Journal of Traditional Chinese Medicine ›› 2022, Vol. 42 ›› Issue (5): 671-680.DOI: 10.19852/j.cnki.jtcm.2022.05.001
• Systematic Review • Next Articles
Effectiveness and safety of tripterygium glycosides tablet (雷公藤多苷片) for lupus nephritis: a systematic review and Meta-analysis
ZHOU Yingyan1, LIANG Huasheng1, YAN Jingyao1, HE Xiaohong1, PAN Lili1, LI Xue1, CHEN Xianghong1, CHEN Xiumin1, YANG Aicheng2( ), HUANG Qingchun1(
), HUANG Qingchun1( )
)
- 1 Department of Rheumatology, the Second Affiliated Hospital, Guangzhou University of Chinese Medicine (Guangdong Provincial Hospital of Chinese Medicine), Guangzhou 510006, China
2 Department of Nephrology, Jiangmen Hospital of Chinese Medicine Affiliated to Jinan University, Jiangmen 529000, China
-
Received:2021-04-02Accepted:2021-07-25Online:2022-10-15Published:2022-09-02 -
Contact:YANG Aicheng,HUANG Qingchun -
About author:YANG Aicheng, Department of Nephrology, Jiangmen Hospital of Chinese Medicine Affiliated to Jinan Uversity, Jiangmen 529000, China. easymu2008@163.com.
HUANG Qingchun, Department of Rheumatology, the Second Affiliated Hospital, Guangzhou University of Chinese Medicine (Guangdong Provincial Hospital of Chinese Medicine), Guangzhou 510006, China. qch1963@163.com.
-
Supported by:Department of Education of Guangdong Province: Study of Biomarkers Discovery for Patients with Relapsed Lupus Nephritis from the Perspective of Urinary Exosomal Microrna and Analysis of the Correlation with Pathogenic Dampness(2018KQNCX051);Guangdong Provincial Hospital of Traditional Chinese Medicine: Study of Biomarkers Discovery for Patients with Relapsed Lupus Nephritis from the Perspective of Urinary Exosomal Microrna and Analysis of the Correlation with Pathogenic Dampness(YN2019QL19);State Key Laboratory of Dampness Syndrome of Chinese Medicine: Early Warning and Intervention(SZ2021ZZ3204)
Cite this article
ZHOU Yingyan, LIANG Huasheng, YAN Jingyao, HE Xiaohong, PAN Lili, LI Xue, CHEN Xianghong, CHEN Xiumin, YANG Aicheng, HUANG Qingchun. Effectiveness and safety of tripterygium glycosides tablet (雷公藤多苷片) for lupus nephritis: a systematic review and Meta-analysis[J]. Journal of Traditional Chinese Medicine, 2022, 42(5): 671-680.
share this article
| Study | Number of patients | Sex ratio (M/F) | Age (years) | Intervention and dose | Duration | Outcome | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Experimental | Control | Experimental | Control | Experimental | Control | Experimental | Control | ||||||
| Wang JJ et al | 40 | 44 | 2 /38 | 2/42 | 34.2±8.7 | 32.0±10.3 | TG, 60 mg/d + GC, 10 mg/d | AZA, 2 mg·kg-1·d-1 + GC, 10 mg/d | 24 months | CR, AEs | |||
| Shi F et al | 28 | 27 | 3/25 | 4/23 | 31.04±4.17 | 30.15±4.37 | TG, 60 mg/d, 10 mg/d | AZA, 1.5-4 mg·kg-1·d-1 | null | CR, PR, 24-hour urinary protein, C3, C4, Cr, AEs | |||
| Dai H, Liu W | 46 | 45 | null | null | null | null | TG, 60 mg/d + GC, 5-10 mg/d | LEF, 20 mg/d + GC, 5-10 mg/d | 6 months | CR, PR, 24-hour urinary protein, C3, Cr, SLEDAI, ALB, AEs | |||
| Hu XW et al | 61 | 61 | 5/56 | 1/60 | 31.5±8.1 | 27.8±8.2 | TG, 60 mg/d + GC, 10 mg/d | LEF, 20 mg/d + GC, 10 mg/d | 24 months | C3, C4, anti-dsDNA, AEs | |||
| Chen GW | 27 | 26 | 3/24 | 3/23 | null | null | TG, 1-1.5 mg·kg-1·d-1, + GC 0.5 mg·kg-1·d-1 | GC 1 mg·kg-1·d-1 | 6 months | CR, PR, 24-hour urinary protein, Cr | |||
| Hong L | 41 | 41 | 10/31 | 11/30 | 30.14±4. 67 | 30. 02±4. 71 | TG, 1.5 mg·kg-1·d-1, + GC, 10 mg/d | GC, 10 mg/d | 12 weeks | 24-hour urinary protein, C3, C4, anti-dsDNA | |||
| Liu F et al | 31 | 31 | 3/28 | 5/26 | 37.9±3.4 | 36.6±3.5 | TG, 1.0 mg·kg-1·d-1, + GC, 1 mg·kg-1·d-1 | GC, 1 mg·kg-1·d-1 | 8 weeks | CR, PR, 24-hour urinary protein, C3, Cr, SLEDAI, ALB, Cr, AEs | |||
| Du YL et al | 34 | 34 | 5/29 | 4/30 | 54.38±3.32 | 32.74±5.1 | TG, 1-1.5 mg·kg-1·d-1, + GC 0.5 mg·kg-1·d-1 | GC 1 mg·kg-1·d-1 | 6 months | CR, PR, AEs | |||
Table 1 Characteristics of the included trials
| Study | Number of patients | Sex ratio (M/F) | Age (years) | Intervention and dose | Duration | Outcome | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Experimental | Control | Experimental | Control | Experimental | Control | Experimental | Control | ||||||
| Wang JJ et al | 40 | 44 | 2 /38 | 2/42 | 34.2±8.7 | 32.0±10.3 | TG, 60 mg/d + GC, 10 mg/d | AZA, 2 mg·kg-1·d-1 + GC, 10 mg/d | 24 months | CR, AEs | |||
| Shi F et al | 28 | 27 | 3/25 | 4/23 | 31.04±4.17 | 30.15±4.37 | TG, 60 mg/d, 10 mg/d | AZA, 1.5-4 mg·kg-1·d-1 | null | CR, PR, 24-hour urinary protein, C3, C4, Cr, AEs | |||
| Dai H, Liu W | 46 | 45 | null | null | null | null | TG, 60 mg/d + GC, 5-10 mg/d | LEF, 20 mg/d + GC, 5-10 mg/d | 6 months | CR, PR, 24-hour urinary protein, C3, Cr, SLEDAI, ALB, AEs | |||
| Hu XW et al | 61 | 61 | 5/56 | 1/60 | 31.5±8.1 | 27.8±8.2 | TG, 60 mg/d + GC, 10 mg/d | LEF, 20 mg/d + GC, 10 mg/d | 24 months | C3, C4, anti-dsDNA, AEs | |||
| Chen GW | 27 | 26 | 3/24 | 3/23 | null | null | TG, 1-1.5 mg·kg-1·d-1, + GC 0.5 mg·kg-1·d-1 | GC 1 mg·kg-1·d-1 | 6 months | CR, PR, 24-hour urinary protein, Cr | |||
| Hong L | 41 | 41 | 10/31 | 11/30 | 30.14±4. 67 | 30. 02±4. 71 | TG, 1.5 mg·kg-1·d-1, + GC, 10 mg/d | GC, 10 mg/d | 12 weeks | 24-hour urinary protein, C3, C4, anti-dsDNA | |||
| Liu F et al | 31 | 31 | 3/28 | 5/26 | 37.9±3.4 | 36.6±3.5 | TG, 1.0 mg·kg-1·d-1, + GC, 1 mg·kg-1·d-1 | GC, 1 mg·kg-1·d-1 | 8 weeks | CR, PR, 24-hour urinary protein, C3, Cr, SLEDAI, ALB, Cr, AEs | |||
| Du YL et al | 34 | 34 | 5/29 | 4/30 | 54.38±3.32 | 32.74±5.1 | TG, 1-1.5 mg·kg-1·d-1, + GC 0.5 mg·kg-1·d-1 | GC 1 mg·kg-1·d-1 | 6 months | CR, PR, AEs | |||
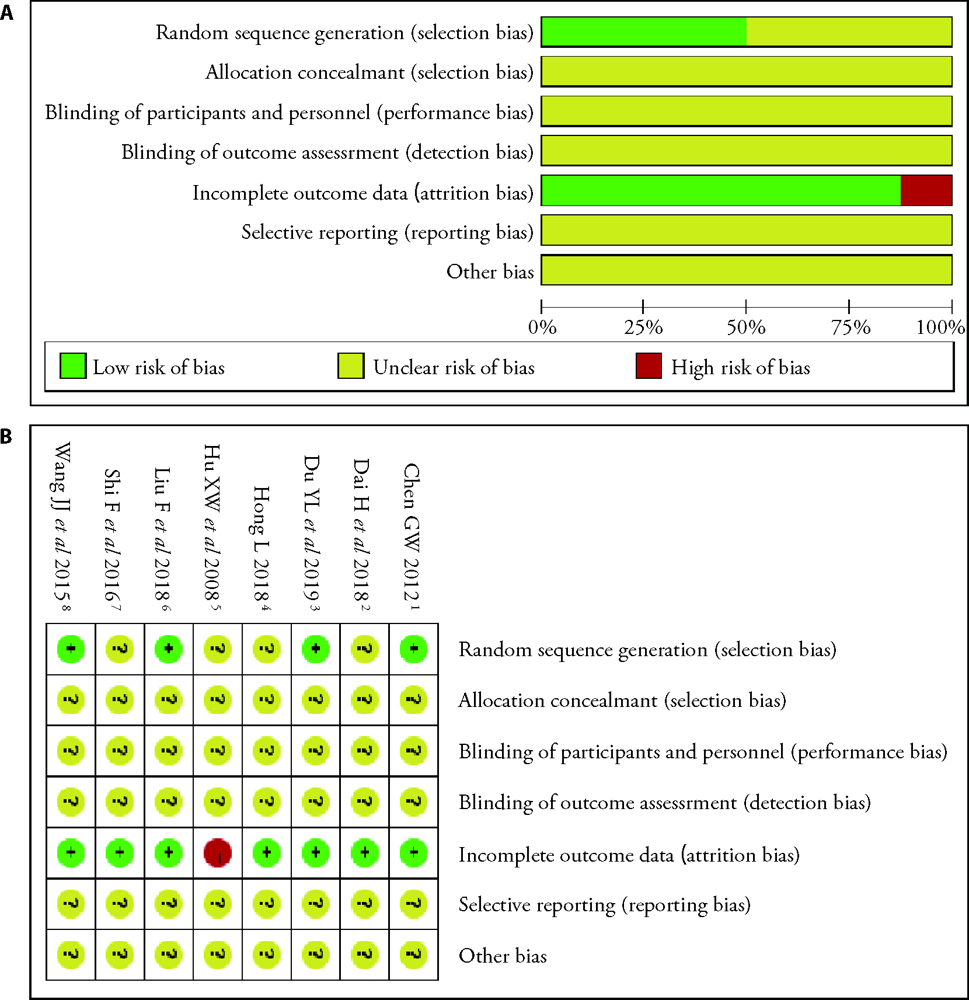
Figure 2 Risk of bias graph and summary A: risk of bias graph: judgements about each risk of bias item presented as percentages across all included studies; B: risk of bias summary: judgements about each risk of bias item for each included study. “?”: unclear risk of bias; “+”: low risk of bias; “-”: high risk of bias

Figure 3 Results of the Meta-analysis of the efficacy of TG tablet combined with GC versus GC A: forest plot of comparison of TR; B. Forest plot of comparison of CR; C: forest plot of comparison of 24-hour urinary protein; D: forest plot of comparison of C3; E: forest plot of comparison of C4; F: forest plot of comparison of serum creatinine. TG: tripterygium glycosides tablet, GC: glucocorticoids, CR: complete remission, TR: total remission [total CR plus partial remission (PR)].

Figure 4 Results of the Meta-analysis of the efficacy of TG tablet combined with GC versus AZA/LEF combined with GC A: forest plot of comparison of TR; B: forest plot of comparison of CR; C: forest plot of comparison of 24-h urinary protein; D: forest plot of comparison of C3; E: forest plot of comparison of C4; F: forest plot of comparison of serum creatinine. TG: tripterygium glycosides tablet; GC: glucocorticoids; AZA: azathioprine; LEF: leflomit; CR: complete remission; TR: total remission [total CR plus partial remission (PR)].
| Therapeutic regimen | Indicator | Number of studies | Q test, P value | Model selected | RR (95% CI) | P value |
|---|---|---|---|---|---|---|
| TG + GC vs GC | Nausea and vomitting | 2 | 0.31 | fixed | 0.44 (0.14, 1.37) | 0.16 |
| Hepatic dysfunction | 2 | 0.71 | fixed | 0.67 (0.12, 3.86) | 0.65 | |
| TG vs AZA | Infection | 2 | 0.74 | fixed | 1.11 (0.72, 1.72) | 0.64 |
| Irregular menstruation | 2 | 0.55 | fixed | 3.57 (1.40, 9.11) | 0.008 | |
| Leucopenia | 2 | 0.40 | fixed | 0.38 (0.17, 0.85) | 0.02 | |
| TG vs LEF | Infection | 2 | 0.37 | fixed | 0.49 (0.15, 1.58) | 0.23 |
| Irregular menstruation | 2 | 0.49 | fixed | 6.69 (2.42, 18.46) | 0.0002 | |
| Leucopenia | 2 | 0.62 | fixed | 0.50 (0.13, 1.93) | 0.31 | |
| Alopecia | 2 | 0.23 | fixed | 0.14 (0.03, 0.77) | 0.02 | |
| hepatic dysfunction | 2 | 0.46 | fixed | 0.69 (0.27, 1.75) | 0.44 | |
| rash | 2 | 0.38 | fixed | 0.09 (0.01, 0.69) | 0.02 |
Table 2 Meta-analysis of the safety of TG tablet in treatment of patients with lupus nephritis
| Therapeutic regimen | Indicator | Number of studies | Q test, P value | Model selected | RR (95% CI) | P value |
|---|---|---|---|---|---|---|
| TG + GC vs GC | Nausea and vomitting | 2 | 0.31 | fixed | 0.44 (0.14, 1.37) | 0.16 |
| Hepatic dysfunction | 2 | 0.71 | fixed | 0.67 (0.12, 3.86) | 0.65 | |
| TG vs AZA | Infection | 2 | 0.74 | fixed | 1.11 (0.72, 1.72) | 0.64 |
| Irregular menstruation | 2 | 0.55 | fixed | 3.57 (1.40, 9.11) | 0.008 | |
| Leucopenia | 2 | 0.40 | fixed | 0.38 (0.17, 0.85) | 0.02 | |
| TG vs LEF | Infection | 2 | 0.37 | fixed | 0.49 (0.15, 1.58) | 0.23 |
| Irregular menstruation | 2 | 0.49 | fixed | 6.69 (2.42, 18.46) | 0.0002 | |
| Leucopenia | 2 | 0.62 | fixed | 0.50 (0.13, 1.93) | 0.31 | |
| Alopecia | 2 | 0.23 | fixed | 0.14 (0.03, 0.77) | 0.02 | |
| hepatic dysfunction | 2 | 0.46 | fixed | 0.69 (0.27, 1.75) | 0.44 | |
| rash | 2 | 0.38 | fixed | 0.09 (0.01, 0.69) | 0.02 |
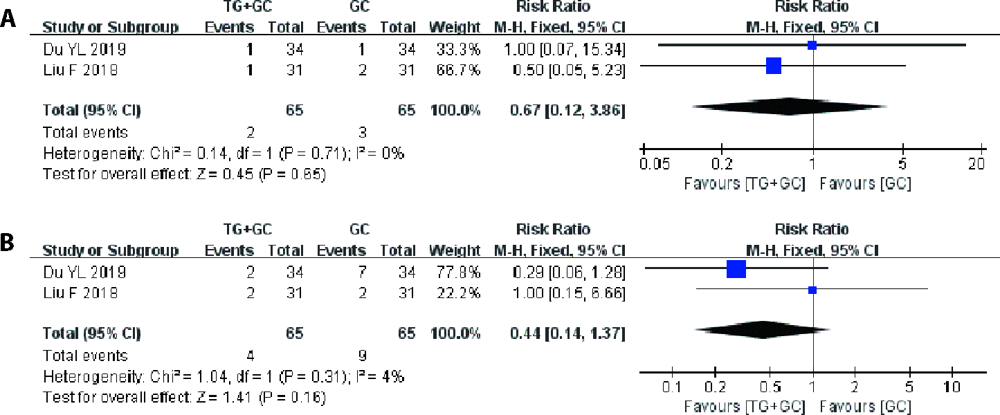
Figure 5 Results of the Meta-analysis of the safety of TG tablet combined with GC versus GC A: forest plot of comparison of hepatic dysfunction; B: forest plot of comparison of nausea and vomitting. TG: tripterygium glycosides tablet, GC: glucocorticoids.

Figure 6 Results of the Meta-analysis of the safety of TG tablet combined with GC versus AZA combined with GC A: forest plot of comparison of infection; B: forest plot of comparison of irregular menstruation; C: forest plot of comparison of leucopenia. TG: tripterygium glycosides tablet; GC: glucocorticoids; AZA: azathioprine.

Figure 7 Results of the Meta-analysis of the safety of TG tablet combined with GC versus LEF combined with GC A: forest plot of comparison of infection; B: forest plot of comparison of irregular menstruation; C: forest plot of comparison of leucopenia; D: forest plot of comparison of alopecia; E: forest plot of comparison of hepatic dysfunction; F: forest plot of comparison of rash. TG: tripterygium glycosides tablet; GC: glucocorticoids; LEF: leflomit.
| 1. |
Gergianaki I, Bortoluzzi A, Bertsias G. Update on the epidemiology, risk factors, and disease outcomes of systemic lupus erythematosus. Best Pract Res Clin Rheumatol 2018; 32: 188-205.
DOI PMID |
| 2. |
Jakes RW, Bae SC, Louthrenoo W, Mok CC, Navarra SV, Kwon N. Systematic review of the epidemiology of systemic lupus erythematosus in the Asia-Pacific region: prevalence, incidence, clinical features, and mortality. Arthritis Care Res (Hoboken) 2012; 64: 159-68.
DOI URL |
| 3. |
Jorge A, Wallace ZS, Zhang Y, Lu N, Costenbader KH, Choi HK. All-cause and cause-specific mortality trends of end-stage renal disease due to lupus nephritis from 1995 to 2014. Arthritis Rheumatol 2019; 71: 403-10.
DOI URL |
| 4. |
Hahn BH, McMahon MA, Wilkinson A, et al. American College of Rheumatology guidelines for screening, treatment, and management of lupus nephritis. Arthritis Care Res (Hoboken) 2012; 64: 797-808.
DOI URL |
| 5. |
Zhang M, Qi C, Zha Y, et al. Leflunomide versus cyclophosphamide in the induction treatment of proliferative lupus nephritis in Chinese patients: a randomized trial. Clin Rheumatol 2019; 38: 859-67.
DOI URL |
| 6. |
Tao X, Davis LS, Lipsky PE. Effect of an extract of the Chinese herbal remedy Tripterygium wilfordii Hook F on human immune responsiveness. Arthritis Rheum 1991; 34: 1274-81.
DOI URL |
| 7. |
Ma J, Dey M, Yang H, et al. Anti-inflammatory and immunosuppressive compounds from Tripterygium wilfordii. Phytochemistry 2007; 68: 1172-8.
DOI URL |
| 8. |
Brinker AM, Ma J, Lipsky PE, Raskin I. Medicinal chemistry and pharmacology of genus Tripterygium (Celastraceae). Phytochemistry 2007; 68: 732-66.
PMID |
| 9. | Wang B, Ma L, Tao X, Lipsky PE. Triptolide, an active component of the Chinese herbal remedy Tripterygium wilfordii Hook F, inhibits production of nitric oxide by decreasing inducible nitric oxide synthase gene transcription. Arthritis Rheum 2004; 50: 2303-995. |
| 10. |
Zhou YY, Xia X, Peng WK, et al. The effectiveness and safety of tripterygium wilfordii hook. F extracts in rheumatoid arthritis: a systematic review and Meta-analysis. Front Pharmacol 2018; 9: 356.
DOI URL |
| 11. |
Zhao X, Tang X, Yan Q, et al. Triptolide ameliorates lupus via the induction of miR-125a-5p mediating Treg upregulation. Int Immunopharmacol 2019; 71: 14-21.
DOI PMID |
| 12. |
Li Q, Li L, Bi L, et al. Kunxian capsules in the treatment of patients with ankylosing spondylitis: A randomized placebo-controlled clinical trial. Trials 2016; 17: 337.
DOI URL |
| 13. | Helmstadter A. Tripterygium wilfordii Hook. F. - how a traditional Taiwanese medicinal plant found its way to the West. Pharmazie 2013; 68: 643-6. |
| 14. |
Liu Q. Triptolide and its expanding multiple pharmacological functions. Int Immunopharmacol 2011; 11: 377-83.
DOI URL |
| 15. |
Yan YH, Shang PZ, Lu QJ, Wu X. Triptolide regulates T cell-mediated immunity via induction of CD11c(low) dendritic cell differentiation. Food Chem Toxicol 2012; 50: 2560-4.
DOI URL |
| 16. | Qiu D, Kao PN. Immunosuppressive and anti-inflammatory mechanisms of triptolide, the principal active diterpenoid from the Chinese medicinal herb Tripterygium wilfordii Hook. F. Drugs R D 2003; 4: 1-18. |
| 17. |
Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and Meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009; 339: b2700.
DOI URL |
| 18. | Review Manager RevMan Computer program. Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014. |
| 19. |
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in Meta-analyses. BMJ 2003; 327: 557-60.
DOI URL |
| 20. | Chen GW. Clinical observation of Tripterygium wilfordii poly-glycosides combined with low dose prednisone in the treatment of lupus nephritis. Zhong Yi Yao Dao Bao 2012; 18: 45-6. |
| 21. | Liu F, Zeng HF, Miu H, Liu X, Sheng MJ. Efficacy of Tripterygium Wilfordii Multi Glucoside combined with hormone and ARB drugs in the treatment of lupus nephritis. Zhong Guo Dang Dai Yi Yao 2018; 25: 144-146, 150. |
| 22. | Hong L. Observation on the effect of Tripterygium wilfordii polyglycosides combined with prednisone in the treatment of lupus nephritis. Zhong Guo Gao Deng Yi Xue Jiao Yu 2018; 32: 140-1. |
| 23. | Wang JJ, Liu ZZ, Chen YH, Hu WX, Liu ZH, Zhang HT. Triptergium wilfordii and azathioprine in maintenance therapy for lupus nephritis-a randomized controlled trial of single center. Shen Zang Bing Yu Tou Xi Shen Yi Zhi Za Zhi 2015; 24: 429-34. |
| 24. | Shi F, Zeng XB, Xie BQ. Comparison of clinical efficacy of tripterygium wilfordii polyglycosides and azathioprine in the treatment of lupus nephritis. Kang Gan Ran Yao Xue 2016; 13: 377-80. |
| 25. | Dai H, Liu W. Efficacy and safety of tripterygium glycosides combined with prednisone in the treatment of lupus nephritis. Xinjiang Yi Xue 2018; 48: 20-2. |
| 26. | Hu XW, Zhang HT, Sun HO, et al. A clinical trial of maintenance treatment for lupus nephritis with leflunomide. Shen Zang Bing Yu Tou Xi Shen Yi Zhi Za Zhi 2008; 17: 224-8, 255. |
| 27. | Du YL, Guo M, Lin J. Clinical efficacy and safety anlysis of tripterygium wilfordii polyglycosides combined with prednisone in the treatment of lupus nephritis. Sichuan Jie Pou Xue Za Zhi 2019; 27: 131-2. |
| 28. |
Jing X, Cheng W, Guo S, Zou Y, Zhang T, He L. Toxic effects of Tripterygium wilfordii Hook F on the reproductive system of adolescent male rats. Biomed Pharmacother 2017; 95: 1338-45.
DOI URL |
| 29. |
Zhang C, Sun PP, Guo HT, et al. Safety profiles of tripterygium wilfordii hook f: a systematic review and Meta-analysis. Front Pharmacol 2016; 7: 402.
PMID |
| 30. |
Li XX, Du FY, Liu HX, Ji JB, Xing J. Investigation of the active components in Tripterygium wilfordii leading to its acute hepatotoxicty and nephrotoxicity. J Ethnopharmacol 2015; 162: 238-43.
DOI PMID |
| 31. |
Ayoub I, Nelson J, Rovin BH. Induction therapy for lupus nephritis: the highlights. Curr Rheumatol Rep 2018; 20: 60.
DOI URL |
| 32. | Song D, Zhong Y, Qian C, et al. Human umbilical cord mesenchymal stem cells therapy in cyclophosphamide-induced premature ovarian failure rat model. Biomed Res Int 2016; 2016: 2517514. |
| 33. | Cao LJ, Yan M, Li HD, Zhang BK, Fang PF. Progress on mechanism of Tripterygium wilfordii-induced liver injury and detoxification mechanism of licorice. Zhong Guo Zhong Yao Za Zhi 2015; 40: 2537-41. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||


