Journal of Traditional Chinese Medicine ›› 2025, Vol. 45 ›› Issue (4): 817-828.DOI: 10.19852/j.cnki.jtcm.2025.04.011
• Original Articles • Previous Articles Next Articles
Efficacy and safety of Suhuang Zhike capsule (苏黄止咳胶囊) for cough variant asthma: a multicenter, single-arm, open-label phase IV real-world clinical trial
ZHANG Hongchun1, LIU Jian1, CHEN Sheng2, ZHANG Wei3, LU Xuechao4, LI Ying1, YU Xueqing5, HUANG Yan6, SU Lianhua7, WEI Baolin8, LI Zhuyin9, PEI Shuai10, LEI Xiang11, YANG Daowen1, GUO Jianning1( )
)
- 1 Department of Traditional Chinese Medicine for Pulmonary Diseases, National Center for Respiratory Medicine; National Centre for Integrative Chinese and Western Medicine; State Key Laboratory of Respiratory Health and Multimorbidity; National Clinical Research Center for Respiratory Diseases; Institute of Respiratory Medicine, Chinese Academy of Medical Sciences; China-Japan Friendship Hospital, Beijing 100029, China
2 Department of Respiratory Diseases, Shenzhen Traditional Chinese Medicine Hospital, Shenzhen 518033, China
3 Department of Pulmonary and Critical Care Medicine, Shandong University of Traditional Chinese Medicine Affiliated Hospital, Jinan 250011, China
4 Department of Respiratory and Critical Care Medicine, Qingdao Hospital of Traditional Chinese Medicine (Qingdao Hiser Hospital), Qingdao 266033, China
5 Department of Pulmonary Diseases, the First Affiliated Hospital of Henan University of Chinese Medicine, Zhengzhou 450000, China
6 Department of Pulmonary Diseases, Inner Mongolia Autonomous Region Traditional Chinese Medicine Hospital, Hohhot 750306, China
7 Department of Pulmonary Diseases, Fangshan Hospital Beijing University of Chinese Medicine, Beijing 102488, China
8 Department of Pulmonary Diseases, the Second Affiliated Hospital of Tianjin University of Traditional Chinese Medicine, Tianjin 300150, China
9 Department of Pulmonary Diseases, the First Affiliated Hospital of Heilongjiang University of Traditional Chinese Medicine, Harbin 150040, China
10 Marketing Department of Yangzijiang Pharmaceutical Group Co., Ltd., Taizhou 225300, China
11 Medical Department, Beijing Qi-Huang Technology Co., Ltd., Beijing 100006, China
-
Received:2024-12-22Accepted:2025-05-18Online:2025-08-15Published:2025-07-25 -
Contact:GUO Jianning -
About author:GUO Jianning, Department of Traditional Chinese Medicine for Pulmonary Diseases, National Center for Respiratory Medicine; National Centre for Integrative Chinese and Western Medicine; State Key Laboratory of Respiratory Health and Multimorbidity; National Clinical Research Center for Respiratory Diseases; Institute of Respiratory Medicine, Chinese Academy of Medical Sciences; China-Japan Friendship Hospital, Beijing 100029, China. Dr.jane93@outlook.com,Telephone: +86-15319537768
First author contact:ZHANG Hongchun and LIU Jian are co-first authors and contributed equally to this work
-
Supported by:Inheritance and Innovation of Traditional Chinese Medicine "Hundred Million" Talent Project (Qihuang Project)(2019-QTL-003)
Cite this article
ZHANG Hongchun, LIU Jian, CHEN Sheng, ZHANG Wei, LU Xuechao, LI Ying, YU Xueqing, HUANG Yan, SU Lianhua, WEI Baolin, LI Zhuyin, PEI Shuai, LEI Xiang, YANG Daowen, GUO Jianning. Efficacy and safety of Suhuang Zhike capsule (苏黄止咳胶囊) for cough variant asthma: a multicenter, single-arm, open-label phase IV real-world clinical trial[J]. Journal of Traditional Chinese Medicine, 2025, 45(4): 817-828.
share this article
| Characteristic | FAS (n =1033) | |
|---|---|---|
| Age (years) | $\bar{x} \pm s$ | 43.59±15.71 |
| Median (Q1, Q3) | 44.00 (29.00, 57.00) | |
| Min, Max | 17.00, 88.00 | |
| Gender [n (%)] | Male | 346 (33.53) |
| Female | 686 (66.47) | |
| Ethnic [n (%)] | Han | 1009 (97.77) |
| Minority | 23 (2.23) | |
| Height (cm, $\bar{x} \pm s$) | 165.71±7.09 | |
| Weight (kg, $\bar{x} \pm s$) | 63.97±10.21 | |
| Body temperature (℃, $\bar{x} \pm s$) | 36.42±0.22 | |
| Pulse (Times/min, $\bar{x} \pm s$) | 74.93±9.33 | |
| Respiratory rates (Times/min, $\bar{x} \pm s$) | 18.09±1.55 | |
| Diastolic blood pressure (mm Hg, $\bar{x} \pm s$) | 76.25±8.53 | |
| Systolic pressure (mm Hg, $\bar{x} \pm s$) | 119.72±11.16 | |
| Positive bronchial provocation test [n (%)] | Yes | 31 (3.00) |
| No | 1002 (97.00) | |
| Positive bronchi relaxation test [n (%)] | Yes | 11 (1.06) |
| No | 1022 (98.94) | |
| The average variability of PEF > 10% [n (%)] | Yes | 0 (0.00) |
| No | 1033 (100.00) | |
| History of diseases [n (%)] | Yes | 244 (23.62) |
| No | 789 (76.38) | |
| Allergic history [n (%)] | Yes | 59 (5.71) |
| No | 974 (94.29) | |
| Anti-asthma treatment was effective [n (%)] | Yes | 71 (6.87) |
| No | 962 (93.13) | |
| Chest X-ray inspection results [n (%)] | Normal | 578 (55.95) |
| Abnormal | 452 (43.76) | |
| Not performed | 2 (0.19) | |
| Missing | 1 (0.10) | |
Table 1 Baseline characteristics of the study population
| Characteristic | FAS (n =1033) | |
|---|---|---|
| Age (years) | $\bar{x} \pm s$ | 43.59±15.71 |
| Median (Q1, Q3) | 44.00 (29.00, 57.00) | |
| Min, Max | 17.00, 88.00 | |
| Gender [n (%)] | Male | 346 (33.53) |
| Female | 686 (66.47) | |
| Ethnic [n (%)] | Han | 1009 (97.77) |
| Minority | 23 (2.23) | |
| Height (cm, $\bar{x} \pm s$) | 165.71±7.09 | |
| Weight (kg, $\bar{x} \pm s$) | 63.97±10.21 | |
| Body temperature (℃, $\bar{x} \pm s$) | 36.42±0.22 | |
| Pulse (Times/min, $\bar{x} \pm s$) | 74.93±9.33 | |
| Respiratory rates (Times/min, $\bar{x} \pm s$) | 18.09±1.55 | |
| Diastolic blood pressure (mm Hg, $\bar{x} \pm s$) | 76.25±8.53 | |
| Systolic pressure (mm Hg, $\bar{x} \pm s$) | 119.72±11.16 | |
| Positive bronchial provocation test [n (%)] | Yes | 31 (3.00) |
| No | 1002 (97.00) | |
| Positive bronchi relaxation test [n (%)] | Yes | 11 (1.06) |
| No | 1022 (98.94) | |
| The average variability of PEF > 10% [n (%)] | Yes | 0 (0.00) |
| No | 1033 (100.00) | |
| History of diseases [n (%)] | Yes | 244 (23.62) |
| No | 789 (76.38) | |
| Allergic history [n (%)] | Yes | 59 (5.71) |
| No | 974 (94.29) | |
| Anti-asthma treatment was effective [n (%)] | Yes | 71 (6.87) |
| No | 962 (93.13) | |
| Chest X-ray inspection results [n (%)] | Normal | 578 (55.95) |
| Abnormal | 452 (43.76) | |
| Not performed | 2 (0.19) | |
| Missing | 1 (0.10) | |
| Outcome | Number [n (missing case)] | Full-day cough symptom score ($\bar{x} \pm s$) | Change vs Baseline ($\bar{x} \pm s$) | P value |
|---|---|---|---|---|
| Baseline | 1026 (0) | 3.8±1.3 | - | - |
| 2 d of treatment | 1013 (13) | 3.4±1.4 | —0.4±1.2 | <0.0001 |
| 3 d of treatment | 1011 (15) | 3.2±1.3 | —0.7±1.3 | <0.0001 |
| 4 d of treatment | 1009 (17) | 2.9±1.3 | —1.0±1.3 | <0.0001 |
| 5 d of treatment | 1007 (19) | 2.5±1.3 | —1.3±1.3 | <0.0001 |
| 6 d of treatment | 1006 (20) | 2.3±1.2 | —1.6±1.3 | <0.0001 |
| 7 d of treatment | 1002 (24) | 2.1±1.1 | —1.8±1.4 | <0.0001 |
| 8 d of treatment | 989 (37) | 2.1±1.1 | —1.8±1.4 | <0.0001 |
| 9 d of treatment | 981 (45) | 2.0±1.1 | —1.9±1.4 | <0.0001 |
| 10 d of treatment | 979 (47) | 1.7±1.1 | —2.1±1.4 | <0.0001 |
| 11 d of treatment | 977 (49) | 1.4±1.1 | —2.4±1.5 | <0.0001 |
| 12 d of treatment | 950 (76) | 1.2±1.1 | —2.6±1.6 | <0.0001 |
| 13 d of treatment | 912 (114) | 1.1±1.1 | —2.8±1.6 | <0.0001 |
| 14 d of treatment | 771 (255) | 0.9±1.1 | —2.9±1.7 | <0.0001 |
Table 2 Cough symptom score
| Outcome | Number [n (missing case)] | Full-day cough symptom score ($\bar{x} \pm s$) | Change vs Baseline ($\bar{x} \pm s$) | P value |
|---|---|---|---|---|
| Baseline | 1026 (0) | 3.8±1.3 | - | - |
| 2 d of treatment | 1013 (13) | 3.4±1.4 | —0.4±1.2 | <0.0001 |
| 3 d of treatment | 1011 (15) | 3.2±1.3 | —0.7±1.3 | <0.0001 |
| 4 d of treatment | 1009 (17) | 2.9±1.3 | —1.0±1.3 | <0.0001 |
| 5 d of treatment | 1007 (19) | 2.5±1.3 | —1.3±1.3 | <0.0001 |
| 6 d of treatment | 1006 (20) | 2.3±1.2 | —1.6±1.3 | <0.0001 |
| 7 d of treatment | 1002 (24) | 2.1±1.1 | —1.8±1.4 | <0.0001 |
| 8 d of treatment | 989 (37) | 2.1±1.1 | —1.8±1.4 | <0.0001 |
| 9 d of treatment | 981 (45) | 2.0±1.1 | —1.9±1.4 | <0.0001 |
| 10 d of treatment | 979 (47) | 1.7±1.1 | —2.1±1.4 | <0.0001 |
| 11 d of treatment | 977 (49) | 1.4±1.1 | —2.4±1.5 | <0.0001 |
| 12 d of treatment | 950 (76) | 1.2±1.1 | —2.6±1.6 | <0.0001 |
| 13 d of treatment | 912 (114) | 1.1±1.1 | —2.8±1.6 | <0.0001 |
| 14 d of treatment | 771 (255) | 0.9±1.1 | —2.9±1.7 | <0.0001 |
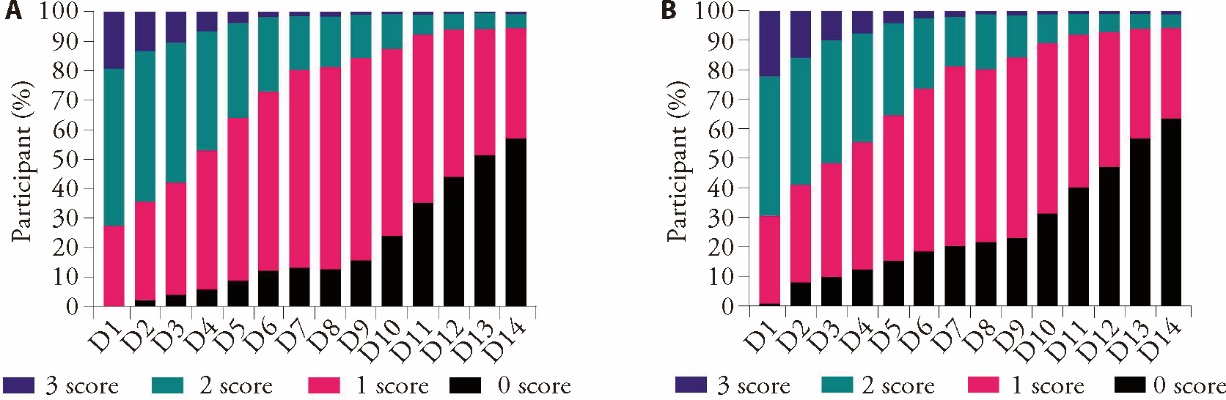
Figure 2 The proportion of patients cough scores changes over the course of treatment A: daytime cough symptom score; B: night cough symptom score. Purple indicates a score of 3, green indicates 2, pink indicates 1, and black indicates 0. Different scores indicate different cough severity.
| Time of treatment (day) | Cough disappearance rate (%, 95% CI) |
|---|---|
| 2 | 5.23 (3.94, 6.79) |
| 3 | 8.11 (6.50, 9.97) |
| 4 | 11.02 (9.16, 13.12) |
| 5 | 15.44 (13.26, 17.82) |
| 6 | 17.43 (15.13, 19.92) |
| 7 | 16.68 (14.41, 19.16) |
| 8 | 19.73 (17.28, 22.37) |
| 9 | 24.69 (22.02, 27.52) |
| 10 | 36.09 (33.08, 39.19) |
| 11 | 47.08 (43.91, 50.27) |
| 12 | 56.38 (53.15, 59.56) |
| 13 | 63.76 (60.53, 66.89) |
| 14 | 67.21 (63.70, 70.58) |
Table 3 Cough disappearance rate at different treatment time points
| Time of treatment (day) | Cough disappearance rate (%, 95% CI) |
|---|---|
| 2 | 5.23 (3.94, 6.79) |
| 3 | 8.11 (6.50, 9.97) |
| 4 | 11.02 (9.16, 13.12) |
| 5 | 15.44 (13.26, 17.82) |
| 6 | 17.43 (15.13, 19.92) |
| 7 | 16.68 (14.41, 19.16) |
| 8 | 19.73 (17.28, 22.37) |
| 9 | 24.69 (22.02, 27.52) |
| 10 | 36.09 (33.08, 39.19) |
| 11 | 47.08 (43.91, 50.27) |
| 12 | 56.38 (53.15, 59.56) |
| 13 | 63.76 (60.53, 66.89) |
| 14 | 67.21 (63.70, 70.58) |
| Full-day cough symptom score | Suhuang Zhike capsule | Combined respiratory system Western Medicine | P value |
|---|---|---|---|
| Baseline | 3.6±1.4 | 2.9±1.4 | <0.0001 |
| 2 d of treatment | 3.5±1.4 | 2.9±1.5 | <0.0001 |
| 3 d of treatment | 3.2±1.3 | 2.7±1.4 | 0.0014 |
| 4 d of treatment | 2.9±1.3 | 2.4±1.4 | 0.0003 |
| 5 d of treatment | 2.6±1.2 | 2.2±1.3 | 0.0026 |
| 6 d of treatment | 2.3±1.2 | 2.0±1.3 | 0.0223 |
| 7 d of treatment | 2.1±1.1 | 1.8±1.2 | 0.0264 |
| 8 d of treatment | 2.1±1.1 | 1.7±1.1 | 0.0027 |
| 9 d of treatment | 2.0±1.0 | 1.9±1.2 | 0.4051 |
| 10 d of treatment | 1.7±1.1 | 1.6±1.2 | 0.3457 |
| 11 d of treatment | 1.4±1.1 | 1.5±1.2 | 0.5501 |
| 12 d of treatment | 1.2±1.1 | 1.4±1.3 | 0.1290 |
| 13 d of treatment | 1.0±1.1 | 1.3±1.2 | 0.0344 |
| 14 d of treatment | 0.9±1.1 | 1.3±1.2 | 0.0026 |
Table 4 Full-day cough symptom score between Suhuang Zhike capsule and Suhuang Zhike capsule combined with Western Medicine of respiratory system ($\bar{x} \pm s$)
| Full-day cough symptom score | Suhuang Zhike capsule | Combined respiratory system Western Medicine | P value |
|---|---|---|---|
| Baseline | 3.6±1.4 | 2.9±1.4 | <0.0001 |
| 2 d of treatment | 3.5±1.4 | 2.9±1.5 | <0.0001 |
| 3 d of treatment | 3.2±1.3 | 2.7±1.4 | 0.0014 |
| 4 d of treatment | 2.9±1.3 | 2.4±1.4 | 0.0003 |
| 5 d of treatment | 2.6±1.2 | 2.2±1.3 | 0.0026 |
| 6 d of treatment | 2.3±1.2 | 2.0±1.3 | 0.0223 |
| 7 d of treatment | 2.1±1.1 | 1.8±1.2 | 0.0264 |
| 8 d of treatment | 2.1±1.1 | 1.7±1.1 | 0.0027 |
| 9 d of treatment | 2.0±1.0 | 1.9±1.2 | 0.4051 |
| 10 d of treatment | 1.7±1.1 | 1.6±1.2 | 0.3457 |
| 11 d of treatment | 1.4±1.1 | 1.5±1.2 | 0.5501 |
| 12 d of treatment | 1.2±1.1 | 1.4±1.3 | 0.1290 |
| 13 d of treatment | 1.0±1.1 | 1.3±1.2 | 0.0344 |
| 14 d of treatment | 0.9±1.1 | 1.3±1.2 | 0.0026 |
| Time of treatment (day) | Cough disappearance/basic disappearance rate (%, 95% CI) | The effective time to cough relief rate (%, 95% CI) |
|---|---|---|
| 0 | 0.00 (0) | 0.00 (0) |
| 1 | 4.46 (3.35, 5.93) | 29.78 (27.07, 32.70) |
| 2 | 5.95 (4.65, 7.59) | 38.31 (35.40, 41.38) |
| 3 | 9.13 (7.50, 11.08) | 51.25 (48.20, 54.37) |
| 4 | 12.61 (10.71, 14.82) | 62.44 (59.45, 65.43) |
| 5 | 17.39 (15.18, 19.87) | 72.15 (69.35, 74.89) |
| 6 | 21.28 (18.88, 23.95) | 79.75 (77.20, 82.18) |
| 8 | 28.93 (26.23, 31.85) | 86.83 (84.64, 88.85) |
| 10 | 43.96 (40.93, 47.11) | 91.09 (89.18, 92.79) |
| 12 | 64.13 (61.12, 67.13) | 93.96 (92.30, 95.37) |
| 14 | 74.72 (71.91, 77.46) | 95.90 (94.45, 97.06) |
| Delete loss rate (%) | 28.27 | 7.21 |
Table 5 The disappearance time of cough and the effective time to cough relief
| Time of treatment (day) | Cough disappearance/basic disappearance rate (%, 95% CI) | The effective time to cough relief rate (%, 95% CI) |
|---|---|---|
| 0 | 0.00 (0) | 0.00 (0) |
| 1 | 4.46 (3.35, 5.93) | 29.78 (27.07, 32.70) |
| 2 | 5.95 (4.65, 7.59) | 38.31 (35.40, 41.38) |
| 3 | 9.13 (7.50, 11.08) | 51.25 (48.20, 54.37) |
| 4 | 12.61 (10.71, 14.82) | 62.44 (59.45, 65.43) |
| 5 | 17.39 (15.18, 19.87) | 72.15 (69.35, 74.89) |
| 6 | 21.28 (18.88, 23.95) | 79.75 (77.20, 82.18) |
| 8 | 28.93 (26.23, 31.85) | 86.83 (84.64, 88.85) |
| 10 | 43.96 (40.93, 47.11) | 91.09 (89.18, 92.79) |
| 12 | 64.13 (61.12, 67.13) | 93.96 (92.30, 95.37) |
| 14 | 74.72 (71.91, 77.46) | 95.90 (94.45, 97.06) |
| Delete loss rate (%) | 28.27 | 7.21 |
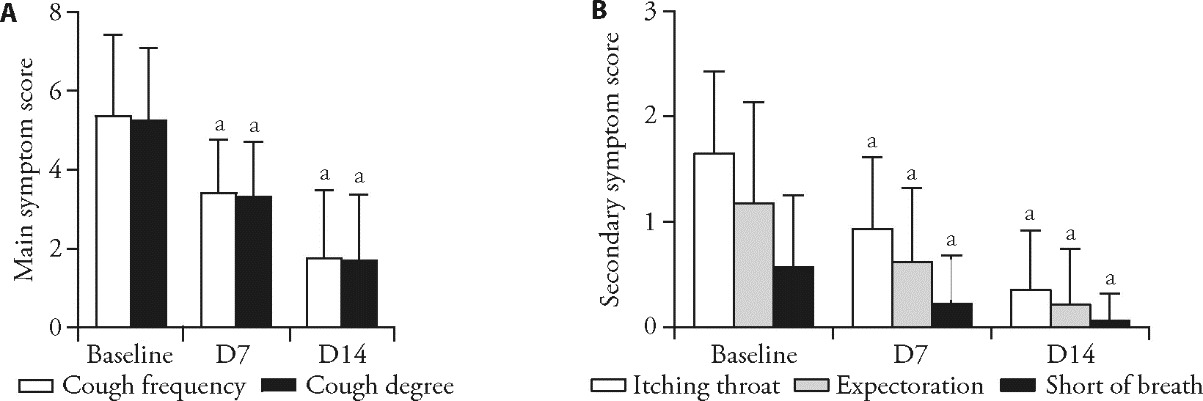
Figure 3 Changes in TCM symptom scores A: primary TCM symptoms, including cough frequency and severity; B: secondary TCM symptoms, including throat itching, expectoration, and shortness of breath, assessed at different time points during treatment. TCM: Traditional Chinese Medicine. Changes in TCM symptom at baseline and post-treatment were described using descriptive statistics and compared before and after treatment using paired t tests. Compared with the baseline, aP < 0.0001.
| Item | Events (n) | Patients (n) | Incidence rate (%) |
|---|---|---|---|
| All AEs | 213 | 125 | 12.18 |
| Related AEs | 30 | 23 | 2.24 |
| Important AEs | 42 | 32 | 3.12 |
| Serious AEs | 3 | 3 | 0.29 |
| Serious related AEs | 0 | 0 | 0 |
| AEs leading to dropout | 8 | 5 | 0.49 |
Table 6 Summary of AEs during treatment (n = 1026)
| Item | Events (n) | Patients (n) | Incidence rate (%) |
|---|---|---|---|
| All AEs | 213 | 125 | 12.18 |
| Related AEs | 30 | 23 | 2.24 |
| Important AEs | 42 | 32 | 3.12 |
| Serious AEs | 3 | 3 | 0.29 |
| Serious related AEs | 0 | 0 | 0 |
| AEs leading to dropout | 8 | 5 | 0.49 |
| Related AEs | Events (n) | Patients (n) | Incidence rate (%) |
|---|---|---|---|
| Total | 30 | 23 | 2.24 |
| Gastrointestinal diseases | 22 | 20 | 1.95 |
| Abdominal discomfort | 9 | 9 | 0.88 |
| Nausea | 8 | 8 | 0.78 |
| Emesis | 2 | 2 | 0.19 |
| Constipation | 1 | 1 | 0.10 |
| Fecal abnormality | 1 | 1 | 0.10 |
| Dry mouth | 1 | 1 | 0.10 |
| Various nervous system diseases | 3 | 3 | 0.29 |
| Dizzy | 2 | 2 | 0.19 |
| Headache | 1 | 1 | 0.10 |
| Diseases of respiratory system, chest and mediastinu | 3 | 3 | 0.29 |
| Dry throat | 3 | 3 | 0.29 |
| Diseases of the skin and subcutaneous tissue | 1 | 1 | 0.10 |
| pruritus | 1 | 1 | 0.10 |
| Heart organ disease | 1 | 1 | 0.10 |
| Palpitation | 1 | 1 | 0.10 |
Table 7 Summary of related AEs
| Related AEs | Events (n) | Patients (n) | Incidence rate (%) |
|---|---|---|---|
| Total | 30 | 23 | 2.24 |
| Gastrointestinal diseases | 22 | 20 | 1.95 |
| Abdominal discomfort | 9 | 9 | 0.88 |
| Nausea | 8 | 8 | 0.78 |
| Emesis | 2 | 2 | 0.19 |
| Constipation | 1 | 1 | 0.10 |
| Fecal abnormality | 1 | 1 | 0.10 |
| Dry mouth | 1 | 1 | 0.10 |
| Various nervous system diseases | 3 | 3 | 0.29 |
| Dizzy | 2 | 2 | 0.19 |
| Headache | 1 | 1 | 0.10 |
| Diseases of respiratory system, chest and mediastinu | 3 | 3 | 0.29 |
| Dry throat | 3 | 3 | 0.29 |
| Diseases of the skin and subcutaneous tissue | 1 | 1 | 0.10 |
| pruritus | 1 | 1 | 0.10 |
| Heart organ disease | 1 | 1 | 0.10 |
| Palpitation | 1 | 1 | 0.10 |
| 1. |
Lai K, Chen R, Lin J, et al. A prospective, multicenter survey on causes of chronic cough in China. Chest 2013; 143: 613-20.
DOI PMID |
| 2. | Song WJ, Chang YS, Faruqi S, et al. The global epidemiology of chronic cough in adults: a systematic review and Meta-analysis. Eur Respir J 2015; 45: 1479-81. |
| 3. | Lai K, Zhan W, Wu F, et al. Clinical and inflammatory characteristics of the chinese apac cough variant asthma cohort. Front Med (Lausanne) 2021; 8: 807385. |
| 4. | Cote A, Russell RJ, Boulet LP, et al. Managing chronic cough due to asthma and naeb in adults and adolescents: chest guideline and expert panel report. Chest 2020; 158: 68-96. |
| 5. | O'Shea O, Stovold E, Cates CJ. Regular treatment with formoterol and an inhaled corticosteroid versus regular treatment with salmeterol and an inhaled corticosteroid for chronic asthma: serious adverse events. Cochrane Database Syst Rev 2021; 4: CD007694. |
| 6. |
Lai K, Long L. Current status and future directions of chronic cough in China. Lung 2020; 198: 23-9.
DOI PMID |
| 7. |
Kyou-Hwan H, Ki Haeng C, Cui SQ, Lily L, Jaejong K. Effectiveness and safety of traditional Chinese herbs in children with cough variant asthma: a systematic review and Meta-analysis. J Tradit Chin Med 2021; 41: 661-8.
DOI |
| 8. |
Fang H, Hong Z, Li D, et al. Formulation of international standards of Chinese medicine technology: clinical practice guide of Chinese medicine for cough. J Tradit Chin Med 2024; 44: 396-402.
DOI |
| 9. | Zhang Y, Miao Q, Chao Y, et al. A multi-centered, randomized-controlled clinical study on suhuang zhike capsule for cough variant asthma. Zhong Yi Za Zhi 2008; 49: 504-6. |
| 10. | Gu C, Peng W, Wang Z, et al. Suhuang zhike capsules for the treatment of cough variant asthma: a meta-analysis. Evid Based Complement Alternat Med 2020; 2020: 9485746. |
| 11. |
Zhang C, Zhang LH, Wu YF, et al. Suhuang antitussive capsule at lower doses attenuates airway hyperresponsiveness, inflammation, and remodeling in a murine model of chronic asthma. Sci Rep 2016; 6: 21515.
DOI PMID |
| 12. | Dai L, Zhuang Y, Lu H, Chen M, Wang X. Effectiveness and safety of suhuang zhike capsule as adjuvant treatment for acute exacerbation of chronic obstructive pulmonary disease: a systematic review and Meta-analysis. J Tradit Chin Med 2023; 43: 231-8. |
| 13. | Liang R, Tong X, Dong Z, et al. Suhuang antitussive capsule ameliorates post-infectious cough in mice through AhR-Nrf 2 pathway. J Ethnopharmacol 2022; 283: 114664. |
| 14. | Ding P, Wang Q, Yao J, Zhou XM, Zhu J. Curative effects of suhuang zhike capsule on postinfectious cough: a Meta-analysis of randomized trials. Evid Based Complement Alternat Med 2016; 2016: 8325162. |
| 15. | Wang J, Han Z, Zhang S, et al. Meta-analysis on randomized controlled trials of treating cough variant asthma in adults with Suhuang Zhike capsule. Shanxi Zhong Yi Yao Da Xue Xue Bao 2021; 22: 79-84. |
| 16. | Asthma Group RS, Chinese Medical Association. Guidelines for the diagnosis and management of cough (2015). Zhong Hua Jie He He Hu Xi Za Zhi 2016; 39: 323-54. |
| 17. | Ming Y, Huang CR, Yu B, et al. Expert consensus on clinical application of Suhuang Zhike capsules intreatment of respiratory diseases. Zhong Guo Zhong Yi Yao Za Zhi 2025; 50: 817-23. |
| 18. | Morice AH, Millqvist E, Bieksiene K, et al. ERS guidelines on the diagnosis and treatment of chronic cough in adults and children. Eur Respir J 2020; 55: 1901136. |
| 19. | Zhan W, Wu F, Zhang Y, et al. Identification of cough-variant asthma phenotypes based on clinical and pathophysiologic data. J Allergy Clin Immunol 2023; 152: 622-32. |
| 20. |
Cox JK, Lockey R, Cardet JC. Cough-variant asthma: a review of clinical characteristics, diagnosis, and pathophysiology. J Allergy Clin Immunol Pract 2025; 13: 490-8.
DOI PMID |
| 21. | Wu X, Liu Q, Chen D, et al. Identification of quality control markers in suhuang antitussive capsule based on HPLC-PDA fingerprint and anti-inflammatory screening. J Pharm Biomed Anal 2020; 180: 113053. |
| 22. | Li S, Liu Q, Liu W, et al. Analysis of volatile constituents in Suhuang Zhike capsules and the intermediateof volatile oils. Zhong Cheng Yao 2017; 39: 2329-34. |
| 23. | Liu Q, Wu X, Chen D, et al. LC-MS analysis of non-volatile components in Suhuang Zike capsules. Zhong Cheng Yao 2019; 41: 1434-45. |
| 24. | Qin W, Wu X, Jia Y, et al. Suhuang antitussive capsule inhibits NLRP 3 inflammasome activation and ameliorates pulmonary dysfunction via suppression of endoplasmic reticulum stress in cough variant asthma. Biomed Pharmacother 2019; 118: 109188. |
| 25. | Jiang H, Bai Z, Ou Y, et al. β-Hydroxybutyric acid upregulated by Suhuang antitussive capsule ameliorates cough variant asthma through GSK3beta/AMPK-Nrf 2 signal axis. J Ethnopharmacol 2023; 307: 116013. |
| 26. | Qin W, Tong X, Liang R, et al. Preservation of mitochondrial homeostasis is responsible for the ameliorative effects of Suhuang antitussive capsule on non-resolving inflammation via inhibition of NF-κB signaling and NLRP 3 inflammasome activation. J Ethnopharmacol 2021; 271: 113827. |
| 27. | Sun X, Li P, Lin H, et al. Efficacy and safety of abelmoschus manihot in treating chronic kidney diseases: a multicentre, open-label and single-arm clinical trial. Phytomedicine 2022; 99: 154011. |
| [1] | XU Jian, LIU Yuntao, LUO Zhihao, ZHAO Zhen, WANG Dawei, LIU Qing. Chinese patent medicine for atherosclerosis: a systematic review and Meta-analysis of randomized controlled trials [J]. Journal of Traditional Chinese Medicine, 2024, 44(6): 1082-1090. |
| [2] | WU Qiaomin, GUAN Xuanke, LIU Jinfeng, WANG Yanli, CHANG Xing, LIU Zhiming, LIU Ruxiu. Compound Tongyang Fumai decoction (通阳复脉方) improves quality of life in sick sinus syndrome: a randomized controlled study [J]. Journal of Traditional Chinese Medicine, 2024, 44(6): 1247-1253. |
| [3] | ZHANG Meizhen, HAO Xiaohui, TANG Yiting, CHEN Yupeng, HE Puyu, ZHAO Liming, PANG Bing, NI Qing. Efficacy and safety of Buyang Huanwu decoction (补阳还五汤) for diabetic peripheral neuropathy: a systematic review and Meta-analysis [J]. Journal of Traditional Chinese Medicine, 2023, 43(5): 841-850. |
| [4] | SUN Wu, ZHAO Yuwei, LIAO Liang, ZHAO Zhonghui, CHEN Shiqi, YAN Xiaoling, WANG Xueyao, CHAO Guojun, ZHOU Jian. Effectiveness and safety of Xuebijing injection for patients with coronavirus disease 2019: a systematic review and Meta-analysis [J]. Journal of Traditional Chinese Medicine, 2023, 43(4): 631-639. |
| [5] | LUO Xin, XIE Jing, HUANG Li, GAN Wenfan, CHEN Ming. Efficacy and safety of activating blood circulation and removing blood stasis of Traditional Chinese Medicine for managing renal fibrosis in patients with chronic kidney disease: a systematic review and Meta-analysis [J]. Journal of Traditional Chinese Medicine, 2023, 43(3): 429-440. |
| [6] | ZHANG Yuehong, SHAO Xianzhi, ZHAO Qianlong, ZHAN Hualong, ZHANG Jianhua, DU Sisi, CHEN Jing, LIU Yingfang, ZHOU Haiwang, CHEN Xinsheng, HONG Ying, LIAN Fengmei, TONG Xiaolin, BA Yuanming. Effectiveness of Xiangsha Liujun pills (香砂六君丸) on decreased digestive function in convalescent patients of coronavirus disease 2019: a randomized, double blind, placebo controlled clinical trial [J]. Journal of Traditional Chinese Medicine, 2023, 43(3): 552-558. |
| [7] | DAI Linfeng, ZHUANG Yan, Lü Hai, CHEN Mingqi, WANG Xing. Effectiveness and safety of Suhuang Zhike capsule (苏黄止咳胶囊) as adjuvant treatment for acute exacerbation of chronic obstructive pulmonary disease: a systematic review and Meta-analysis [J]. Journal of Traditional Chinese Medicine, 2023, 43(2): 231-238. |
| [8] | ZHOU Yingyan, LIANG Huasheng, YAN Jingyao, HE Xiaohong, PAN Lili, LI Xue, CHEN Xianghong, CHEN Xiumin, YANG Aicheng, HUANG Qingchun. Effectiveness and safety of tripterygium glycosides tablet (雷公藤多苷片) for lupus nephritis: a systematic review and Meta-analysis [J]. Journal of Traditional Chinese Medicine, 2022, 42(5): 671-680. |
| [9] | LIN Yi, LI Xun, WANG Zi, ZHENG Xiaoran, HANG Haiyan, LI Lingling. Efficacy and safety of external application of Chinese herbal medicine for psoriasis vulgaris: a systematic review of randomized controlled trials [J]. Journal of Traditional Chinese Medicine, 2022, 42(4): 493-504. |
| [10] | HUANG Yusi, YANG Jiju, LI Xinyi, HAO Huifeng, LI Chong, ZHANG Fan, LIN Haiming, XIE Xianfei, HE Ke, TIAN Guihua. Effectiveness and safety of electroacupuncture for the treatment of pain after laparoscopic surgery: a systematic review [J]. Journal of Traditional Chinese Medicine, 2022, 42(4): 505-512. |
| [11] | LIU Ying, ZOU Wen, XIAN Qingfei, DENG Xin, ZHANG Fuchun, WANG Li, LI Yonghong, LUN Wenhui, WANG Jian. Efficacy and safety of Mianyi granules (+mianyi+) for reversal of immune nonresponse following antiretroviral therapy of human immunodeficiency virus-1: a randomized, double-blind, multi-center, placebo-controlled trial [J]. Journal of Traditional Chinese Medicine, 2022, 42(3): 432-438. |
| [12] | CHEN Yunhu, FAN Lihua, ZHANG Tao, LIU Xueqian. Effectiveness of Zhuling decoction (猪苓汤) on diuretic resistance in patients with heart failure: a randomized, controlled trial [J]. Journal of Traditional Chinese Medicine, 2022, 42(3): 439-445. |
| [13] | WANG Yifei, YANG Yi, WANG Yu, ZHANG Jinling, ZHAI Weihang, LI Shaoyuan, WU Mozheng, HE Jianghong, RONG Peijing. Transcutaneous auricular vague nerve stimulation improved brain connection activity on patients of disorders of consciousness: a pilot study [J]. Journal of Traditional Chinese Medicine, 2022, 42(3): 463-471. |
| [14] | MA Tingting, WU Jie, YANG Lijie, FENG Fen, YANG Huilin, ZHANG Jinhua, ZHONG Yanjin, NING Qing, HUANG Lirong, LIN Youbing, YAN Jue, CHEN Guiquan, HOU Tianshu, WANG Li, REN Yuanfang, TAN Jing. Ginger-indirect moxibustion plus acupuncture versus acupuncture alone for chronic fatigue syndrome: a randomized controlled trial [J]. Journal of Traditional Chinese Medicine, 2022, 42(2): 242-249. |
| [15] | Rina SHA, Lu TANG, Yawei DU, Shengxian WU, Huawei SHI, Hongxin ZOU, Xuran ZHANG, Xinglu DONG, Li ZHOU. Effectiveness and safety of Ginkgo biloba extract (GBE50) in the treatment of dizziness caused by cerebral arteriosclerosis: a multi-center, double-blind, randomized controlled trial [J]. Journal of Traditional Chinese Medicine, 2022, 42(1): 83-89. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||