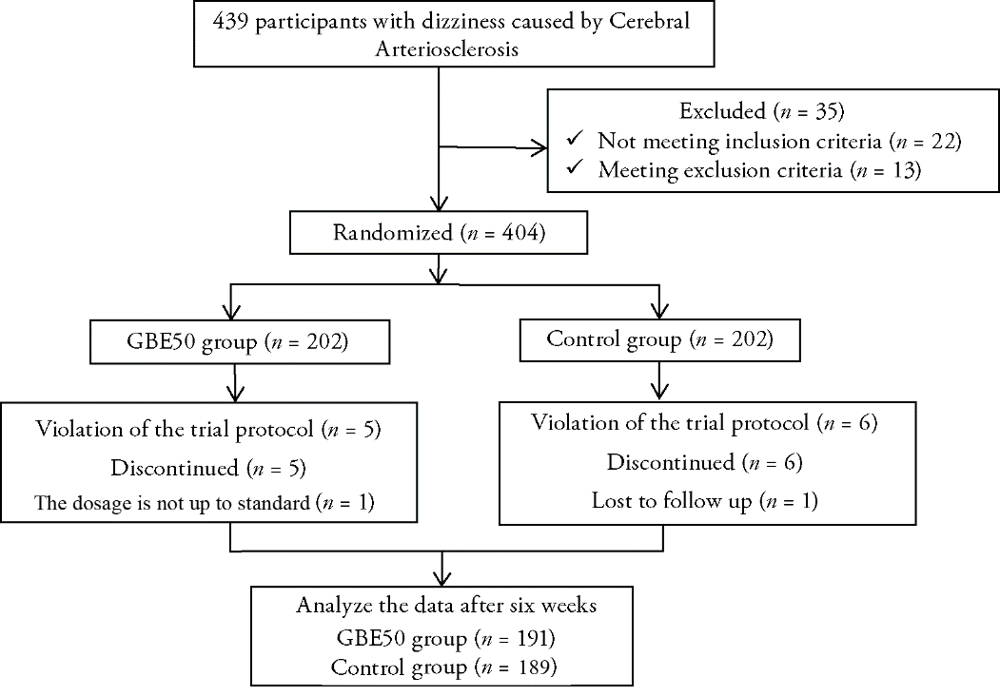
Journal of Traditional Chinese Medicine ›› 2022, Vol. 42 ›› Issue (1): 83-89.DOI: 10.19852/j.cnki.jtcm.20211214.001
• Research Articles • Previous Articles Next Articles
Effectiveness and safety of Ginkgo biloba extract (GBE50) in the treatment of dizziness caused by cerebral arteriosclerosis: a multi-center, double-blind, randomized controlled trial
Rina SHA1, Lu TANG2, Yawei DU2, Shengxian WU2, Huawei SHI2, Hongxin ZOU1, Xuran ZHANG1, Xinglu DONG2( ), Li ZHOU2(
), Li ZHOU2( )
)
- 1 The First clinical medical college, Beijing University of Chinese Medicine, Beijing 100029, China
2 Department of Neurology, Dongzhimen Hospital, Beijing University of Chinese Medicine, Beijing 100700, China
-
Received:2021-02-26Accepted:2021-05-20Online:2022-02-15Published:2021-12-14 -
Contact:Xinglu DONG,Li ZHOU -
About author:ZHOU Li, Department of Neurology, Dongzhimen Hospital, Beijing University of Chinese Medicine, Beijing 100700, China. Zhouljk7211@163.com
DONG Xinglu, Department of Neurology, Dongzhimen Hospital, Beijing University of Chinese Medicine, Beijing 100700, China. arthasdxl@163.com;
-
Supported by:National Major Scientific and Technological Special Project for "Significant New Drugs development"(2017ZX09304019);National Major Scientific and Technological Special Project for Clinical Evaluation Technology Platform of "Disease, Symptom Pattern and Symptom Correlation" of New Traditional Chinese Medicine
Cite this article
Rina SHA, Lu TANG, Yawei DU, Shengxian WU, Huawei SHI, Hongxin ZOU, Xuran ZHANG, Xinglu DONG, Li ZHOU. Effectiveness and safety of Ginkgo biloba extract (GBE50) in the treatment of dizziness caused by cerebral arteriosclerosis: a multi-center, double-blind, randomized controlled trial[J]. Journal of Traditional Chinese Medicine, 2022, 42(1): 83-89.
share this article
| Project | GBE50 group (n = 191) | Control group (n = 189) | P value |
|---|---|---|---|
| Male (n) | 84 | 72 | 0.2437 |
| Age (years) | 59±6 | 59±6 | 0.6476 |
| Height (cm) | 166±7 | 165±7 | 0.5668 |
| Weight (kg) | 67±9 | 66±9 | 0.5176 |
| SBP (mm Hg) | 127±7 | 128±7 | 0.3493 |
| DBP (mm Hg) | 77±7 | 77±7 | 0.7792 |
| HR (bpm) | 70±8 | 70±10 | 0.9577 |
| Combined disease (n) | 118 | 114 | 0.7700 |
Table 1 Baseline characteristics in GBE50 and control group ($\bar{x}$ ± s)
| Project | GBE50 group (n = 191) | Control group (n = 189) | P value |
|---|---|---|---|
| Male (n) | 84 | 72 | 0.2437 |
| Age (years) | 59±6 | 59±6 | 0.6476 |
| Height (cm) | 166±7 | 165±7 | 0.5668 |
| Weight (kg) | 67±9 | 66±9 | 0.5176 |
| SBP (mm Hg) | 127±7 | 128±7 | 0.3493 |
| DBP (mm Hg) | 77±7 | 77±7 | 0.7792 |
| HR (bpm) | 70±8 | 70±10 | 0.9577 |
| Combined disease (n) | 118 | 114 | 0.7700 |
| Time | Group | n | Ineffective (n) | Effective (n) | Obvious effective (n) | Recovery (n) | Total rate (%) |
|---|---|---|---|---|---|---|---|
| 2 weeks | GBE50 | 191 | 165 | 26 | 0 | 0 | 13.61 |
| Control | 189 | 167 | 22 | 0 | 0 | 11.64 | |
| 4 weeks | GBE50 | 191 | 62 | 119 | 9 | 1 | 67.54 |
| Control | 189 | 73 | 109 | 5 | 2 | 61.38 | |
| 6 weeks | GBE50 | 191 | 14 | 67 | 54 | 56 | 92.67a |
| Control | 189 | 32 | 79 | 41 | 37 | 83.07 |
Table 2 Comparison of Evaluation of the effectiveness of Traditional Chinese Medicine symptom patterns between two groups after 2, 4 and 6 weeks
| Time | Group | n | Ineffective (n) | Effective (n) | Obvious effective (n) | Recovery (n) | Total rate (%) |
|---|---|---|---|---|---|---|---|
| 2 weeks | GBE50 | 191 | 165 | 26 | 0 | 0 | 13.61 |
| Control | 189 | 167 | 22 | 0 | 0 | 11.64 | |
| 4 weeks | GBE50 | 191 | 62 | 119 | 9 | 1 | 67.54 |
| Control | 189 | 73 | 109 | 5 | 2 | 61.38 | |
| 6 weeks | GBE50 | 191 | 14 | 67 | 54 | 56 | 92.67a |
| Control | 189 | 32 | 79 | 41 | 37 | 83.07 |
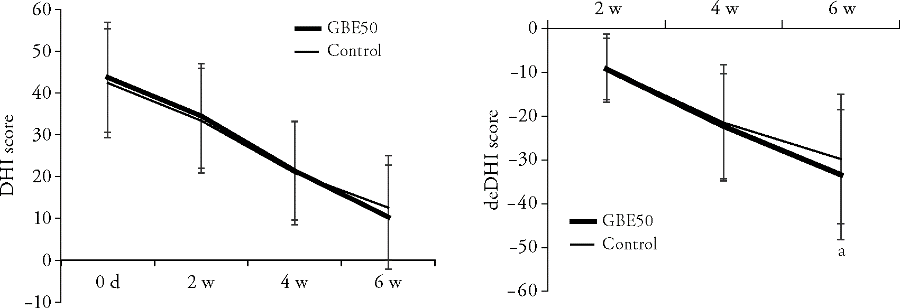
Figure 2 Comparison of the DHI score of two groups after 2, 4 and 6 weeks of treatment A: comparison of the DHI score between the GBE50 group and the control group at 2, 4 and 6 weeks; B: comparison of difference between the DHI score and baseline score of two groups at 2, 4 and 6 weeks. DHI: dizziness handicap inventory. Data are presented as mean ± standard deviation. aP < 0.05 vs the control group after 6 weeks.
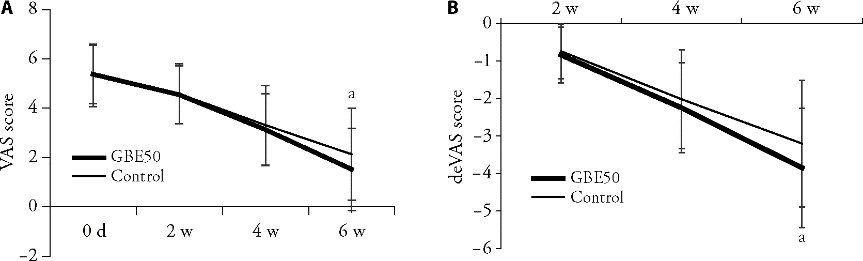
Figure 3 Comparison of severity VAS scores of dizziness symptoms at 2, 4 and 6 weeks of treatment A: comparison of the VAS score between the GBE50 group and the control group at 2, 4 and 6 weeks; B: comparison of difference between the VAS score and baseline score of two groups at 2, 4 and 6 weeks. Data are presented as mean ± standard deviation. Compared with the control group, aP < 0.01 vs the control group after 6 weeks.
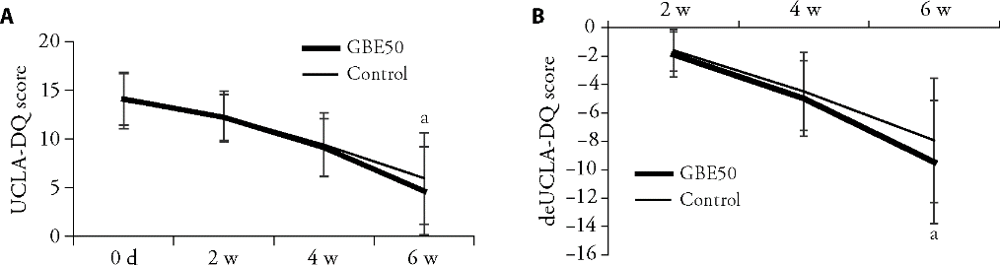
Figure 4 Comparison of UCLA-DQ scores at 2, 4 and 6 weeks of treatment A: comparison of the UCLA-DQ score between the GBE50 group and the control group at 2, 4 and 6 weeks; B: comparison of difference between the UCLA-DQ score and baseline score of two groups at 2, 4 and 6 weeks. Data are presented as mean ± standard deviation.aP < 0.01 vs the control group after 6 weeks.
| Item | 2 weeks | 4 weeks | 6 weeks | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| GBE50 (n = 191) | Control group (n = 189) | GBE50 (n = 191) | Control group (n = 189) | GBE50 (n = 191) | Control group (n = 189) | |||||
| Dizziness | –0.5±0.9 | –0.4±0.8 | –1.5±1.2a | –1.3±1.1 | –2.7±1.4b | –2.1±1.4 | ||||
| Dazzling and blurring of vision | –0.2±0.7 | –0.2±0.6 | –1.0±1.0 | –0.8±1.0 | –1.8±1.0 b | –1.5±1.1 | ||||
| Headache | –0.2±0.4 | –0.2±0.4 | –0.5±0.6 | –0.5±0.6 | –0.9±0.6b | –0.8±0.6 | ||||
| Tinnitus | –0.4±0.5 | –0.3±0.5 | –0.6±0.6 | –0.6±0.6 | –0.9±0.7 | –0.8±0.7 | ||||
| Insomnia | –0.2±0.5 | –0.2±0.4 | –0.4±0.6 | –0.4±0.6 | –0.8±0.7 | –0.7±0.7 | ||||
| Amnesia | –0.3±0.5 | –0.2±0.4 | –0.5±0.6a | –0.3±0.5 | –0.8±0.7b | –0.6±0.7 | ||||
| Numbness of the limbs | –0.4±0.6 | –0.4±0.5 | –0.7±0.7 | –0.6±0.7 | –1.0±0.9 | –0.9±0.8 | ||||
Table 3 Comparison of the single-item symptom score of TCM for 2, 4 and 6 weeks of treatment ($\bar{x}$ ± s)
| Item | 2 weeks | 4 weeks | 6 weeks | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| GBE50 (n = 191) | Control group (n = 189) | GBE50 (n = 191) | Control group (n = 189) | GBE50 (n = 191) | Control group (n = 189) | |||||
| Dizziness | –0.5±0.9 | –0.4±0.8 | –1.5±1.2a | –1.3±1.1 | –2.7±1.4b | –2.1±1.4 | ||||
| Dazzling and blurring of vision | –0.2±0.7 | –0.2±0.6 | –1.0±1.0 | –0.8±1.0 | –1.8±1.0 b | –1.5±1.1 | ||||
| Headache | –0.2±0.4 | –0.2±0.4 | –0.5±0.6 | –0.5±0.6 | –0.9±0.6b | –0.8±0.6 | ||||
| Tinnitus | –0.4±0.5 | –0.3±0.5 | –0.6±0.6 | –0.6±0.6 | –0.9±0.7 | –0.8±0.7 | ||||
| Insomnia | –0.2±0.5 | –0.2±0.4 | –0.4±0.6 | –0.4±0.6 | –0.8±0.7 | –0.7±0.7 | ||||
| Amnesia | –0.3±0.5 | –0.2±0.4 | –0.5±0.6a | –0.3±0.5 | –0.8±0.7b | –0.6±0.7 | ||||
| Numbness of the limbs | –0.4±0.6 | –0.4±0.5 | –0.7±0.7 | –0.6±0.7 | –1.0±0.9 | –0.9±0.8 | ||||
| Category | GBE50 (n = 201) | Control group (n = 202) |
|---|---|---|
| Adverse events | 20 (9.95) | 30 (14.85) |
| Adverse events associated with research drugs | 1 (0.50) | 0 |
| Severe adverse events | 0 | 1 (0.25) |
Table 4 Adverse events in experiment and control groups [n (%)]
| Category | GBE50 (n = 201) | Control group (n = 202) |
|---|---|---|
| Adverse events | 20 (9.95) | 30 (14.85) |
| Adverse events associated with research drugs | 1 (0.50) | 0 |
| Severe adverse events | 0 | 1 (0.25) |
| [1] | Neuhauser H. The epidemiology of dizziness and vertigo. Handb Clin Neurol 2016;137:67-82. |
| [2] | Karatas M. Vascular vertigo: epidemiology and clinical syndromes. Neurologist 2011;17:1-10. |
| [3] | Yang X. Current status and countermeasures of vertigo diagnosis in domestic neurology. Zhong Yi Cu Zhong Za Zhi 2015;10:373-81. |
| [4] | Xue H, Chong Y, Jiang ZD. Etiological analysis on patients with vertigo or dizziness. Zhong Hua Yi Xue Za Zhi 2018;98:1227-30. |
| [5] | Flusty B, Havenon AD, Prabhakaran S, et al. Intracranial atherosclerosis treatment: past, present, and future. Stroke 2020;51:49-53. |
| [6] | Murdin L, Hussain K, Schilder AGM. Betahistine for symptoms of vertigo. Cochrane Database Syst Rev 2016; 2016: CD010696. |
| [7] | Huang Z, Hu YQ, Chen LM, et al. Research progress of Xuefu Zhuyu Decoction on vertigo. Shan Xi Zhong Yi 2020;41:1169-72. |
| [8] | Wang XZ, Han QY, Guo XF, et al. Complete Book of Diagnosis and Treatment of Stroke and Encephalopathy. Beijing: China Medical Science Press, 2000: 334-5. |
| [9] | The Minister of Health of the People's Republic of China. The second part of the Guiding Principle of Clinical Research on New Drugs of Chinese Medicine. Beijing: the Chinese Ministry of Public Health, 1995: 210-2. |
| [10] | Zheng XY. Clinical research guidelines for new Chinese medicine. Beijing: Medical Science and Technology Press, 2002: 383-5. |
| [11] | Lu WX. Cinical trial of Xingling granules for vertigo. Zhong Cheng Yao 1998;20(10):22-5. |
| [12] | Li HM. Comparison of the curative effect of Xuesaitong soft capsule and ginkgo ketone ester dripping pills in the treatment of dizziness caused by Cerebral Arteriosclerosis. Zhong Guo Lao Nian Bao Jian Yi Xue 2018;16:105-6. |
| [13] | Zhang J, Yang KP, Wei XX, et al. Efficacy of ginkgo ketone dripping pills in treating cerebral arteriosclerosis and vertigo. Zhong Hua Shi Yong Yi Xue 2014;9(22):143-4. |
| [14] | Liu CY, Wang B, He DY, et al. Current status of Traditional Chinese and Western Medicine research on chronic cerebral insufficiency. Zhong Guo Lao Nian Xue Za Zhi 2014;34:1722-4. |
| [15] | Gui SQ, Yu Z, Qu S, et al. Study on the academic thought of Renzhai Zhi Zhi Fang. Zhong Guo Zhong Yi Ji Chu Yi Xue Za Zhi 2016;22:744-7. |
| [16] | Zhang ZJ. Treatise on Febrile Diseases. People’s Medical Publishing House Co., Ltd., 2005: 252. |
| [17] | Zhang GQ, Chen L, Zhang LY. Study on TCM syndrome differentiation and syndrome factors of vascular headache based on data mining. Ya Tai Chuan Tong Yi Yao 2017;13:67-9. |
| [18] | Li ZP, Zhao DS, Wang FY. A review on numbness in TCM syndrome. Zhong Guo Lin Chuang Yan Jiu 2015;7:144-6. |
| [19] | Li D, Shao YY, Zhao M. Study on distribution law of TCM syndrome and syndrome element of insomnia based on literature. Huan Qiu Zhong Yi Yao 2020;13:384-8. |
| [20] | Ekwall A, Lindberg A, Magnusson M. Dizzy-why not take a walk? Low level physical activity improves quality of life among elderly with dizziness. Gerontology 2009;55:652-9. |
| [21] | Dros J, Maarsingh OR, Beem L, et al. Impact of dizziness on everyday life in older primary care patients: a cross-sectional study. Health Qual Life Outcomes 2011;9:44. |
| [22] | Jacobson GP, Newman CW. The development of the dizziness handicap inventory. Arch Otolaryngol Head Neck Surg 1990;116:424-7. |
| [23] | Honrubia V, Bell TS, Harris MR, et al. Quantitative evaluation of dizziness characteristics and impact on quality of life. Am J Otol 1996;17:595-602. |
| [24] | Duracinsky M, Mosnier I, Bouccara D, et al. Literature review of questionnaires assessing vertigo and dizziness, and their impact on patients' quality of life. Value Health 2007;10:273-84. |
| [1] | QIN Xiaoyu, WANG Chunai, XUE Jianjun, ZHANG Jie, LU Xiaoting, DING Shengshuang, GE Long, WANG Minzhen. Efficacy of electroacupuncture on myocardial protection and postoperative rehabilitation in patients undergoing cardiac surgery with cardiopulmonary bypass: a systematic review and Meta-analysis [J]. Journal of Traditional Chinese Medicine, 2024, 44(1): 1-15. |
| [2] | DAI Xiaoling, ZHANG Anming, LIN Hui, SHI Bei, REN Yi, WEN Hongzhu, FEI Xiaoyan, LIN Jiang. Qingchang suppositry (清肠栓) induced remission in patients with mild-to-moderate ulcerative proctitis: a multicenter, prospective, randomized, parallel-controlled clinical trial [J]. Journal of Traditional Chinese Medicine, 2024, 44(1): 156-162. |
| [3] | WANG Yichen, WU Shiyi, WANG Zhengyan, CHANG Wenling, XIE Zhihao, TANG Xing, ZHAO Songmei, ZHOU Jing, CHEN Zehong, WANG Chao, YANG Chunxia. Efficacy of Zhumian Tang formula granules (助眠汤配方颗粒) combined with eszopiclone for the treatment of poor sleep quality: a multi-center, randomized controlled, superiority trial [J]. Journal of Traditional Chinese Medicine, 2024, 44(1): 163-171. |
| [4] | YANG Yi, YE Huijun, ZHENG Huiling, JIN Lihua. Clinical observation on 90 cases of primary dysmenorrhea treated by buccal acupuncture therapy: a randomized controlled study [J]. Journal of Traditional Chinese Medicine, 2024, 44(1): 172-181. |
| [5] | DAI Zeqi, LIAO Xing, GUAN Yueyue, ZENG Zixiu, TANG Jun, HU Jing. Bloodletting puncture in the treatment of acute ischemic stroke: protocol for a mixed-method study of a multi-center randomized controlled trial and focus group [J]. Journal of Traditional Chinese Medicine, 2023, 43(6): 1259-1267. |
| [6] | XU Yani, ZHANG Yutong, HE Weile, DAI Linglin, TANG Ding, WANG Jialing, ZHANG Xufen, CHEN Qin, CHEN Lifang, WANG Zhanglian, ZHAN Mingjie. Efficiency and safety of acupuncture for women with premature ovarian insufficiency: study protocol for a randomized controlled trial [J]. Journal of Traditional Chinese Medicine, 2023, 43(6): 1268-1274. |
| [7] | XU Xiangru, ZHOU Yi, CHEN Gang, LEI Ming, ZHANG Wen, WU Xinxin, PU Yuting, CHEN Caiyu, SUN Yuting, ZHOU Shuang, FANG Bangjiang. Clinical efficacy of Buzhong Yiqi decoction (补中益气汤) in the treatment of hospital-acquired pneumonia with multi-drug resistant bacteria: a prospective, randomized, multicenter controlled trial [J]. Journal of Traditional Chinese Medicine, 2023, 43(5): 1010-1018. |
| [8] | ZHAO Ming, LUO Yimiao, WANG Huichan, CAO Yu, MA Lina, PEI Hui, LI Hao. Guilingji capsule (龟龄集胶囊) for Alzheimer's disease: secondary analysis of a randomized non-inferiority controlled trial [J]. Journal of Traditional Chinese Medicine, 2023, 43(5): 1019-1025. |
| [9] | ZHANG Meizhen, HAO Xiaohui, TANG Yiting, CHEN Yupeng, HE Puyu, ZHAO Liming, PANG Bing, NI Qing. Efficacy and safety of Buyang Huanwu decoction (补阳还五汤) for diabetic peripheral neuropathy: a systematic review and Meta-analysis [J]. Journal of Traditional Chinese Medicine, 2023, 43(5): 841-850. |
| [10] | YANG Yuqing, CHEN Yuhuan, LI Chunxiao, LING Xiao, WANG Panpan, GUO Jing, ZHANG Yingying. Effectiveness and safety of Pingxiao capsule (平消胶囊) as adjuvant therapy in treatment of breast cancer: a systematic review and Meta-analysis [J]. Journal of Traditional Chinese Medicine, 2023, 43(5): 851-859. |
| [11] | SUN Wu, ZHAO Yuwei, LIAO Liang, ZHAO Zhonghui, CHEN Shiqi, YAN Xiaoling, WANG Xueyao, CHAO Guojun, ZHOU Jian. Effectiveness and safety of Xuebijing injection for patients with coronavirus disease 2019: a systematic review and Meta-analysis [J]. Journal of Traditional Chinese Medicine, 2023, 43(4): 631-639. |
| [12] | WANG Chao, WU Qiong, LI Ping, WANG Zhigang, LOU Xusheng, LI Yuanyuan, ZHANG Lin. Effect of Traditional Chinese Medicine combined with Western Medicine on blood lipid levels and inflammatory factors in patients with angina pectoris in coronary heart disease identified as intermingled phlegm and blood stasis syndrome: a network Meta-analysis [J]. Journal of Traditional Chinese Medicine, 2023, 43(4): 640-649. |
| [13] | ZHANG Xinghe, LI Qifu, YI Rong, XING Chonghui, JIN Yuhao, MENG Jiangqiong, FENG Jialei, ZHAO Siwen, LIANG Fanrong, GUO Taipin. Effect of catgut embedding at acupoints versus non-acupoints in abdominal obesity: a randomized clinical trial [J]. Journal of Traditional Chinese Medicine, 2023, 43(4): 780-786. |
| [14] | ZHANG Yuehong, SHAO Xianzhi, ZHAO Qianlong, ZHAN Hualong, ZHANG Jianhua, DU Sisi, CHEN Jing, LIU Yingfang, ZHOU Haiwang, CHEN Xinsheng, HONG Ying, LIAN Fengmei, TONG Xiaolin, BA Yuanming. Effectiveness of Xiangsha Liujun pills (香砂六君丸) on decreased digestive function in convalescent patients of coronavirus disease 2019: a randomized, double blind, placebo controlled clinical trial [J]. Journal of Traditional Chinese Medicine, 2023, 43(3): 552-558. |
| [15] | ZHANG Yuehong, DONG Dandan, YAN Youqin, ZHANG Hao, WANG Guangli, ZHOU Wei, LI Wei, QIU Li, LI Tingming, LIU Quan, XIA Ping, MAO Lina, YANG Danlin, YANG Lu, LIAN Fengmei, TONG Xiaolin, BA Yuanming. Effectiveness and safety of Jinshuibao capsules (金水宝胶囊) in treatment of residual cardiopulmonary symptoms in convalescent patients of coronavirus disease 2019: a pilot randomized, double-blind, placebo-controlled clinical trial [J]. Journal of Traditional Chinese Medicine, 2023, 43(1): 134-139. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||