Aguila JL, Saef B, et al. Polygenic risk score of sporadic late-onset Alzheimer's disease reveals a shared architecture with the familial and early-onset forms. Alzheimers Dement 2018; 14: 205-14.
DOI PMID |
| 14. |
Krauskopf J, Bergdahl IA, Johansson A, et al. Blood transcriptome response to environmental metal exposure reveals potential biological processes related to Alzheimer's disease. Front Public Health 2020; 8: 557587.
DOI URL |
| 15. |
Minhas PS, Latif-Hernandez A, McReynolds MR, et al. Restoring metabolism of myeloid cells reverses cognitive decline in ageing. Nature 2021; 590: 122-8.
DOI |
| 16. |
Howard R, McShane R, Lindesay J, et al. Donepezil and memantine for moderate-to-severe Alzheimer's disease. N Engl J Med 2012; 366: 893-903.
DOI URL |
| 17. | Birks JS, Grimley Evans J. Rivastigmine for Alzheimer's disease. Cochrane Database Syst Rev 2015; 4: Cd001191. |
| 18. |
Vaz M, Silvestre S. Alzheimer's disease: recent treatment strategies. Eur J Pharmacol 2020; 887: 173554.
DOI URL |
| 19. |
Panza F, Lozupone M, Dibello V, et al. Are antibodies directed against amyloid-β (Aβ) oligomers the last call for the Aβ hypothesis of Alzheimer's disease? Immunotherapy 2019; 11: 3-6.
DOI PMID |
| 20. |
Frozza RL, Lourenco MV, De Felice FG. Challenges for Alzheimer's disease therapy: insights from novel mechanisms beyond memory defects. Front Neurosci 2018; 12: 37.
DOI URL |
| 21. |
Li S, Wu Z, Le W. Traditional Chinese medicine for dementia. Alzheimers Dement 2021; 17: 1066-71.
DOI PMID |
| 22. |
Sreenivasmurthy SG, Liu JY, Song JX, et al. Neurogenic Traditional Chinese Medicine as a promising strategy for the treatment of Alzheimer's disease. Int J Mol Sci 2017; 18: 272.
DOI URL |
| 23. |
Zhao SJ, Tian JS, Tai G, et al. (1)H NMR-based metabolomics revealed the protective effects of Guilingji on the testicular dysfunction of aging rats. J Ethnopharmacol 2019; 238: 111839.
DOI URL |
| 24. |
Du K, Gao XX, Feng Y, et al. Integrated adrenal and testicular metabolomics revealed the protective effects of Guilingji on the Kidney-Yang deficiency syndrome rats. J Ethnopharmacol 2020; 255: 112734.
DOI URL |
| 25. |
Soma S, Suematsu N, Sato AY, et al. Acetylcholine from the nucleus basalis magnocellularis facilitates the retrieval of well-established memory. Neurobiol Learn Mem 2021; 183: 107484.
DOI URL |
| 26. |
Shalaby R, Flores-Romero H, García-Sáez AJ. The mysteries around the BCL-2 family member BOK. Biomolecules 2020; 10: 1638.
DOI URL |
| [1] | QIN Xiaoyu, WANG Chunai, XUE Jianjun, ZHANG Jie, LU Xiaoting, DING Shengshuang, GE Long, WANG Minzhen. Efficacy of electroacupuncture on myocardial protection and postoperative rehabilitation in patients undergoing cardiac surgery with cardiopulmonary bypass: a systematic review and Meta-analysis [J]. Journal of Traditional Chinese Medicine, 2024, 44(1): 1-15. |
| [2] | DAI Xiaoling, ZHANG Anming, LIN Hui, SHI Bei, REN Yi, WEN Hongzhu, FEI Xiaoyan, LIN Jiang. Qingchang suppositry (清肠栓) induced remission in patients with mild-to-moderate ulcerative proctitis: a multicenter, prospective, randomized, parallel-controlled clinical trial [J]. Journal of Traditional Chinese Medicine, 2024, 44(1): 156-162. |
| [3] | WANG Yichen, WU Shiyi, WANG Zhengyan, CHANG Wenling, XIE Zhihao, TANG Xing, ZHAO Songmei, ZHOU Jing, CHEN Zehong, WANG Chao, YANG Chunxia. Efficacy of Zhumian Tang formula granules (助眠汤配方颗粒) combined with eszopiclone for the treatment of poor sleep quality: a multi-center, randomized controlled, superiority trial [J]. Journal of Traditional Chinese Medicine, 2024, 44(1): 163-171. |
| [4] | YANG Yi, YE Huijun, ZHENG Huiling, JIN Lihua. Clinical observation on 90 cases of primary dysmenorrhea treated by buccal acupuncture therapy: a randomized controlled study [J]. Journal of Traditional Chinese Medicine, 2024, 44(1): 172-181. |
| [5] | DAI Zeqi, LIAO Xing, GUAN Yueyue, ZENG Zixiu, TANG Jun, HU Jing. Bloodletting puncture in the treatment of acute ischemic stroke: protocol for a mixed-method study of a multi-center randomized controlled trial and focus group [J]. Journal of Traditional Chinese Medicine, 2023, 43(6): 1259-1267. |
| [6] | XU Yani, ZHANG Yutong, HE Weile, DAI Linglin, TANG Ding, WANG Jialing, ZHANG Xufen, CHEN Qin, CHEN Lifang, WANG Zhanglian, ZHAN Mingjie. Efficiency and safety of acupuncture for women with premature ovarian insufficiency: study protocol for a randomized controlled trial [J]. Journal of Traditional Chinese Medicine, 2023, 43(6): 1268-1274. |
| [7] | XU Xiangru, ZHOU Yi, CHEN Gang, LEI Ming, ZHANG Wen, WU Xinxin, PU Yuting, CHEN Caiyu, SUN Yuting, ZHOU Shuang, FANG Bangjiang. Clinical efficacy of Buzhong Yiqi decoction (补中益气汤) in the treatment of hospital-acquired pneumonia with multi-drug resistant bacteria: a prospective, randomized, multicenter controlled trial [J]. Journal of Traditional Chinese Medicine, 2023, 43(5): 1010-1018. |
| [8] | ZHANG Meizhen, HAO Xiaohui, TANG Yiting, CHEN Yupeng, HE Puyu, ZHAO Liming, PANG Bing, NI Qing. Efficacy and safety of Buyang Huanwu decoction (补阳还五汤) for diabetic peripheral neuropathy: a systematic review and Meta-analysis [J]. Journal of Traditional Chinese Medicine, 2023, 43(5): 841-850. |
| [9] | YANG Yuqing, CHEN Yuhuan, LI Chunxiao, LING Xiao, WANG Panpan, GUO Jing, ZHANG Yingying. Effectiveness and safety of Pingxiao capsule (平消胶囊) as adjuvant therapy in treatment of breast cancer: a systematic review and Meta-analysis [J]. Journal of Traditional Chinese Medicine, 2023, 43(5): 851-859. |
| [10] | ZHANG Xiaoying, WANG Ruixuan, WANG Yiqing, XU Fanxing, YAN Tingxu, WU Bo, ZHANG Ming, JIA Ying. Spinosin protects Neuro-2a/APP695 cells from oxidative stress damage by inactivating p38 [J]. Journal of Traditional Chinese Medicine, 2023, 43(5): 868-875. |
| [11] | WANG Chao, WU Qiong, LI Ping, WANG Zhigang, LOU Xusheng, LI Yuanyuan, ZHANG Lin. Effect of Traditional Chinese Medicine combined with Western Medicine on blood lipid levels and inflammatory factors in patients with angina pectoris in coronary heart disease identified as intermingled phlegm and blood stasis syndrome: a network Meta-analysis [J]. Journal of Traditional Chinese Medicine, 2023, 43(4): 640-649. |
| [12] | ZHANG Xinghe, LI Qifu, YI Rong, XING Chonghui, JIN Yuhao, MENG Jiangqiong, FENG Jialei, ZHAO Siwen, LIANG Fanrong, GUO Taipin. Effect of catgut embedding at acupoints versus non-acupoints in abdominal obesity: a randomized clinical trial [J]. Journal of Traditional Chinese Medicine, 2023, 43(4): 780-786. |
| [13] | ZHANG Yuehong, DONG Dandan, YAN Youqin, ZHANG Hao, WANG Guangli, ZHOU Wei, LI Wei, QIU Li, LI Tingming, LIU Quan, XIA Ping, MAO Lina, YANG Danlin, YANG Lu, LIAN Fengmei, TONG Xiaolin, BA Yuanming. Effectiveness and safety of Jinshuibao capsules (金水宝胶囊) in treatment of residual cardiopulmonary symptoms in convalescent patients of coronavirus disease 2019: a pilot randomized, double-blind, placebo-controlled clinical trial [J]. Journal of Traditional Chinese Medicine, 2023, 43(1): 134-139. |
| [14] | YAO Yao, ZHAO Zhenni, CHEN Fengqin, LENG Yufei, PANG Xiangtian, XU Xiao, SUN Zhiling. Effectiveness of moxibustion alone on lumbar disc herniation: a Meta-analysis of randomized controlled trials [J]. Journal of Traditional Chinese Medicine, 2023, 43(1): 14-26. |
| [15] | JIN Yutong, WU Lingtao, GUO Yanglu, XIA Chong, YU Binyan, XUAN Lihua. Long-term efficacy of point application therapy on different acupoints and durations in the treatment of asthma: a randomized controlled trial [J]. Journal of Traditional Chinese Medicine, 2023, 43(1): 146-153. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||