Journal of Traditional Chinese Medicine ›› 2023, Vol. 43 ›› Issue (6): 1200-1208.DOI: 10.19852/j.cnki.jtcm.20231008.002
• Research Articles • Previous Articles Next Articles
Bo′s abdominal acupuncture improves disordered metabolism in obese type 2 diabetic rats through regulating fibroblast growth factor 21 and its related adipokines
QIN Xihui1, PANG Jianli2( ), XIONG Guan3, FENG Jie4(
), XIONG Guan3, FENG Jie4( )
)
- 1 School of Pharmaceutical Sciences, Guangxi Medical University, Nanning 530021, China
2 Department of Endocrinology, Ruikang Hospital Affiliated to Guangxi University of Traditional Chinese Medicine, Nanning 530011, China
3 Department of Gynecology and obstetrics, the Eighth People’s Hospital of Nanning, Nanning 530007, China
4 School of Pharmaceutical Sciences, Guangxi Medical University, Nanning 530021, China
-
Received:2022-08-11Accepted:2022-11-23Online:2023-10-25Published:2023-10-08 -
Contact:Dr. PANG Jianli, Department of Ruikang Hospital Affiliated, Guangxi University of Traditional Chinese Medicine, Nanning 530011, China. pangjianli@sina.com; Prof. FENG Jie, School of Pharmaceutical Sciences, Guangxi Medical University, Nanning 530021, China. ezjiefeng@hotmail.com. Telephone: +86-07712183132, +86-13557313544 -
Supported by:National Natural Science Foundation of China(81560731);to Explore the Mechanism of the Regulation of Bo′s Abdominal Acupuncture on Obesity Type 2 Diabetes based on fibroblast growth factor 21 Signaling Pathway
Cite this article
QIN Xihui, PANG Jianli, XIONG Guan, FENG Jie. Bo′s abdominal acupuncture improves disordered metabolism in obese type 2 diabetic rats through regulating fibroblast growth factor 21 and its related adipokines[J]. Journal of Traditional Chinese Medicine, 2023, 43(6): 1200-1208.
share this article
| Step | Temperature (℃) | Time | Cycle |
|---|---|---|---|
| UDG activation | 50 | 2 min | Hold |
| Dual-lock DNA polymerase | 95 | 2 min | Hold |
| Denature | 95 | 15 s | 40 |
| Anneal/extend | 60 | 1 min |
Table 1 qPCR reaction condition
| Step | Temperature (℃) | Time | Cycle |
|---|---|---|---|
| UDG activation | 50 | 2 min | Hold |
| Dual-lock DNA polymerase | 95 | 2 min | Hold |
| Denature | 95 | 15 s | 40 |
| Anneal/extend | 60 | 1 min |
| Gene | Sequence | Tm (℃) |
|---|---|---|
| FGF21 | Forward Primer 5′-TCTCCTGCTGCCTGTCTTCCTG-3′ | 61.9 |
| Reverse Primer 5′-TCGGTGTCCTGGTCGTCATCTG-3′ | 61.7 | |
| PPAR-γ | Forward Primer 5′-CGCCAAGGTGCTCCAGAAGATG-3′ | 61.4 |
| Reverse Primer 5′- AGGGTGAAGGCTCATATCTGTCTCC-3′ | 60.3 | |
| PPAR-α | Forward Primer 5′-GCCAAGAGAATCCACGAAGCCTAC-3′ | 60.3 |
| Reverse Primer 5′-TGTTGCTAGTCTTTCCTGCGAGTATG-3′ | 58.6 | |
| Leptin | Forward Primer 5′-GTTCCTGTGGCTTTGGTCCTATCTG-3′ | 59.9 |
| Reverse Primer 5′- TGATCCTGGTGACAATGGTCTTGATG-3′ | 58.8 | |
| ADP | Forward Primer 5′- ATGTATCACTCAGCATTCAGCGTAGG-3′ | 58.5 |
| Reverse Primer 5′-CTGCCGTCATAATGATTCTGTTGGTTG-3′ | 58.2 | |
| AMPK | Forward Primer 5′- ATGATGAGGTGGTGGAGCAGAGG-3′ | 61.5 |
| Reverse Primer 5′-GGTTCTCGGCTGTGCTGGAATC-3′ | 60.7 | |
| β-actin | Forward Primer 5′-GGTTCTCGGCTGTGCTGGAATC-3′ | 60.6 |
| Reverse Primer 5′- AGGAAGAGGATGCGGCAGTGG-3′ | 62.9 |
Table 2 Primer sequence and Tm of each gene
| Gene | Sequence | Tm (℃) |
|---|---|---|
| FGF21 | Forward Primer 5′-TCTCCTGCTGCCTGTCTTCCTG-3′ | 61.9 |
| Reverse Primer 5′-TCGGTGTCCTGGTCGTCATCTG-3′ | 61.7 | |
| PPAR-γ | Forward Primer 5′-CGCCAAGGTGCTCCAGAAGATG-3′ | 61.4 |
| Reverse Primer 5′- AGGGTGAAGGCTCATATCTGTCTCC-3′ | 60.3 | |
| PPAR-α | Forward Primer 5′-GCCAAGAGAATCCACGAAGCCTAC-3′ | 60.3 |
| Reverse Primer 5′-TGTTGCTAGTCTTTCCTGCGAGTATG-3′ | 58.6 | |
| Leptin | Forward Primer 5′-GTTCCTGTGGCTTTGGTCCTATCTG-3′ | 59.9 |
| Reverse Primer 5′- TGATCCTGGTGACAATGGTCTTGATG-3′ | 58.8 | |
| ADP | Forward Primer 5′- ATGTATCACTCAGCATTCAGCGTAGG-3′ | 58.5 |
| Reverse Primer 5′-CTGCCGTCATAATGATTCTGTTGGTTG-3′ | 58.2 | |
| AMPK | Forward Primer 5′- ATGATGAGGTGGTGGAGCAGAGG-3′ | 61.5 |
| Reverse Primer 5′-GGTTCTCGGCTGTGCTGGAATC-3′ | 60.7 | |
| β-actin | Forward Primer 5′-GGTTCTCGGCTGTGCTGGAATC-3′ | 60.6 |
| Reverse Primer 5′- AGGAAGAGGATGCGGCAGTGG-3′ | 62.9 |
| Group | n | FBG (mmol/L) | HOMA-IR | ISI |
|---|---|---|---|---|
| Control | 10 | 5.00±0.15 | 2.38±0.19 | -3.98±0.08 |
| Negative | 10 | 18.98±0.57a | 11.73±0.79a | -5.58±0.07a |
| Met | 10 | 6.92±0.25b | 3.61±0.16b | -4.40±0.05b |
| BOAA | 10 | 9.58±0.53bc | 5.28±0.42bc | -4.70±0.12bc |
| COA | 10 | 16.40±0.73b | 9.19±0.87b | -5.37±0.07b |
Table 3 Levels of FBG, HOMA-IR and ISI
| Group | n | FBG (mmol/L) | HOMA-IR | ISI |
|---|---|---|---|---|
| Control | 10 | 5.00±0.15 | 2.38±0.19 | -3.98±0.08 |
| Negative | 10 | 18.98±0.57a | 11.73±0.79a | -5.58±0.07a |
| Met | 10 | 6.92±0.25b | 3.61±0.16b | -4.40±0.05b |
| BOAA | 10 | 9.58±0.53bc | 5.28±0.42bc | -4.70±0.12bc |
| COA | 10 | 16.40±0.73b | 9.19±0.87b | -5.37±0.07b |
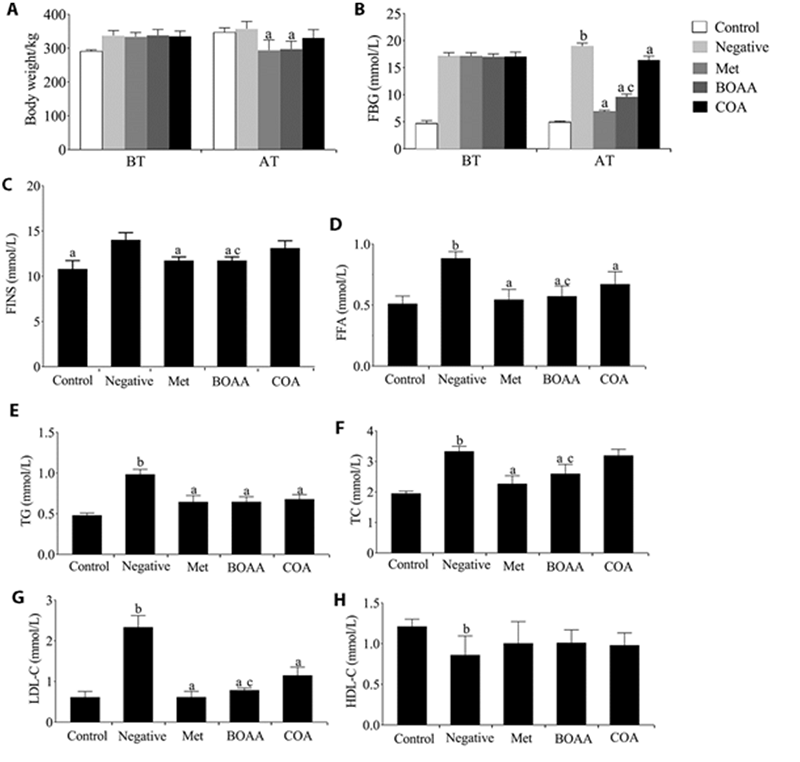
Figure 1 Effects of the body weight, blood glucose, FINS, FFA, TG, TC, LDL-C and HDL-C levels in different groups A: body weight; B: blood glucose; C: FINS; D: FAA; E: TG; F: TC; G: LDL-C; H: HDL-C. BT: before treatment; AT: after treatment. Control group: no treatment is given; negative group: treated with high fat feed; Met group: treated with metformin; COA group: treated with COA; BOAA group: treated with BOAA. BOAA: Bo′s abdominal acupuncture; COA: conventional acupuncture; FBG: fasting blood glucose; FINS: fasting insulin; FFA: free fatty acids; TC: total cholesterol; TG: total triglycerides; HDL-C: high-density lipoprotein; LDL-C: low-density lipoprotein. Data are expressed as mean ± standard deviation (n = 10). Compared with the negative group, aP < 0.05; compared with the control group, bP < 0.05; BOAA group compared with the COA group, cP < 0.05.
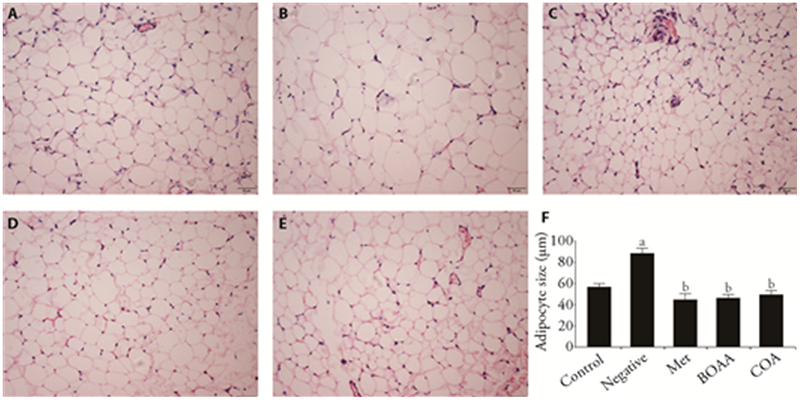
Figure 2 Histopathological observations of adipose tissue A: control group; B: negative group; C: met group; D: BOAA group; E: COA group; F: adipocyte size. Control group: no treatment is given; negative group: treated with high fat feed; Met group: treated with metformin; COA group: treated with COA; BOAA group: treated with BOAA. BOAA: Bo′s abdominal acupuncture; COA: conventional acupuncture. Data are expressed as mean ± standard deviation (n = 10). Compared with the control group, aP < 0.05; compared with the negative group, bP < 0.05; BOAA group compared with the COA group, cP < 0.05. Samples were stained with hematoxylin-eosin and photographed at 20 × 10 magnification.
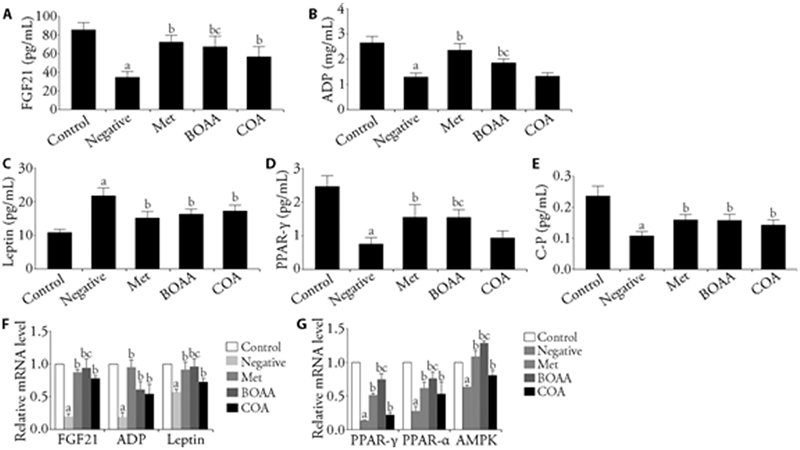
Figure 3 Serum levels of FGF21, ADP leptin, PPAR-γ and C-P, and the mRNA expressions of FGF21, ADP, leptin, PPAR-γ, PPAR-α and AMPK in adipose tissue A: FGF21; B: ADP; C: Leptin; D: PPAR-γ; E: C-P; F-G: relative mRNA level. A-E: serum levels of FGF21, ADP leptin, PPAR-γ and C-P. F-G: q-PCR was used to analyze the mRNA expression levels of FGF21, ADP, leptin, PPAR-γ, PPAR-α and AMPK in adipose tissue. BOAA: Bo′s abdominal acupuncture; COA: conventional acupuncture; FGF21: fibroblast growth factor 21; PPAR-α: peroxisome proliferator-activated receptor α; PPAR-γ: peroxisome proliferator-activated receptor γ; AMPK: adenosine 5‘-monophosphate (AMP)-activated protein kinase; C-P: C-peptide; ADP: adiponectin. Control group: no treatment is given; negative group: treated with high fat feed; Met group: treated with metformin; COA group: treated with COA; BOAA group: treated with BOAA. Data are expressed as mean ± standard deviation (n = 10). Compared with the control group, aP < 0.05; compared with the negative group, bP < 0.05; BOAA group compared with the COA group, cP < 0.05.
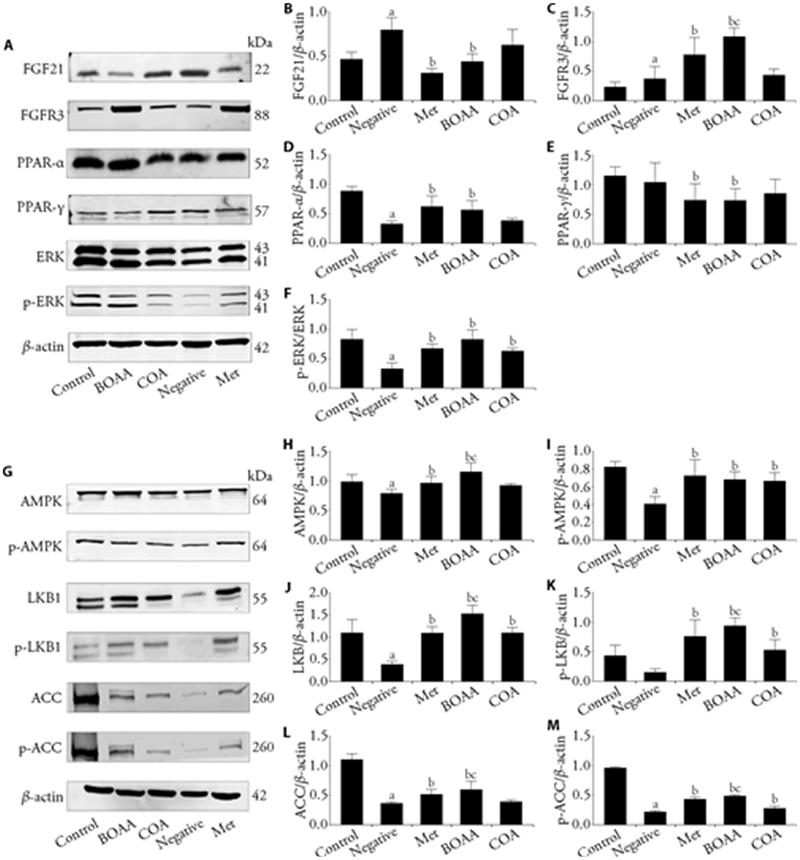
Figure 4 Proteins expression of FGF21/PPAR-γ/ERK/AMPK signaling pathway in adipose tissue A-F: Western blot was used to analyze the protein levels of (B) FGF21, (C) FGFR3, (D) PPAR-α, (E) PPAR-γ, (F) ERK in adipose tissue. G-M: Western blot was used to analyze the protein levels of (H) AMPK, (I) p-AMPK, (J) LKB1, (K) p-LKB1, (L) ACC, (M) p-ACC in adipose tissue. BOAA: Bo′s abdominal acupuncture; COA: conventional acupuncture; FGF21:fibroblast growth factor 21; FGFR3:fibroblast growth factor receptors3; PPAR-α:peroxisome proliferator-activated receptor α; PPAR-γ: peroxisome proliferator-activated receptor γ; ERK: extracellular signal-regulated kinase; AMPK: adenosine 5‘-monophosphate-activated protein kinase; p-AMPK: phosphorylated adenosine 5‘-monophosphate-activated protein kinase; LKB1: liver kinase B1; p-LKB1: phosphorylated liver kinase B1; ACC: acetyl-CoA carboxylase; p-ACC: phosphorylated acetyl-CoA carboxylase. Control group: no treatment is given; negative group: treated with high fat feed; Met group: treated with metformin; COA group: treated with COA; BOAA group: treated with BOAA. Data are expressed as mean ± standard deviation (n = 10). Compared with the control group, aP < 0.05; compared with the negative group, bP < 0.05; BOAA group compared with the COA group, cP < 0.05.
| 1. |
Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol 2018; 14: 88-98.
DOI PMID |
| 2. | Malone JI, Hansen BC. Does obesity cause type 2 diabetes mellitus (T2DM)? Or is it the opposite? Pediatr Diabetes 2019; 20: 5-9. |
| 3. |
Xiao M, Tang Y, Wang S, et al. The role of fibroblast growth factor 21 in diabetic cardiovascular complications and related epigenetic mechanisms. Front Endocrinol (Lausanne) 2021; 12: 598008.
DOI URL |
| 4. |
Maximus PS, Al Achkar Z, Hamid PF, Hasnain SS, Peralta CA. Adipocytokines: are they the theory of everything? Cytokine 2020; 133: 155144-54.
DOI URL |
| 5. |
Esmaili S, Hemmati M, Karamian M. Physiological role of adiponectin in different tissues: a review. Arch Physiol Biochem 2020; 126: 67-73.
DOI PMID |
| 6. |
Achari AE, Jain SK. Adiponectin, a Therapeutic target for obesity, diabetes, and endothelial dysfunction. Int J Mol Sci 2017; 18: 1321-8.
DOI URL |
| 7. |
Liu W, Zhou XH, Li YF, et al. Serum leptin, resistin, and adiponectin levels in obese and non-obese patients with newly diagnosed type 2 diabetes mellitus: a population-based study. Medicine (Baltimore) 2020; 99: e19052.
DOI URL |
| 8. | Wang LK, Wang H, Wu XL, et al. Relationships among resistin, adiponectin, and leptin and microvascular complications in patients with type 2 diabetes mellitus. J Int Med Res 2020; 48: 300060519870407. |
| 9. |
Geng L, Lam KSL, Xu A. The therapeutic potential of FGF21 in metabolic diseases: from bench to clinic. Nat Rev Endocrinol 2020; 16: 654-67.
DOI |
| 10. |
Liu QR, Wang S, Wei M, et al. Improved FGF21 sensitivity and restored FGF21 signaling pathway in high-fat diet/streptozotocin-induced diabetic rats after duodenal-jejunal bypass and sleeve gastrectomy. Front Endocrinol (Lausanne) 2019; 10: 566-79.
DOI URL |
| 11. |
Andersen B, Straarup EM, Heppner KM, et al. FGF21 decreases body weight without reducing food intake or bone mineral density in high-fat fed obese rhesus macaque monkeys. Int J Obes (Lond) 2018; 42 (6): 1151-60.
DOI |
| 12. |
She QY, Bao JF, Wang HZ, et al. Fibroblast growth factor 21: A "rheostat" for metabolic regulation. Metabolism 2022; 130: 155166.
DOI URL |
| 13. |
Wang N, Sun B, Guo H, et al. Association of elevated plasma FGF21 and activated FGF 21 signaling in visceral white adipose tissue and improved insulin sensitivity in gestational diabetes mellitus subtype: a case-control study. Front Endocrinol (Lausanne) 2021; 12: 795520.
DOI URL |
| 14. |
Sun L, Yan J, Goh HJ, et al. Fibroblast Growth Factor-21, leptin, and adiponectin responses to acute cold-induced brown adipose tissue activation. J Clin Endocrinol Metab 2020; 105: e520-31.
DOI URL |
| 15. | Park SY, Lee HJ, Song JH, et al. Dimethyl itaconate attenuates palmitate-induced insulin resistance in skeletal muscle cells through the AMPK/FGF21/PPARδ-mediated suppression of inflammation. Life Sci 2021; 287: 120129. |
| 16. |
Liu M, Chen J, Ren Q, et al. Acupuncture and related techniques for type 2 diabetes mellitus: a systematic review protocol. Medicine (Baltimore) 2019; 98: e14059.
DOI URL |
| 17. |
Martinez B, Peplow PV. Treatment of insulin resistance by acupuncture: a review of human and animal studies. Acupunct Med 2016; 34: 310-9.
DOI PMID |
| 18. | Yin G, Shen GM, Jiang AJ, Li JY. Acupuncture intervention induced improvement of oxidative stress by regulating PKCβ/P66shc signaling in obese diabetic rats. Zhen Ci Yan Jiu 2021; 46: 642-8. |
| 19. | Duan HR, Li R, Song SS, Hu SQ, Zhuang ST, Li QY. Electroacupuncture improves glucose and lipid metabolism by regulating APN/AMPK/PPARα signaling of skeletal muscle in Zucker diabetic obese rats. Zhen Ci Yan Jiu 2021; 46: 907-13. |
| 20. |
Fasipe OJ, Ayoade OG, Enikuomehin AC. Severity grade assessment classifications for both insulin resistance syndrome and status of pancreatic beta cell function in clinical practice using homeostasis model assessment method indices. Can J Diabetes 2020; 44: 663-9.
DOI PMID |
| 21. | Hua XB. The acupoints of animals. Zhong Yi Za Zhi 1987; 33: 65-6. |
| 22. | Cheng M, Zhang X, Shi YL, Shang PP, Pu GX, Xu YD. Survey of acupoint localization in experimental rats and mice. Shanghai Zhen Jiu Za Zhi 2021; 40: 640-6. |
| 23. |
Li F, Chen J, Liu Y, et al. Deficiency of cathelicidin attenuates high-fat diet plus alcohol-induced liver injury through FGF21/adiponectin regulation. Cells 2021; 10: 3333.
DOI URL |
| 24. |
Makarova E, Kazantseva A, Dubinina A, et al. Fibroblast growth factor 21 (FGF21) administration sex-specifically affects blood insulin levels and liver steatosis in obese ay mice. Cells 2021; 10: 3440.
DOI URL |
| 25. |
Yanai H, Yoshida H. Beneficial effects of adiponectin on glucose and lipid metabolism and atherosclerotic progression: mechanisms and perspectives. Int J Mol Sci 2019; 20: 1190.
DOI URL |
| 26. |
Pan Q, Lin S, Li Y, et al. A novel GLP-1 and FGF 21 dual agonist has therapeutic potential for diabetes and non-alcoholic steatohepatitis. EBioMedicine 2021; 63: 103202.
DOI URL |
| 27. | Mazuecos L, Pintado C, Rubio B, Guisantes-Batán E, Andrés A, Gallardo N. Leptin, acting at central level, increases FGF21 expression in white adipose tissue via PPARβ/δ. Int J Mol Sci 2021; 22: 4624. |
| 28. |
Keinicke H, Sun G, Mentzel CMJ, et al. FGF21 regulates hepatic metabolic pathways to improve steatosis and inflammation. Endocr Connect 2020; 9: 755-68.
DOI PMID |
| 29. |
Kim KW, Shin WC, Choi MS, et al. Effects of acupuncture on anthropometric and serum metabolic parameters in premenopausal overweight and obese women: a randomized, patient- and assessor-blind, sham-controlled clinical trial. Acupunct Med 2021; 39: 30-40.
DOI URL |
| 30. | Gong L, Zou Z, Huang L, Guo S, Xing D. Photobiomodulation therapy decreases free fatty acid generation and release in adipocytes to ameliorate insulin resistance in type 2diabetes. Cell Signal 2020; 67: 09491. |
| 31. |
Flippo KH, Potthoff MJ. Metabolic messengers: FGF21. Nat Metab 2021; 3: 309-17.
DOI PMID |
| 32. |
Hong ES, Lim C, Choi HY, et al. Plasma fibroblast growth factor 21 levels increase with ectopic fat accumulation and its receptor levels are decreased in the visceral fat of patients with type 2 diabetes. BMJ Open Diabetes Res Care 2019; 7: e000776.
DOI URL |
| 33. |
Markan KR, Naber MC, Small SM. FGF21 resistance is not mediated by downregulation of beta-klotho expression in white adipose tissue. Mol Metab 2017; 6: 602-10.
DOI PMID |
| 34. |
Dai Q, Fan X, Meng X, et al.FGF21 promotes ischaemic angiogenesis and endothelial progenitor cells function under diabetic conditions in an AMPK/NAD+-dependent manner. J Cell Mol Med 2021; 25: 3091-102.
DOI PMID |
| 35. | Xie T, So WY, Li XY, Leung PS. Fibroblast growth factor 21 protects against lipotoxicity-induced pancreatic β-cell dysfunction via regulation of AMPK signaling and lipid metabolism. Clin Sci (Lond) 2019; 133: 2029-44. |
| 36. |
Zhang N, Liu C, Zhang Y, et al. Liraglutide regulates lipid metabolism via FGF21- LKB1- AMPK- ACC1 pathway in white adipose tissues and macrophage of type 2 diabetic mice. Biochem Biophys Res Commun 2021; 548: 120-6.
DOI URL |
| 37. |
Lewis JE, Monnier C, Marshall H, et al. Whole-body and adipose tissue-specific mechanisms underlying the metabolic effects of fibroblast growth factor 21 in the Siberian hamster. Mol Metab 2020; 31: 45-54.
DOI PMID |
| 38. | Katsu-Jiménez Y, Giménez-Cassina A. Fibroblast growth factor-21 promotes ketone body utilization in neurons through activation of AMP-dependent kinase. Mol Cell Neurosci 2019; 101: 103415-27. |
| 39. |
Ifrim CF, Antochi AD, Barbilian AG. Acupuncture and the retrospect of its modern research. Rom J Morphol Embryol 2019; 60: 411-8.
PMID |
| 40. | Yan SY, Xiong ZY, Liu XY, Liu CZ, Liu BY. Review of clinical research in acupuncture and moxibustion from 2010 to 2020 and future prospects. Zhong Guo Zhen Jiu 2022; 42: 116-8. |
| 41. | Zhou K, Wu Q, Yue J, et al. Electroacupuncture suppresses spinal nerve ligation-induced neuropathic pain via regulation of synaptic plasticity through upregulation of basic fibroblast growth factor expression. Acupunct Med 2022; 9645284211066499. |
| 42. | Liu XX, Zhang LZ, Zhang HH, et al. Low-frequency electroacupuncture improves disordered hepatic energy metabolism in insulin-resistant Zucker diabetic fatty rats via the AMPK/mTORC1/p70S6K signaling pathway. Acupunct Med 2022;9645284211070301. |
| [1] | ZHU Linghui, SUN Ziwei, GUAN Yuanyuan, LIU Meiyi, ZHENG Yi, YU Ruoxi, WANG Qi, LI Lingru. Differences in vascular endothelial function and serum proteome between obese people with phlegm-dampness constitution and balanced constitution [J]. Journal of Traditional Chinese Medicine, 2024, 44(1): 188-196. |
| [2] | ZHAO HuiYan, JUN Purumea, LEE Chaewon, HAN Chang-Hyun. Acupoint catgut embedding for simple obesity in animal studies: a systematic review and Meta-analysis [J]. Journal of Traditional Chinese Medicine, 2023, 43(5): 860-867. |
| [3] | ZHANG Xinghe, LI Qifu, YI Rong, XING Chonghui, JIN Yuhao, MENG Jiangqiong, FENG Jialei, ZHAO Siwen, LIANG Fanrong, GUO Taipin. Effect of catgut embedding at acupoints versus non-acupoints in abdominal obesity: a randomized clinical trial [J]. Journal of Traditional Chinese Medicine, 2023, 43(4): 780-786. |
| [4] | YE Xiaomei, XING Xiaowei, YUAN Kangrui, WANG Dongming, WU Dudu, CHEN Zhi, YU Zhiqiang. Astragaloside IV ameliorates insulin induced insulin resistance in HepG2 cells through reactive oxygen species mediated c-Jun N-terminal kinase pathway [J]. Journal of Traditional Chinese Medicine, 2023, 43(1): 60-67. |
| [5] | WEI Jiali, LAI Lily, LIN Zhechao, LIU Jianping, HAN Mei. Acupoint catgut embedding versus acupuncture for simple obesity: a systematic review and Meta-analysis of randomized controlled trials [J]. Journal of Traditional Chinese Medicine, 2022, 42(6): 839-847. |
| [6] | YE Wujie, XING Jingyu, YU Zekai, HU Xingang, ZHAO Yan. Systematic review and Meta-analysis of acupuncture and acupoint catgut embedding for the treatment of abdominal obesity [J]. Journal of Traditional Chinese Medicine, 2022, 42(6): 848-857. |
| [7] | Lakkana Rerksuppaphol, Sanguansak Rerksuppaphol. Efficacy of short duration versus conventional electroacupuncture in the treatment of obesity: a randomized crossover study [J]. Journal of Traditional Chinese Medicine, 2022, 42(2): 256-263. |
| [8] | CHEN Xi, HUANG Weixuan, Lü Hongmei, QIN Jian, KE Bin, SHEN Weizeng. Identification of Cald1 as a novel regulator of Linggui Zhugan decoction(苓桂术甘汤) for improving insulin resistance in vivo and in vitro [J]. Journal of Traditional Chinese Medicine, 2021, 41(5): 706-716. |
| [9] | YANG Le, YANG Baocun, ZHANG Caixia, TONg Jie, FENG Geli, XIAO Gaiqin. Protective effects of acupuncture and LGNHFD on expressions of vascular endothelial growth factor, basic fibroblast growth factor,and cluster of differentiation 34 in rats with cerebral ischemia-reperfusion injury [J]. Journal of Traditional Chinese Medicine, 2021, 41(3): 463-469. |
| [10] | YIN Yundong, FANG Zhaohui, WU Yuanyuan, YOU Liangzhen. Effect of Shenzhu Tiaopi granule(参术调脾颗粒) on hepatic insulin resistance in diabetic Goto-Kakizakirats via liver kinase B1/adenosine 5'-monophosphate/mammalian target of rapamycin signaling pathway [J]. Journal of Traditional Chinese Medicine, 2021, 41(1): 107-116. |
| [11] | Sun Chen, Wang Li, Sun Jing, Wang Zheng, Tang Zhishu. Hypoglycemic and hypolipidemic effects of rutin on hyperglycemic rats [J]. Journal of Traditional Chinese Medicine, 2020, 40(4): 640-645. |
| [12] | Se-Eun Lee, Hyungwoo Kim, Chang-Hyun Kim, Chiyeon Lim, Suin Cho. Effect of methanol extract of Schisandrae Fructus on high fat diet induced hyperlipidemic mice [J]. Journal of Traditional Chinese Medicine, 2019, 39(06): 818-825. |
| [13] | Wei Yan, Hong Yuizhi, Hou Pengchao, Xu Xinpeng. Effect of sugar-free Qishan granules on glucose and lipid metabolism and insulin resistance in a rat model of prediabetes [J]. Journal of Traditional Chinese Medicine, 2019, 39(04): 535-541. |
| [14] | Tian Chunyu, Wang Ya, La Xiaojin, Li Ji'an, Zhang Biwei. Spleen-kidney supplementing formula alleviates insulin resistance via regulating AKT/glycogen synthase kinase 3β pathway in rats with type 2 diabetic induced by high-fat diet [J]. Journal of Traditional Chinese Medicine, 2019, 39(02): 199-206. |
| [15] | Wang Haiyan, Li Linyi, Qin Lingling, Wang Dongchao, Jiang Yueying, Wu Xinli, Xu Tunhai, Liu Tonghua. Mixture of five herbal extracts ameliorates pioglitazone-induced aggravation of hepatic steatosis via activating the adiponectin receptor 2/AMP-activated protein kinase signal pathway in diabetic KKAy mice [J]. Journal of Traditional Chinese Medicine, 2017, 37(05): 588-598. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||

