Journal of Traditional Chinese Medicine ›› 2024, Vol. 44 ›› Issue (6): 1153-1167.DOI: 10.19852/j.cnki.jtcm.20240927.004
• Research Articles • Previous Articles Next Articles
Transcriptome sequencing-based study on the mechanism of action of Jintiange capsules (金天格胶囊) in regulating synovial mesenchymal stem cells exosomal miRNA and articular chondrocytes mRNA for the treatment of osteoarthritis
CHEN Zhongying1, ZHANG Xue1, ZHANG Xiaofei1, ZOU Junbo1, YUAN Puwei2( ), SHI Yajun1(
), SHI Yajun1( )
)
- 1 Shaanxi Province Key Laboratory of New Drugs and Chinese Medicine Foundation Research, Pharmacy College, Shaanxi University of Chinese Medicine, Xianyang 712046, China
2 The First Clinical Medical College, Shaanxi University of Chinese Medicine, Xianyang 712046, China
-
Accepted:2023-12-05Online:2024-12-15Published:2024-09-27 -
Contact:SHI Yajun, Shaanxi Province Key Laboratory of New Drugs and Chinese Medicine Foundation Research, Pharmacy College, Shaanxi University of Chinese Medicine, Xianyang 712046, China. 2051004@sntcm.edu.cn Telephone: +86-15319070696
YUAN Puwei, the First Clinical Medical College, Shaanxi University of Chinese Medicine, Xianyang 712046, China. Spine_surgeon@163.com -
Supported by:Shaanxi Province Key R&D Program(2022SF-238);Chinese Medicine Pharmaceutical Key Discipline of Shaanxi province(303061107);Discipline Innovation team Project of Shaanxi University of Chinese Medicine(2019-YL11);Shaanxi Province Key subject of pharmacy engineering of Shaanxi Provincial Traditional Chinese Medicine administration(2017001)
Cite this article
CHEN Zhongying, ZHANG Xue, ZHANG Xiaofei, ZOU Junbo, YUAN Puwei, SHI Yajun. Transcriptome sequencing-based study on the mechanism of action of Jintiange capsules (金天格胶囊) in regulating synovial mesenchymal stem cells exosomal miRNA and articular chondrocytes mRNA for the treatment of osteoarthritis[J]. Journal of Traditional Chinese Medicine, 2024, 44(6): 1153-1167.
share this article
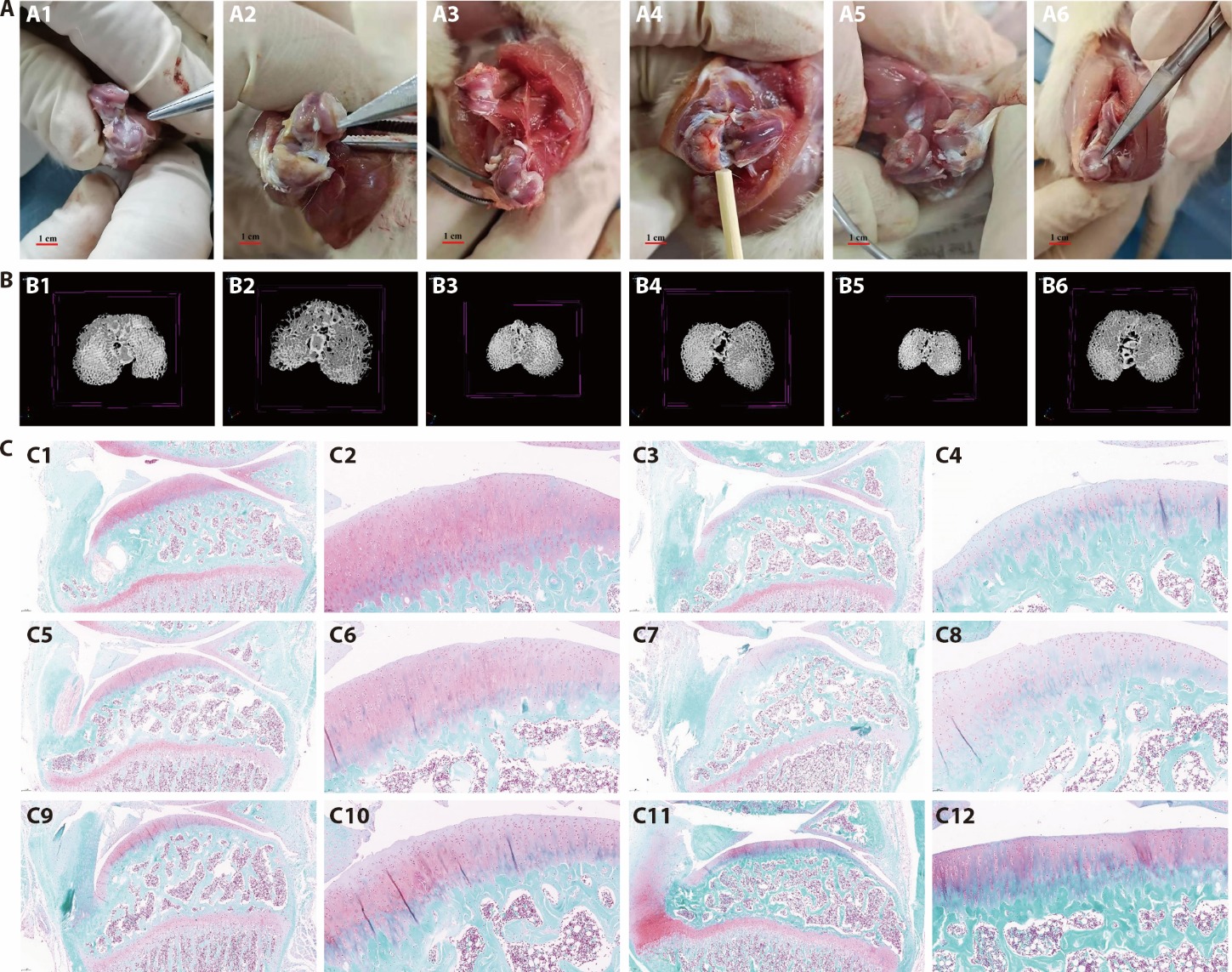
Figure 1 Pharmacodynamic investigation of SMSCs regulating ACs through exosomal miRNAs A: gross observation of articular cartilage in rats; A1: Blank group; A2: Model group; A3: Diacerein Capsules group; A4: 0.324 g/kg JTGs group; A5: 0.648 g/kg JTGs group; A6: 1.296 g/kg JTGs group; B: micro-CT subchondral bone imaging of rat knee specimens; B1: Blank group; B2: Model group; B3: Diacerein Capsules group; B4: 0.324 g/kg JTGs group; B5: 0.648 g/kg JTGs group; B6: 1.296 g/kg JTGs group; C: Staining diagram of the Safranin fast green; C1: Blank group (×5); C2: Blank group (×20); C3: Model group (×5); C4: Model group (×20); C5: Diacerein Capsules group (×5); C6: Diacerein Capsules group (×20); C7: 0.324 g/kg JTGs group (×5); C8: 0.324 g/kg JTGs group (×20); C9: 0.648 g/kg JTGs group (×5); C10: 0.648 g/kg JTGs group (×20); C11: 1.296 g/kg JTGs group (×5); C12: 1.296 g/kg JTGs group (×20). Blank and model groups: 0.5% CMC-Na at a daily dose of 5 mL/kg for 30 d; diacerein capsules group: diacerein at a daily dose of 9 mg/kg for 30 d; 0.324 g/kg JTGs group: the JTGs at a daily dose of 0.324 g/kg for 30 d; 0.648 g/kg JTGs group: the JTGs at a daily dose of 0.648 g/kg for 30 d; 1.296 g/kg JTGs group: the JTGs at a daily dose of 1.296 g/kg for 30 d. SMSCs: synovial mesenchymal stem cells; ACs: articular chondrocytes; JTGs: Jintiange capsules. Dyeing method of all pictures are the Safranin fast green staining method.
| Bone microstructure index | Blank group (n = 6) | Model group (n = 6) | Diacerein Capsules group (n = 6) | JTGs (0.324 g/kg) (n = 6) | JTGs (0.648 g/kg) (n = 6) | JTGs (1.296 g/kg) (n = 6) |
|---|---|---|---|---|---|---|
| BV/TV | 66.28±6.53 | 47.90±4.87a | 62.70±12.68c | 53.82±4.88 | 60.65±10.66b | 59.68±6.21b |
| BS/TV | 19.08±0.35 | 15.18±2.24a | 18.48±1.66c | 18.05±3.15b | 18.45±1.73c | 18.27±1.76b |
| BS/BV | 24.95±1.87 | 39.17±6.12a | 26.31±5.39c | 33.98±6.09 | 28.04±4.00c | 30.27±2.37c |
| Tb.Sp | 75.66±31.69 | 137.79±20.97a | 80.83±30.09c | 121.84±39.54 | 87.92±36.77b | 105.88±36.33 |
| Tb.Th | 159.20±10.60 | 109.36±16.40a | 147.27±23.38c | 128.52±5.75b | 140.12±19.32c | 136.30±11.47c |
| Tb.N | 4.68± 0.16 | 3.93±0.32a | 4.52±0.20c | 4.22±0.30 | 4.45±0.16c | 4.34±0.35b |
Table 1 Results of micro-CT analysis of morphological parameters of subchondral bone microstructure ($\bar{x}±s$)
| Bone microstructure index | Blank group (n = 6) | Model group (n = 6) | Diacerein Capsules group (n = 6) | JTGs (0.324 g/kg) (n = 6) | JTGs (0.648 g/kg) (n = 6) | JTGs (1.296 g/kg) (n = 6) |
|---|---|---|---|---|---|---|
| BV/TV | 66.28±6.53 | 47.90±4.87a | 62.70±12.68c | 53.82±4.88 | 60.65±10.66b | 59.68±6.21b |
| BS/TV | 19.08±0.35 | 15.18±2.24a | 18.48±1.66c | 18.05±3.15b | 18.45±1.73c | 18.27±1.76b |
| BS/BV | 24.95±1.87 | 39.17±6.12a | 26.31±5.39c | 33.98±6.09 | 28.04±4.00c | 30.27±2.37c |
| Tb.Sp | 75.66±31.69 | 137.79±20.97a | 80.83±30.09c | 121.84±39.54 | 87.92±36.77b | 105.88±36.33 |
| Tb.Th | 159.20±10.60 | 109.36±16.40a | 147.27±23.38c | 128.52±5.75b | 140.12±19.32c | 136.30±11.47c |
| Tb.N | 4.68± 0.16 | 3.93±0.32a | 4.52±0.20c | 4.22±0.30 | 4.45±0.16c | 4.34±0.35b |
| Survival rate | Control group (n = 3) | IL-1β group (n = 3) | 5 % JTGs medicated serum group (n = 3) | 10 % JTGs medicated serum group (n = 3) | 15 % JTGs medicated serum group (n = 3) |
|---|---|---|---|---|---|
| SMSCs | 1.000±0.000 | 0.146±0.063a | 0.844±0.052b | 0.972±0.067b | 0.672±0.209b |
| ACs | 1.000±0.000 | 0.136±0.017a | 0.742±0.132b | 0.930±0.078b | 0.714±0.134b |
Table 2 Screening results of the drug-containing serum concentrations of JTGs ($\bar{x}±s$)
| Survival rate | Control group (n = 3) | IL-1β group (n = 3) | 5 % JTGs medicated serum group (n = 3) | 10 % JTGs medicated serum group (n = 3) | 15 % JTGs medicated serum group (n = 3) |
|---|---|---|---|---|---|
| SMSCs | 1.000±0.000 | 0.146±0.063a | 0.844±0.052b | 0.972±0.067b | 0.672±0.209b |
| ACs | 1.000±0.000 | 0.136±0.017a | 0.742±0.132b | 0.930±0.078b | 0.714±0.134b |
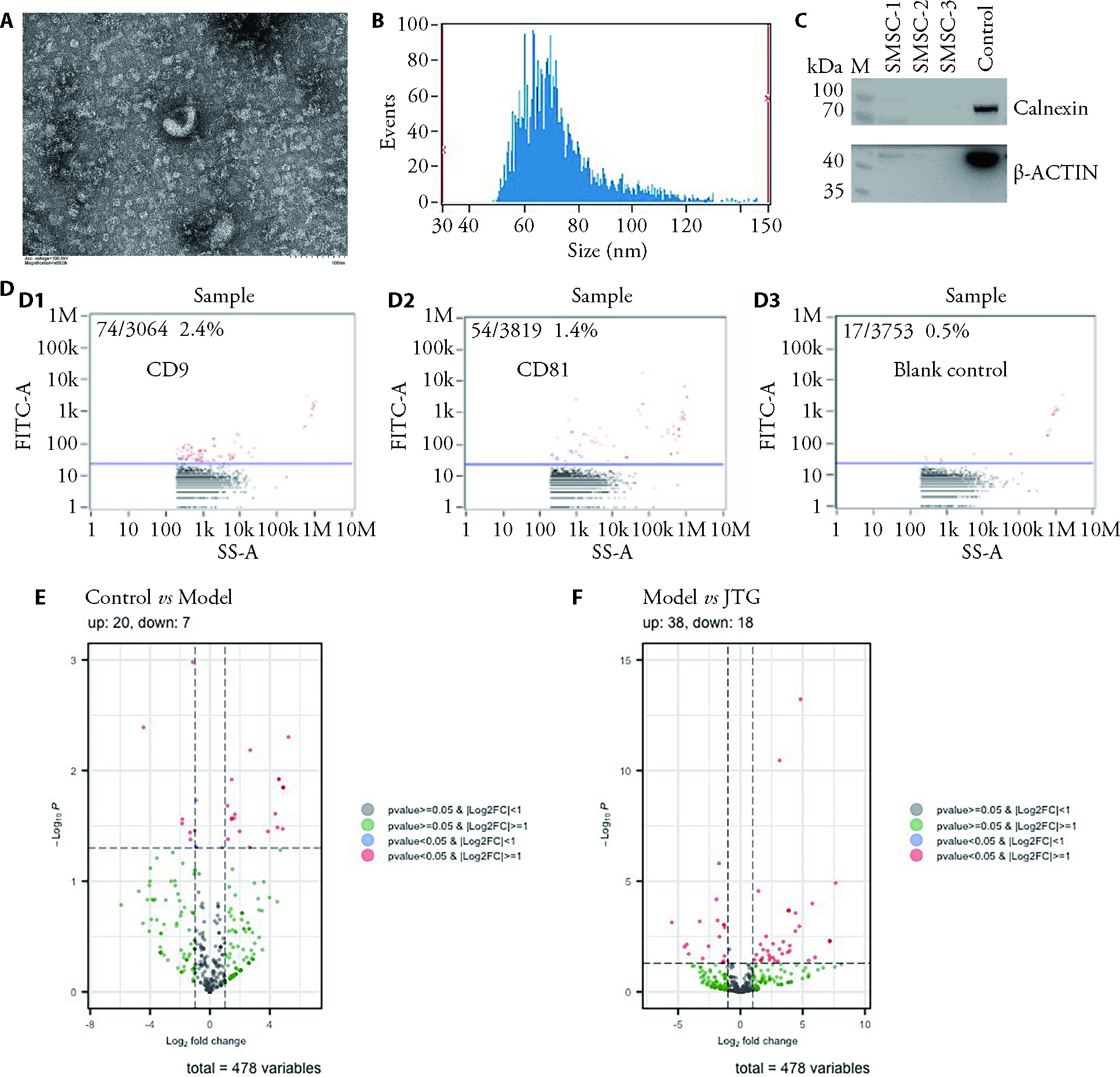
Figure 2 Identification of SMSC-Exos and their differentially expressed gene screening results A: SMSC-Exos transmission electron microscope; B: Exosome particle size analysis; C: Western blots to detect the expression of calnexin and actin protein in SMSC; D: Nanoflow method to detect CD9 (D1), CD81 (D2) and blank control (D3) protein expression; E: Volcano map of SMSC-Exos miRNA differentially expressed genes in normal group compared with model group; F: Volcano map of SMSC-Exos miRNA differentially expressed genes in the model group compared with the JTGs group. M: Marker; control: 293T cell; 30 μg/lane; CD9 and CD81: exosome marker proteins; Control: 10% fetal bovine serum; Model: 10% fetal bovine serum + 10 ng/ml of IL-1β; JTG: 10% drug-containing serum + 10 ng/mL of IL-1β. SMSC: synovial mesenchymal stem cells; SMSC-Exos: synovial mesenchymal stem cell exosomes; JTG: Jintiange capsules; IL-1β: Interleukin-1beta. The miRNAs with significant differences were identified using the criteria of |log2FC| ≥ 1 and P < 0.05.
| miRNA | Up / down | log2FC | P value |
|---|---|---|---|
| rno-miR-342-3p | down | -3.263143907 | 0.000652152 |
| rno-miR-146b-5p | down | -1.688859604 | 0.003207717 |
| rno-miR-501-3p | down | -4.154136556 | 0.019277237 |
| rno-miR-23a-3p | down | -1.275428183 | 0.024102849 |
| rno-miR-214-3p | down | -1.432467001 | 0.047196955 |
| rno-miR-222-3p | Up | -1.150188066 | 0.001046597 |
| rno-miR-30e-3p | Up | -4.438090482 | 0.004065579 |
| rno-miR-676 | Up | -1.859923545 | 0.030041356 |
| rno-miR-192-5p | Up | -1.302132946 | 0.042020132 |
Table 3 Significantly differentially expressed miRNAs
| miRNA | Up / down | log2FC | P value |
|---|---|---|---|
| rno-miR-342-3p | down | -3.263143907 | 0.000652152 |
| rno-miR-146b-5p | down | -1.688859604 | 0.003207717 |
| rno-miR-501-3p | down | -4.154136556 | 0.019277237 |
| rno-miR-23a-3p | down | -1.275428183 | 0.024102849 |
| rno-miR-214-3p | down | -1.432467001 | 0.047196955 |
| rno-miR-222-3p | Up | -1.150188066 | 0.001046597 |
| rno-miR-30e-3p | Up | -4.438090482 | 0.004065579 |
| rno-miR-676 | Up | -1.859923545 | 0.030041356 |
| rno-miR-192-5p | Up | -1.302132946 | 0.042020132 |
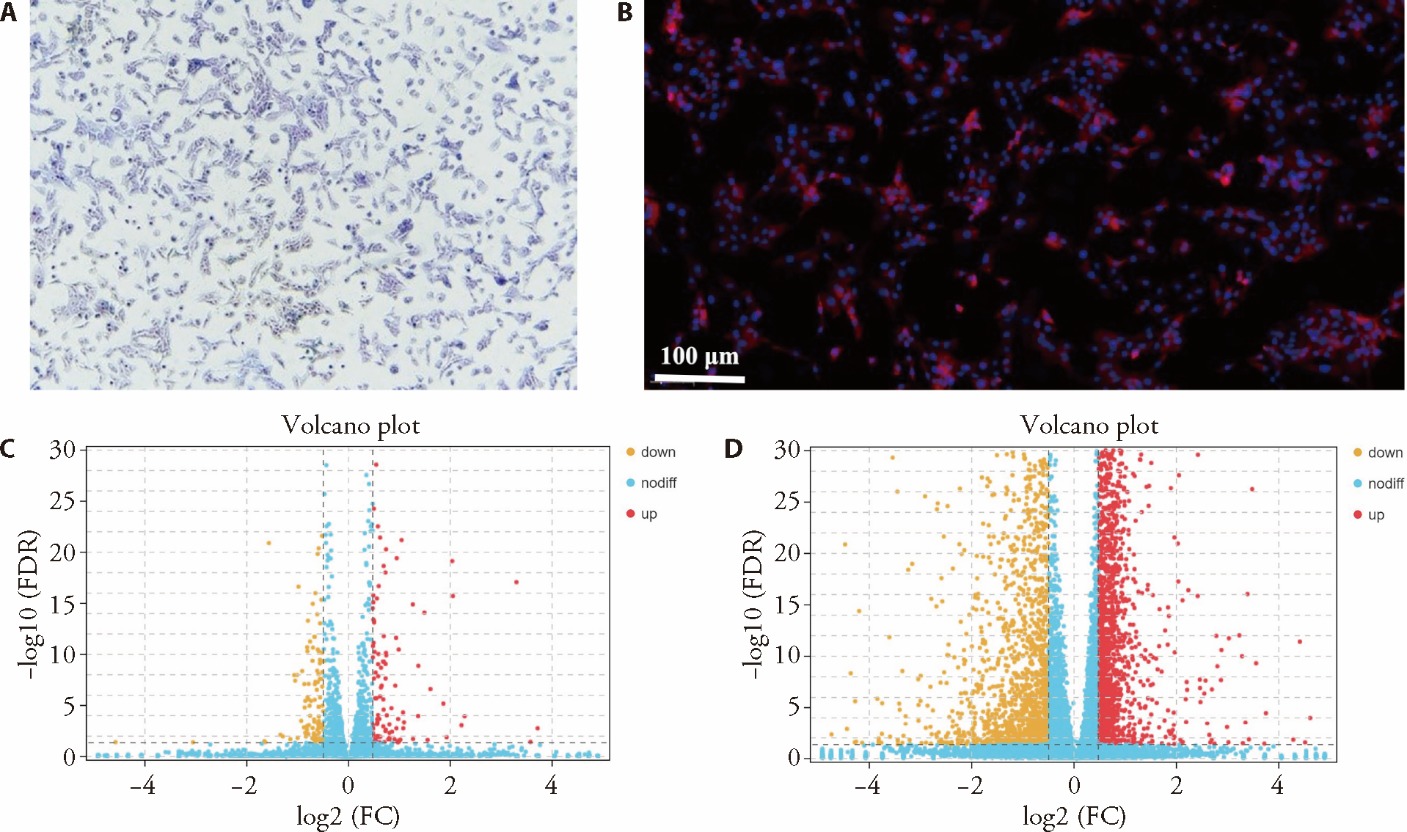
Figure 3 Identification of ACs and their differentially expressed gene screening results A: result of toluidine blue ACs staining (×50); B: result of type Ⅱ collagen immunofluorescence staining; C: volcano plot of ACs differentially expressed genes in the normal group compared with the model group; D: Volcano map of ACs differentially expressed genes in the model group compared with the JTGs group. ACs: articular chondrocytes. The identification of ACs was conducted through toluidine blue staining and immunofluorescence staining for type II collagen. The mRNA of ACs exhibiting significant differences was identified based on the criteria of |log2FC| ≥ 0.5 and P < 0.05.
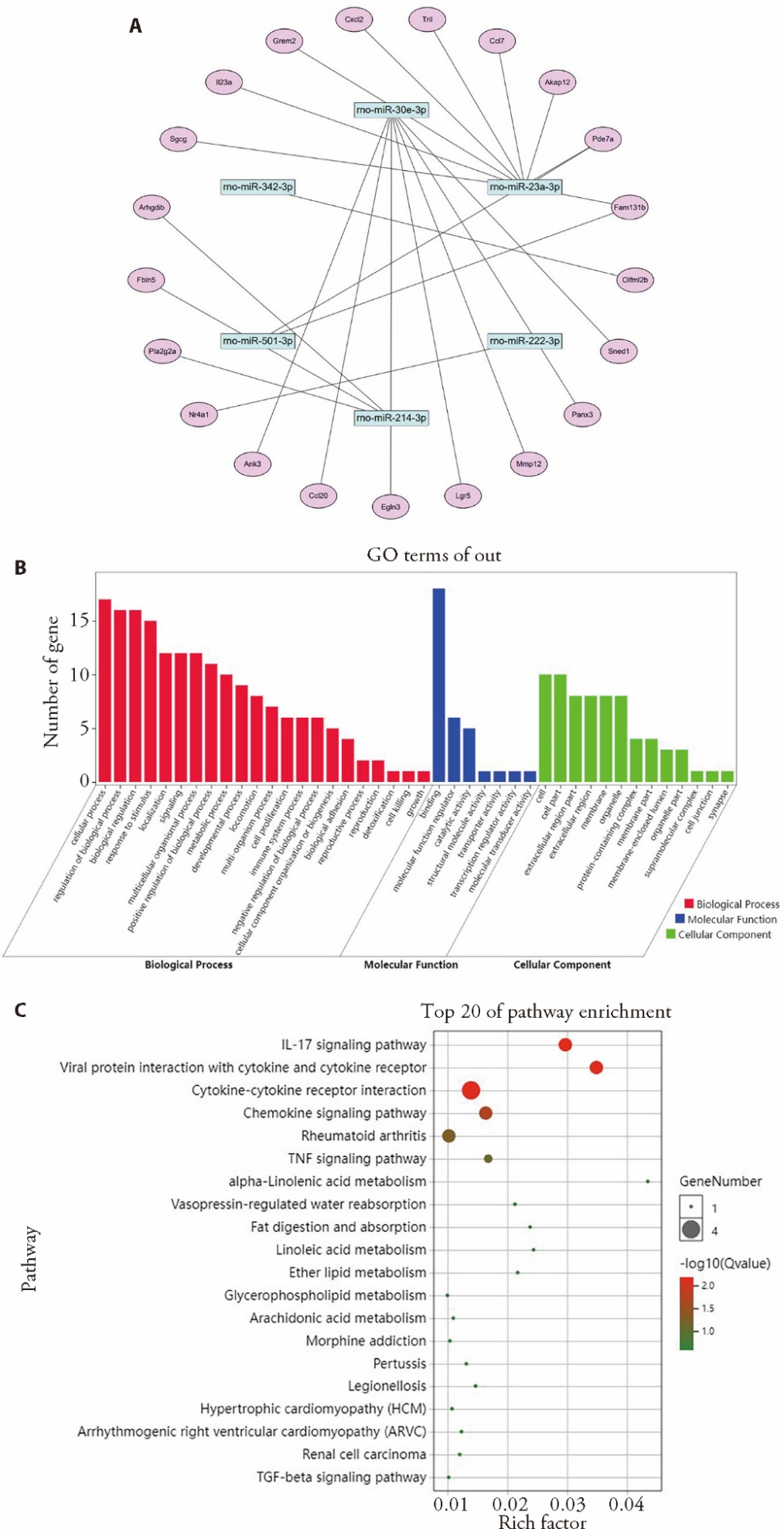
Figure 4 Screening of differentially expressed genes and their GO and KEGG enrichment analysis A: miRNA and mRNA co-intersection differentially expressed genes; B: result of GO enrichment analysis of co-intersecting differentially expressed genes; C: result of KEGG enrichment analysis of co-intersecting differentially expressed genes. GO: Gene Ontology; KEGG: Kyoto Encyclopedia of Genes and Genomes. The miRNA-mRNA regulatory network was constructed by Cytoscape software.
| Group | Ccl7 (n = 3) | Akap12 (n = 3) | Egln3 (n = 3) | Arhgdib (n = 3) | Ccl20 (n = 3) | Mmp12 (n = 3) | Pla2g2a (n = 3) | Grem2 (n = 3) | Nr4a1 (n = 3) |
|---|---|---|---|---|---|---|---|---|---|
| Control | 1.000±0.036 | 1.000±0.028 | 1.000±0.012 | 1.000±0.131 | 1.000±0.070 | 1.000± 0.137 | 1.000±0.047 | 1.000±0.119 | 1.000±0.128 |
| Model | 1.699±0.055a | 0.633±0.034c | 1.727± 0.050a | 1.995±0.117a | 2.722±0.241a | 1.555±0.184a | 1.889±0.044a | 1.404±0.046a | 1.683±0.218a |
| JTG | 0.948±0.059b | 1.364±0.236b | 0.923± 0.024b | 0.445±0.045b | 0.643±0.023b | 0.809±0.009b | 0.220±0.014b | 0.461±0.032b | 0.650±0.031b |
Table 4 Expression and trend of differential genes in common intersection
| Group | Ccl7 (n = 3) | Akap12 (n = 3) | Egln3 (n = 3) | Arhgdib (n = 3) | Ccl20 (n = 3) | Mmp12 (n = 3) | Pla2g2a (n = 3) | Grem2 (n = 3) | Nr4a1 (n = 3) |
|---|---|---|---|---|---|---|---|---|---|
| Control | 1.000±0.036 | 1.000±0.028 | 1.000±0.012 | 1.000±0.131 | 1.000±0.070 | 1.000± 0.137 | 1.000±0.047 | 1.000±0.119 | 1.000±0.128 |
| Model | 1.699±0.055a | 0.633±0.034c | 1.727± 0.050a | 1.995±0.117a | 2.722±0.241a | 1.555±0.184a | 1.889±0.044a | 1.404±0.046a | 1.683±0.218a |
| JTG | 0.948±0.059b | 1.364±0.236b | 0.923± 0.024b | 0.445±0.045b | 0.643±0.023b | 0.809±0.009b | 0.220±0.014b | 0.461±0.032b | 0.650±0.031b |
| 1. | Sellam J, Berenbaum F. The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nat rev rheumato 2010; 6: 625-35. |
| 2. |
Scanzello C, Goldring S. The role of synovitis in osteoarthritis pathogenesis. Bone 2012; 51: 249-57.
DOI PMID |
| 3. | Zhao Y, Li A, Ni L, Wang W, Shi X. The research progress of tiger bone and artificial tiger bone for the treatment of osteoporosis. Zhong Guo Gu Zhi Shu Song Za Zhi 2012; 18: 95-8. |
| 4. |
Sun J, Yang X, Hu Y. Efficacy of Jintiange Capsules in the treatment of osteoporosis: a network Meta-analysis. Orthop Surg 2019; 11: 176-86.
DOI PMID |
| 5. |
Li N, Gao J, Mi L, et al. Synovial membrane mesenchymal stem cells: past life, current situation, and application in bone and joint diseases. Stem Cell Res Ther 2020; 11: 381.
DOI PMID |
| 6. | Qiong J, Xia Z, Jing L, Haibin W. Synovial mesenchymal stem cells effectively alleviate osteoarthritis through promoting the proliferation and differentiation of meniscus chondrocytes. Eur Rev Med Pharmacol Sci 2020; 24: 1645-55. |
| 7. | Guo S, Tao S, Yin W, Qi X, Sheng J, Zhang C. Exosomes from human synovial-derived mesenchymal stem cells prevent glucocorticoid-induced osteonecrosis of the femoral head in the rat. Int J Biol Sci 2016; 12: 1262-72. |
| 8. |
Zhu Y, Wang Y, Zhao B, et al. Comparison of exosomes secreted by induced pluripotent stem cell-derived mesenchymal stem cells and synovial membrane-derived mesenchymal stem cells for the treatment of osteoarthritis. Stem Cell Res Ther 2017; 8: 64.
DOI PMID |
| 9. | Tao S, Yuan T, Zhang Y, Yin W, Guo S, Zhang C. Exosomes derived from miR-140-5p-overexpressing human synovial mesenchymal stem cells enhance cartilage tissue regeneration and prevent osteoarthritis of the knee in a rat model. Theranostics 2017; 7: 180-95. |
| 10. | Wang Z, Yan K, Ge G, et al. Exosomes derived from miR-155-5p-overexpressing synovial mesenchymal stem cells prevent osteoarthritis via enhancing proliferation and migration, attenuating apoptosis, and modulating extracellular matrix secretion in chondrocytes. Cell Biol Toxicol 2021; 37: 85-96. |
| 11. | Zeng Z, Dai Y, Deng S, Zou S, Dou T, Wei F. Synovial mesenchymal stem cell-derived extracellular vesicles alleviate chondrocyte damage during osteoarthritis through microRNA-130b-3p-mediated inhibition of the LRP12/AKT/β-catenin axis. Immunopharmacol Immunotoxicol 2022; 44: 247-60. |
| 12. | Zheng T, Li Y, Zhang X, Xu J, Luo M. Exosomes Derived from miR-212-5p overexpressed human synovial mesenchymal stem cells suppress chondrocyte degeneration and inflammation by targeting ELF3. Front Bioeng Biotechnol 2022; 10: 816209. |
| 13. | Qiu M, Liu D, Fu Q. MiR-129-5p shuttled by human synovial mesenchymal stem cell-derived exosomes relieves IL-1β induced osteoarthritis via targeting HMGB1. Life Sci 2021; 269: 118987. |
| 14. |
Kong R, Zhang J, Ji L, et al. Synovial mesenchymal stem cell-derived exosomal microRNA-320c facilitates cartilage damage repair by targeting ADAM19-dependent Wnt signalling in osteoarthritis rats. Inflammopharmacology 2023; 31: 915-26.
DOI PMID |
| 15. | Wanchen Y, Shijun W, Xuming J, Haijun Z, Xin Z, Yanjiao C. Influence of water decoction of huangqi on immune function in rats with deficiency cold pattern: a study based on transcriptome sequencing technique. Beijing Zhong Yi Yao Da Xue Xue Bao 2018; 41: 759-70. |
| 16. | Zhang X, Song JY, Hu YL, et al. Research progress of the regulation on active compound biosynthesis by the bHLH transcription factors in plants. Yao Xue Xue Bao 2014; 49: 435-42. |
| 17. |
Liao Y, Long J, Gallo C, Mirando A, Hilton M. Isolation and culture of murine primary chondrocytes: costal and growth plate cartilage. Methods Mol Biol 2021; 2230: 415-23.
DOI PMID |
| 18. |
Haseeb A, Lefebvre V. Isolation of mouse growth plate and articular chondrocytes for primary cultures. Methods Mol Biol 2021; 2245: 39-51.
DOI PMID |
| 19. | Chen S, Zhou Y, Chen Y, Gu J. Fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018; 34: i884-90. |
| 20. | Langmead B, Salzberg S. Fast gapped-read alignment with Bowtie 2. Nat Methods 2012; 9: 357-9. |
| 21. | Ru Y, Kechris K, Tabakoff B, et al. The multiMiR R package and database: integration of microRNA-target interactions along with their disease and drug associations. Nucleic Acids Res 2014; 42: e133. |
| 22. | Ritchie M, Phipson B, Wu D, et al. Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res 2015; 43: e47. |
| 23. |
Pouran B, Arbabi V, Bleys R, René van Weeren P, Zadpoor A, Weinans H. Solute transport at the interface of cartilage and subchondral bone plate: effect of micro-architecture. J Biomech 2017; 52: 148-54.
DOI PMID |
| 24. | Du LL, Yuan PW, Yang W, Li XF, Gao QM. Advantages of micro CT in three-dimensional reconstruction of specimens and its application in animal models of osteoarthritis. Zhong Guo Zu Zhi Gong Cheng Yan Jiu 2022; 26: 1931-6. |
| 25. | Wei Y, Zheng D, Guo X, Zhao M, Gao L, Bai L. Transient receptor potential channel, vanilloid 5, induces chondrocyte apoptosis in a rat osteoarthritis model through the mediation of Ca2+ influx. Cell Physiol Biochem 2018; 46: 687-98. |
| 26. |
Asghar S, Litherland G, Lockhart J, Goodyear C, Crilly A. Exosomes in intercellular communication and implications for osteoarthritis. Rheumatology (Oxford) 2020; 59: 57-68.
DOI PMID |
| 27. | D'Arrigo D, Roffi A, Cucchiarini M, Moretti M, Candrian C, Filardo G. Secretome and extracellular vesicles as new biological therapies for knee osteoarthritis: a systematic review. J Clin Med 2019; 8: 1867. |
| 28. |
Ni Z, Kuang L, Chen H, et al. The exosome-like vesicles from osteoarthritic chondrocyte enhanced mature IL-1β production of macrophages and aggravated synovitis in osteoarthritis. Cell Death Dis 2019; 10: 522.
DOI PMID |
| 29. | Ding LB, Wang HJ, Li Y, et al. Electroacupuncture stimulating neixiyan (EX-LE5) and dubi (ST35) alleviates osteoarthritis in rats induced by anterior cruciate ligament transaction affecting DNA methylation regulated transcription of miR-146a and miR-140-5p. J Tradit Chin Med 2023; 43: 983-90. |
| 30. |
Kopańska M, Szala D, Czech J, et al. MiRNA expression in the cartilage of patients with osteoarthritis. J Orthop Surg Res 2017; 12: 51.
DOI PMID |
| 31. | Yang Y, Li P, Zhu S, Bi R. Comparison of early-stage changes of osteoarthritis in cartilage and subchondral bone between two different rat models. PeerJ 2020; 8: e8934. |
| 32. | Wei ZY, Zhang ZL. Interpretation and application of micro-CT to obtain microstructure index in bone metabolism research. Zhong Guo Zu Zhi Gong Cheng Yan Jiu 2018; 11: 200-5. |
| 33. | Snelling S, Bas S, Puskas G, et al. Presence of IL-17 in synovial fluid identifies a potential inflammatory osteoarthritic phenotype. PloS one 2017; 12: e0175109. |
| 34. |
Raghu H, Lepus C, Wang Q, et al. CCL2/CCR2, but not CCL5/CCR5, mediates monocyte recruitment, inflammation and cartilage destruction in osteoarthritis. Ann Rheum Dis 2017; 76: 914-22.
DOI PMID |
| 35. |
Alaaeddine N, Antoniou J, Moussa M, et al. The chemokine CCL20 induces proinflammatory and matrix degradative responses in cartilage. Inflamm Res 2015; 64: 721-31.
DOI PMID |
| 36. |
Shi X, Ye H, Yao X, Gao Y. The involvement and possible mechanism of NR4A1 in chondrocyte apoptosis during osteoarthritis. Am J Transl Res 2017; 9: 746-54.
PMID |
| 37. | Meng J, Ma X, Ma D, Xu C. Microarray analysis of differential gene expression in temporomandibular joint condylar cartilage after experimentally induced osteoarthritis. Osteoarthritis cartilage 2005; 13: 1115-25. |
| 38. |
Yang S, Ryu J, Oh H, et al. NAMPT (visfatin), a direct target of hypoxia-inducible factor-2α, is an essential catabolic regulator of osteoarthritis. Ann Rheum Dis 2015; 74: 595-602.
DOI PMID |
| 39. | Lai C, Liao B, Peng S, Fang P, Bao N, Zhang L. Synovial fibroblast-miR-214-3p-derived exosomes inhibit inflammation and degeneration of cartilage tissues of osteoarthritis rats. Mol Cell Biochem 2023; 478: 637-49. |
| 40. | Kang L, Yang C, Song Y, et al. MicroRNA-23a-3p promotes the development of osteoarthritis by directly targeting SMAD3 in chondrocytes. Biochem Biophys Res Commun 2016; 478: 467-73. |
| 41. |
Dong Y, Li T, Li Y, Ren S, Fan J, Weng X. MicroRNA-23a-3p inhibitor decreases osteonecrosis incidence in a rat model. Mol Med Rep 2017; 16: 9331-6.
DOI PMID |
| 42. | Hu H, Dong L, Bu Z, et al. MiR-23a-3p-abundant small extracellular vesicles released from gelma/nanoclay hydrogel for cartilage regeneration. J Extracell Vesicles 2020; 9: 1778883. |
| 43. | Akbaba T, Akkaya-Ulum Y, Tavukcuoglu Z, Bilginer Y, Ozen S, Balci-Peynircioglu B. Inflammation-related differentially expressed common miRNAs in systemic autoinflammatory disorders patients can regulate the clinical course. Clin Exp Rheumatol 2021: 109-17. |
| 44. | Chen Y, Wang Z, Chen X, Peng X, Nie Q. CircNFIC balances inflammation and apoptosis by sponging miR-30e-3p and regulating DENND1B expression. Genes (Basel) 2021; 12: 1829. |
| 45. | Cao Y, Tang S, Nie X, Han W, Zhu Z, Ding C. OP0246 MiR-214-3P protects against osteoarthritis by directly targeting NF-ĸB pathway. Ann Rheum Dis 2020; 79: 155. 2-155. |
| 46. | Li H. Physiologic and pathophysiologic roles of AKAP12. Sci Prog 2022; 105: 368504221109212. |
| 47. | Shepherd C, Reynard LN, Loughlin J. Genotype at the MGP OA risk locus correlates with an expression quantitative trait locus operating on MGP in cartilage and with dna methylation at a cluster of regulatory CpGs within arhgdib. Osteoarthr Cartilage 2018; 26: S154-S155. |
| 48. |
Müller I, Melville D, Tanwar V, et al. Functional modeling in zebrafish demonstrates that the atrial-fibrillation-associated gene GREM2 regulates cardiac laterality, cardiomyocyte differentiation and atrial rhythm. Dis Model Mech 2013; 6: 332-41.
DOI PMID |
| 49. | Wang C, Xiao F, Wang C, et al. Gremlin2 Suppression Increases the BMP-2-Induced Osteogenesis of human bone marrow-derived mesenchymal stem cells via the BMP-2/Smad/Runx2 signaling pathway. J Cell Biochem 2017; 118: 286-97. |
| 50. | Kim J, Jeon J, Shin M, et al. Regulation of the catabolic cascade in osteoarthritis by the zinc-ZIP8-MTF1 axis. Cell 2014; 156: 730-43. |
| 51. |
Xiong Y, Ran J, Xu L, et al. Reactivation of NR4A 1 restrains chondrocyte inflammation and ameliorates osteoarthritis in rats. Front Cell Dev Biol 2020; 8: 158.
DOI PMID |
| 52. | Tsolis K, Bei E, Papathanasiou I, et al. Comparative proteomic analysis of hypertrophic chondrocytes in osteoarthritis. Clin Proteomics 2015; 12: 12. |
| [1] | CHEN Dandan, JIN Qianhong, SHEN Yuanjuan, WANG Qing, DAI Zhengxiang. Scraping therapy for knee osteoarthritis: a systematic review and Meta-analysis [J]. Journal of Traditional Chinese Medicine, 2024, 44(4): 633-641. |
| [2] | ZHU Wenting, GUO Changqing, DU Mei, MA Yunxuan, CUI Yongqi, CHEN Xilin, GUO Changqing. Acupotomy alleviates knee osteoarthritis in rabbit by regulating chondrocyte mitophagy via Pink1-Parkin pathway [J]. Journal of Traditional Chinese Medicine, 2024, 44(3): 468-477. |
| [3] | ZHOU Mingwang, DONG Zhuanli, WEI Changhao, FENG Lufang, WANG Xiaoping, LIU Haiping, JI Xing, YANG Kehu, LI Shenghua. Efficacy and safety of extracorporeal shock wave therapy combined with sodium hyaluronate in treatment of knee osteoarthritis: a systematic review and Meta-analysis [J]. Journal of Traditional Chinese Medicine, 2024, 44(2): 243-250. |
| [4] | DING Luobin, WANG Huajun, LI Yao, LI Jia, LI Ling, GAO Yangping, GUAN Jian, GENG Weiqiang. Electroacupuncture stimulating Neixiyan (EX-LE5) and Dubi (ST35) alleviates osteoarthritis in rats induced by anterior cruciate ligament transaction via affecting DNA methylation regulated transcription of miR-146a and miR-140-5p [J]. Journal of Traditional Chinese Medicine, 2023, 43(5): 983-990. |
| [5] | Chen Xilin, GUO Yan, LU Juan, QIN Luxue, HU Tingyao, ZENG Xin, WANG Xinyue, ZHANG Anran, ZHUANG Yuxin, ZHONG Honggang, GUO Changqing. Acupotomy ameliorates subchondral bone absorption and mechanical properties in rabbits with knee osteoarthritis by regulating bone morphogenetic protein 2-Smad1 pathway [J]. Journal of Traditional Chinese Medicine, 2023, 43(4): 734-743. |
| [6] | GUO Zhuang, WANG Junwen, LI Zhonglong, CHEN Zhongjie, CHEN Li, YAN Shiyan, LU Hongrong, LI Zhigeng, LI Guanying. Protocol to establish auxiliary diagnostic model for knee osteoarthritis functional testing equipment [J]. Journal of Traditional Chinese Medicine, 2023, 43(2): 379-385. |
| [7] | JIANG Zong, YAO Xiaoling, MA Wukai, TANG Fang. Molecular mechanism analysis of Miao medicine Jinwujiangu decoction (金乌健骨方) in treating osteoarthritis based on a network pharmacology approach [J]. Journal of Traditional Chinese Medicine, 2022, 42(4): 576-585. |
| [8] | QIN Luxue, GUO Changqing, ZHAO Ruili, WANG Tong, WANG Junmei, GUO Yan, ZHANG Wei, HU Tingyao, CHEN Xilin, ZHANG Qian, ZHANG Dian, XU Yue. Acupotomy inhibits aberrant formation of subchondral bone through regulating osteoprotegerin/receptor activator of nuclear factor-κB ligand pathway in rabbits with knee osteoarthritis induced by modified Videman method [J]. Journal of Traditional Chinese Medicine, 2022, 42(3): 389-399. |
| [9] | GUO Xiao, YANG Yunhao, LIAO Dongmei, PANG Fang, YANG Zhixue, ZHU Zhengwei, LUO Ao, TANG Chenglin. Effect of manipulation on cartilage in rats with knee osteoarthritis based on the Rho-associated protein kinase/LIM kinase 1/Cofilin signaling pathways [J]. Journal of Traditional Chinese Medicine, 2022, 42(2): 194-199. |
| [10] | YAO Nan, CHEN Guocai, LU Yanyan, XU Xuemeng, ZHAO Chuanxi, HUANG Xuejun, LIU Wengang, PENG Sha, WU Huai. Bushen Qiangjin capsule(补肾强筋胶囊) inhibits the Wnt/β-catenin pathway to ameliorate papain-induced knee osteoarthritis in rats [J]. Journal of Traditional Chinese Medicine, 2021, 41(6): 935-942. |
| [11] | LIU Di, WU Yongli, LI Chun, WANG Minglei, MA Xiaoxiu, LIU Junwei, ZHANG Yanling, YANG Lei. Warming moxibustion attenuates inflammation and cartilage degradation in experimental rabbit knee osteoarthritis [J]. Journal of Traditional Chinese Medicine, 2021, 41(6): 959-967. |
| [12] | LAI Qingzhong, ZHANG Xianjie, CHEN Meilan, HAN Zhicun. Salvia miltiorrhiza-asarum ointment combined with Chinese medical massage alleviates symptoms of osteoarthritis in a rat model through the Notch1/matrix metalloproteinase-13 signaling pathway [J]. Journal of Traditional Chinese Medicine, 2021, 41(4): 600-607. |
| [13] | Fang Ting, Li Qi, Zhou Fanyuan, Liu Fushui, Liu Zhongyong, Zhao Meimei, Chen Mei, You Jianyu, Jin Yuli, Xie Jinmei. Effect and safety of acupotomy in treatment of knee osteoarthritis:a systematic review and Meta-analysis [J]. Journal of Traditional Chinese Medicine, 2020, 40(3): 355-364. |
| [14] | Zhang Lele, Yuan Haixin. Effectiveness and clinical benefit of a therapy of combined non-pharmaceutical Traditional Chinese Medicine for knee osteoarthritis: a randomized controlled study [J]. Journal of Traditional Chinese Medicine, 2020, 40(3): 447-454. |
| [15] | He Xiaojin, Wang Lei, Zhou Xueping, Xu Luzhou, Cao Jing, Wang Ruirui, Wang Min, Xie Guoqian. Effect of Gubi prescription on caveolin-1 expression and phosphoinositide 3 kinase/protein kinase B and Fas signal pathways in rats with knee osteoarthritis [J]. Journal of Traditional Chinese Medicine, 2020, 40(2): 224-235. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||

