Journal of Traditional Chinese Medicine ›› 2025, Vol. 45 ›› Issue (6): 1376-1384.DOI: 10.19852/j.cnki.jtcm.2025.06.015
• Original Articles • Previous Articles Next Articles
Traditional Chinese Medicine treatment with syndrome differentiation for patients with severe community-acquired pneumonia: a multicenter, randomized, placebo-controlled trial
LI Jiansheng1( ), WANG Haifeng1, ZHANG Kang1, XIE Kai1, LI Suyun1, ZHANG Chenxi2, ZHANG Yaqing3
), WANG Haifeng1, ZHANG Kang1, XIE Kai1, LI Suyun1, ZHANG Chenxi2, ZHANG Yaqing3
- 1 Department of Respiratory and Critical Care Medicine, The First Affiliated Hospital of Henan University of Chinese Medicine, Zhengzhou 450000, China; Co-construction Collaborative Innovation Center for Chinese Medicine and Respiratory Diseases by Henan & Education Ministry of China, Henan University of Chinese Medicine, Zhengzhou 450046, China
2 Co-construction Collaborative Innovation Center for Chinese Medicine and Respiratory Diseases by Henan & Education Ministry of China, Henan University of Chinese Medicine, Zhengzhou 450046, China
3 Intensive Care Unit, Zhengzhou 15th People's Hospital, Zhengzhou 450000, China
-
Received:2025-05-22Accepted:2025-08-25Online:2025-12-15Published:2025-11-24 -
Contact:Prof. LI Jiansheng, Department of Respiratory and Critical Care Medicine, the First Affiliated Hospital of Henan University of Chinese Medicine, Zhengzhou 450000, China. li_js8@163.com.Telephone: +86-371-66248624 -
About author:LI Jiansheng and WANG Haifeng are co-first authors and contributed equally to this work -
Supported by:Mechanism of Qingre jiedu Huatan Formula Regulating Macrophage Mitochondrial Autophagy / NLR family pyrin domain containing 3 Inflammasome Activation and Intervening intestinal-Lung Bacterial Translocation in the Treatment of Severe Pneumonia(82074411);Prevention and Treatment of Respiratory Critical Illness with Traditional Chinese Medicine(22IRTSTHN029);a Randomized Controlled Trial(STG-ZYX02-202204)
Cite this article
LI Jiansheng, WANG Haifeng, ZHANG Kang, XIE Kai, LI Suyun, ZHANG Chenxi, ZHANG Yaqing. Traditional Chinese Medicine treatment with syndrome differentiation for patients with severe community-acquired pneumonia: a multicenter, randomized, placebo-controlled trial[J]. Journal of Traditional Chinese Medicine, 2025, 45(6): 1376-1384.
share this article
| Characteristic | Combination group (n = 91) | Conventional group (n = 92) | P value | |
|---|---|---|---|---|
| Age (mean±SD, years) | 64.6±14.8 | 62.9±15.6 | 0.439b | |
| 18-60 [n (%)] | 26 (28.6) | 31 (33.7) | 0.280c | |
| >60 [n (%)] | 65 (71.4) | 61 (66.3) | ||
| Male [n (%)] | 62 (68.1) | 68 (73.9) | 0.389c | |
| Body mass index [median (IQR), kg/m2] | 22.85 (19.86, 25.62) | 22.84 (20.24, 25.06) | 0.778d | |
| TCM syndrome differentiation [n (%)] | phlegm-heat obstruction in the lung | 54 (59.3) | 54 (58.7) | 0.929c |
| pulmonary stagnation of phlegm | 35 (38.5) | 35 (38.0) | 0.954c | |
| pathogenic heat trapped in the pericardium | 2 (2.2) | 3 (3.3) | 0.659c | |
| Clinical signs [median (IQR)] | Temperature (℃) | 37.00 (36.70, 38.10) | 37.20 (36.63, 38.28) | 0.973d |
| Respiratory rate (breaths/min) | 23.0 (20.0, 29.0) | 24.0 (20.0, 29.8) | 0.509d | |
| Heart rate (beats/min) | 98.0 (82.0, 110.0) | 97.5 (84.0, 110.0) | 0.492d | |
| Systolic blood pressure (mm Hg) | 124.0 (114.0, 135.0) | 128.0 (115.0, 137.8) | 0.398d | |
| Comorbidities [n (%)] | Hypertension | 33 (36.3) | 38 (41.3) | 0.484c |
| Cerebrovascular disease | 24 (24.7) | 26 (27.6) | 0.775c | |
| Diabetes mellitus | 23 (25.9) | 21 (23.0) | 0.698c | |
| Coronary artery disease | 21 (22.4) | 22 (24.1) | 0.894c | |
| Chronic pulmonary disease | 15 (16.5) | 17 (18.5) | 0.722c | |
| Serum levels [median (IQR)] | Creatinine (μmol/L) | 64.15 (44.15, 81.35) | 71.00 (56.95, 92.70) | 0.074d |
| Platelets (× 109/L) | 213.00 (132.50, 279.75) | 188.00 (120.25, 258.50) | 0.376d | |
| White blood cell count (× 109/L) | 10.17 (7.47, 13.60) | 10.16 (6.70, 15.17) | 0.92d | |
| PaO2/FiO2 (mm Hg) | 182.95 (135.78, 270.50) | 187.50 (145.03, 254.75) | 0.685d | |
| Severity criteria | ICU admission [n (%)] | 40 (44.0) | 50 (54.3) | 0.160c |
| SOFA score [median (IQR)] | 4.0 (2.0, 6.0) | 4.5 (2.25, 6.0) | 0.423d | |
| PSI score (mean±SD) | 107.9±35.2 | 106.4±42.5 | 0.992b | |
| Risk class [n (%)] a | Ⅰ-Ⅲ | 35 (38.4) | 34 (37.0) | 0.992c |
| Ⅳ | 34 (37.4) | 35 (38.0) | ||
| Ⅴ | 22 (24.2) | 23 (25.0) | ||
Table 1 Baseline characteristics of the intention-to-treat population
| Characteristic | Combination group (n = 91) | Conventional group (n = 92) | P value | |
|---|---|---|---|---|
| Age (mean±SD, years) | 64.6±14.8 | 62.9±15.6 | 0.439b | |
| 18-60 [n (%)] | 26 (28.6) | 31 (33.7) | 0.280c | |
| >60 [n (%)] | 65 (71.4) | 61 (66.3) | ||
| Male [n (%)] | 62 (68.1) | 68 (73.9) | 0.389c | |
| Body mass index [median (IQR), kg/m2] | 22.85 (19.86, 25.62) | 22.84 (20.24, 25.06) | 0.778d | |
| TCM syndrome differentiation [n (%)] | phlegm-heat obstruction in the lung | 54 (59.3) | 54 (58.7) | 0.929c |
| pulmonary stagnation of phlegm | 35 (38.5) | 35 (38.0) | 0.954c | |
| pathogenic heat trapped in the pericardium | 2 (2.2) | 3 (3.3) | 0.659c | |
| Clinical signs [median (IQR)] | Temperature (℃) | 37.00 (36.70, 38.10) | 37.20 (36.63, 38.28) | 0.973d |
| Respiratory rate (breaths/min) | 23.0 (20.0, 29.0) | 24.0 (20.0, 29.8) | 0.509d | |
| Heart rate (beats/min) | 98.0 (82.0, 110.0) | 97.5 (84.0, 110.0) | 0.492d | |
| Systolic blood pressure (mm Hg) | 124.0 (114.0, 135.0) | 128.0 (115.0, 137.8) | 0.398d | |
| Comorbidities [n (%)] | Hypertension | 33 (36.3) | 38 (41.3) | 0.484c |
| Cerebrovascular disease | 24 (24.7) | 26 (27.6) | 0.775c | |
| Diabetes mellitus | 23 (25.9) | 21 (23.0) | 0.698c | |
| Coronary artery disease | 21 (22.4) | 22 (24.1) | 0.894c | |
| Chronic pulmonary disease | 15 (16.5) | 17 (18.5) | 0.722c | |
| Serum levels [median (IQR)] | Creatinine (μmol/L) | 64.15 (44.15, 81.35) | 71.00 (56.95, 92.70) | 0.074d |
| Platelets (× 109/L) | 213.00 (132.50, 279.75) | 188.00 (120.25, 258.50) | 0.376d | |
| White blood cell count (× 109/L) | 10.17 (7.47, 13.60) | 10.16 (6.70, 15.17) | 0.92d | |
| PaO2/FiO2 (mm Hg) | 182.95 (135.78, 270.50) | 187.50 (145.03, 254.75) | 0.685d | |
| Severity criteria | ICU admission [n (%)] | 40 (44.0) | 50 (54.3) | 0.160c |
| SOFA score [median (IQR)] | 4.0 (2.0, 6.0) | 4.5 (2.25, 6.0) | 0.423d | |
| PSI score (mean±SD) | 107.9±35.2 | 106.4±42.5 | 0.992b | |
| Risk class [n (%)] a | Ⅰ-Ⅲ | 35 (38.4) | 34 (37.0) | 0.992c |
| Ⅳ | 34 (37.4) | 35 (38.0) | ||
| Ⅴ | 22 (24.2) | 23 (25.0) | ||
| Outcome | Intention-to-treat populations | Per-protocol populations | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Combination group (n = 91) | Conventional group (n = 92) | Between-group different (95% CI) | P value | Combination group (n = 83) | Conventional group (n = 87) | Between-group different (95% CI) | P value | ||||
| Treatment failure [n (%)]a | 18 (19.8) | 34 (37.0) | 0.17 (0.04, 0.30) | 0.010 | 14 (16.9) | 31 (36.5) | 0.19 (0.04, 0.32) | 0.006c | |||
| 28d mortality [n (%)] | 16 (17.6) | 29 (31.5) | 0.14 (0.02, 0.26) | 0.029 | 13 (15.7) | 27 (31.0) | 0.15 (0.03, 0.27) | 0.018c | |||
| 90-d mortality [n (%)] | 21 (23.1) | 31 (33.7) | 0.11 (-0.02, 0.24) | 0.111 | 18 (21.7) | 29 (33.3) | 0.12 (-0.02, 0.25) | 0.090c | |||
| Time to clinical stability (mean±SD, days)b | 11.8±1.1 | 17.8±1.2 | 0.001 | 9.6±0.8 | 17.9±1.2 | 0.001d | |||||
| Clinical stability [n (%)] | 61 (67.0) | 37 (40.2) | 0.27 (0.13, 0.41) | 0.001 | 59 (71.1) | 36 (41.1) | 0.30 (0.15, 0.44) | 0.001c | |||
| Length of hospital stay [median (IQR), days] | 17.0 (10.0, 23.0) | 16.0 (10.2, 25.0) | 0 (-3.00, 3.00) | 0.901 | 17.0 (10.0, 22.0) | 16.0 (11.0, 26.0) | -2.00 (-5.00, 1.00) | 0.231e | |||
Table 2 Outcomes using descriptive statistics for the intention-to-treat and per-protocol populations
| Outcome | Intention-to-treat populations | Per-protocol populations | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Combination group (n = 91) | Conventional group (n = 92) | Between-group different (95% CI) | P value | Combination group (n = 83) | Conventional group (n = 87) | Between-group different (95% CI) | P value | ||||
| Treatment failure [n (%)]a | 18 (19.8) | 34 (37.0) | 0.17 (0.04, 0.30) | 0.010 | 14 (16.9) | 31 (36.5) | 0.19 (0.04, 0.32) | 0.006c | |||
| 28d mortality [n (%)] | 16 (17.6) | 29 (31.5) | 0.14 (0.02, 0.26) | 0.029 | 13 (15.7) | 27 (31.0) | 0.15 (0.03, 0.27) | 0.018c | |||
| 90-d mortality [n (%)] | 21 (23.1) | 31 (33.7) | 0.11 (-0.02, 0.24) | 0.111 | 18 (21.7) | 29 (33.3) | 0.12 (-0.02, 0.25) | 0.090c | |||
| Time to clinical stability (mean±SD, days)b | 11.8±1.1 | 17.8±1.2 | 0.001 | 9.6±0.8 | 17.9±1.2 | 0.001d | |||||
| Clinical stability [n (%)] | 61 (67.0) | 37 (40.2) | 0.27 (0.13, 0.41) | 0.001 | 59 (71.1) | 36 (41.1) | 0.30 (0.15, 0.44) | 0.001c | |||
| Length of hospital stay [median (IQR), days] | 17.0 (10.0, 23.0) | 16.0 (10.2, 25.0) | 0 (-3.00, 3.00) | 0.901 | 17.0 (10.0, 22.0) | 16.0 (11.0, 26.0) | -2.00 (-5.00, 1.00) | 0.231e | |||
| Outcome | Intention-to-treat populations | Per-protocol populations | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Unadjusted OR or HR (95% CI) | P valuec | Adjusted OR or HR (95% CI)d | P valuec | Unadjusted OR or HR (95% CI) | P valuec | Adjusted OR or HR (95% CI)d | P valuec | ||
| Treatment failurea | 0.42 (0.22, 0.82) | 0.011 | 0.43 (0.21, 0.88) | 0.021 | 0.37 (0.18, 0.76) | 0.007 | 0.41 (0.19, 0.89) | 0.025 | |
| 28-d mortality | 0.46 (0.23, 0.93) | 0.030 | 0.52 (0.24, 1.09) | 0.083 | 0.38 (0.18, 0.812) | 0.012 | 0.45 (0.20, 1.01) | 0.051 | |
| 90-d mortality | 0.59 (0.31, 1.13) | 0.113 | 0.63 (0.31, 1.28) | 0.196 | 0.56 (0.28, 1.12) | 0.101 | 0.67 (0.31, 1.44) | 0.306 | |
| Time to clinical stabilityb | 2.08 (1.38, 3.14) | 0.001 | 2.29 (1.50, 3.50) | 0.001 | 2.55 (1.67, 3.90) | 0.001 | 2.43 (1.58, 3.73) | 0.001 | |
| Clinical stability | 0.33 (0.18, 0.61) | 0.001 | 0.27 (0.13, 0.55) | 0.001 | 0.29 (0.15, 0.54) | 0.001 | 0.25 (0.12, 0.53) | 0.001 | |
| Length of hospital stay | 0.67 (0.36, 1.25) | 0.209 | 0.87 (0.47, 1.66) | 0.673 | 0.69 (0.35, 1.35) | 0.277 | 0.97 (0.47, 1.99) | 0.924 | |
Table 3 Outcomes using logistic regression or Cox proportional hazards models
| Outcome | Intention-to-treat populations | Per-protocol populations | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Unadjusted OR or HR (95% CI) | P valuec | Adjusted OR or HR (95% CI)d | P valuec | Unadjusted OR or HR (95% CI) | P valuec | Adjusted OR or HR (95% CI)d | P valuec | ||
| Treatment failurea | 0.42 (0.22, 0.82) | 0.011 | 0.43 (0.21, 0.88) | 0.021 | 0.37 (0.18, 0.76) | 0.007 | 0.41 (0.19, 0.89) | 0.025 | |
| 28-d mortality | 0.46 (0.23, 0.93) | 0.030 | 0.52 (0.24, 1.09) | 0.083 | 0.38 (0.18, 0.812) | 0.012 | 0.45 (0.20, 1.01) | 0.051 | |
| 90-d mortality | 0.59 (0.31, 1.13) | 0.113 | 0.63 (0.31, 1.28) | 0.196 | 0.56 (0.28, 1.12) | 0.101 | 0.67 (0.31, 1.44) | 0.306 | |
| Time to clinical stabilityb | 2.08 (1.38, 3.14) | 0.001 | 2.29 (1.50, 3.50) | 0.001 | 2.55 (1.67, 3.90) | 0.001 | 2.43 (1.58, 3.73) | 0.001 | |
| Clinical stability | 0.33 (0.18, 0.61) | 0.001 | 0.27 (0.13, 0.55) | 0.001 | 0.29 (0.15, 0.54) | 0.001 | 0.25 (0.12, 0.53) | 0.001 | |
| Length of hospital stay | 0.67 (0.36, 1.25) | 0.209 | 0.87 (0.47, 1.66) | 0.673 | 0.69 (0.35, 1.35) | 0.277 | 0.97 (0.47, 1.99) | 0.924 | |
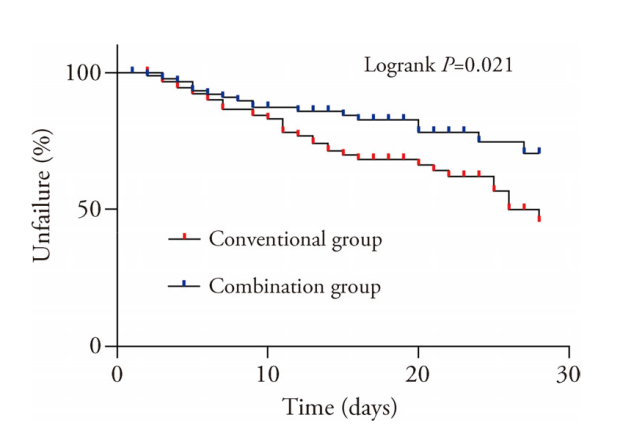
Figure 2 Kaplan-Meier analysis of the effect on the time to treatment failure Combination group: patients were given conventional medicine + traditional Chinese herbal granules 1 packets × twice daily for 28 d; conventional group: patients were given conventional medicine + placebo herbal 1 packets × twice daily for 28 d. The test method was Kaplan-Meier method. Data is expressed in percentage. Conventional group (n = 92), combination group (n = 91).
| 1. |
Torres A, Cilloniz C, Niederman MS, et al. Pneumonia. Nat Rev Dis Primers 2021; 7: 25.
DOI PMID |
| 2. |
Ramirez JA, Wiemken TL, Peyrani P, et al. Adults hospitalized with pneumonia in the United States: incidence, epidemiology, and mortality. Clin Infect Dis 2017; 65: 1806-12.
DOI PMID |
| 3. |
Song JH, Huh K, Chung DR. Community-acquired pneumonia in the Asia-Pacific region. Semin Respir Crit Care Med 2016; 37: 839-54.
DOI URL |
| 4. |
Ferreira-Coimbra J, Sarda C, Rello J. Burden of community-acquired pneumonia and unmet clinical needs. Adv Ther 2020; 37: 1302-18.
DOI PMID |
| 5. | Nair GB, Niederman MS. Updates on community acquired pneumonia management in the ICU. Pharmacol Ther 2021; 217: 107663. |
| 6. | Bartos H, Dzupova O. Severe community-acquired pneumonia in intensive care. Epidemiol Mikrobiol Imunol 2020; 69: 159-63. |
| 7. |
Zaragoza R, Vidal-Cortes P, Aguilar G, et al. Update of the treatment of nosocomial pneumonia in the ICU. Crit Care 2020; 24: 383.
DOI |
| 8. |
Olson G, Davis AM. Diagnosis and treatment of adults with community-acquired pneumonia. Jama 2020; 323: 885-6.
DOI PMID |
| 9. | Wang J, Song YL. Advances in severe community-acquired pneumonia. Chin Med J (Engl) 2019; 132: 1891-3. |
| 10. | Sun WZ, Sun GR. General principle of high-quality academic development of Traditional Chinese Medicine: "carrying on the essence, while pursuing innovations". J Tradit Chin Med 2023; 43: 1-2. |
| 11. | Hu Q, Yu T, Li J, Yu Q, Zhu L, Gu Y. End-to-end syndrome differentiation of Yin deficiency and Yang deficiency in Traditional Chinese Medicine. Comput Methods Programs Biomed 2019; 174: 9-15. |
| 12. |
Li ZX, Wang XR, Ulloa L, et al. Complementary and alternative medicine on cognitive defects and neuroinflammation after sepsis. J Tradit Chin Med 2024; 44: 408-16.
DOI |
| 13. | Ma HD, Deng YR, Tian Z, Lian ZX. Traditional Chinese Medicine and immune regulation. Clin Rev Allergy Immunol 2013; 44: 229-41. |
| 14. |
Song Y, Yao C, Yao Y, et al. Xuebijing injection versus placebo for critically ill patients with severe community-acquired pneumonia: a randomized controlled trial. Crit Care Med 2019; 47: e735-43.
DOI URL |
| 15. | Cai H, Luo S, Cai X, et al. Effect of Fu Zheng Jie Du formula on outcomes in patients with severe pneumonia receiving prone ventilation: a retrospective cohort study. Front Pharmacol 2024; 15: 1428817. |
| 16. |
Wang H, Li J, Yu X, Li SY. Integrated traditional Chinese and conventional medicine in treatment of severe community-acquired pneumonia: study protocol for a randomized placebo-controlled trial. Trials 2018; 19: 620.
DOI PMID |
| 17. | Qu JM, Cao B. Guidelines for the diagnosis and treatment of adult community acquired pneumonia in China (2016 edition). Zhonghua Jie He He Hu XI Za Zhi 2016; 39: 241-2. |
| 18. | Li JS, Wang ZW, Li SY. Diagnostic criteria for Traditional Chinese Medicine syndromes of community-acquired pneumonia (2011 edition). Zhong Yi Za Zhi 2011; 52: 2158-9. |
| 19. | Yu XQ, Xie Y, Li JS. Guidelines for Traditional Chinese Medicine in the diagnosis and treatment of community acquired pneumonia (2018 revised edition). Zhong Yi Za Zhi 2019; 60: 350-60. |
| 20. |
Torres A, Sibila O, Ferrer M, et al. Effect of corticosteroids on treatment failure among hospitalized patients with severe community-acquired pneumonia and high inflammatory response: a randomized clinical trial. JAMA 2015; 313: 677-86.
DOI PMID |
| 21. |
Halm EA, Fine MJ, Marrie TJ, et al. Time to clinical stability in patients hospitalized with community-acquired pneumonia: implications for practice guidelines. JAMA 1998; 279: 1452-7.
DOI URL |
| 22. |
Luo X, Xie J, Huang L, Gan WF, Chen M. Efficacy and safety of activating blood circulation and removing blood stasis of Traditional Chinese Medicine for managing renal fibrosis in patients with chronic kidney disease: a systematic review and Meta-analysis. J Tradit Chin Med 2023; 43: 429-40.
DOI |
| 23. |
Liu Z, Yan S, Wu J, et al. Acupuncture for chronic severe functional constipation: a randomized trial. Ann Intern Med 2016; 165: 761-9.
DOI PMID |
| 24. |
Luo Y, Wang CZ, Hesse-Fong J, Lin JG, Yuan CS. Application of Chinese medicine in acute and critical medical conditions. Am J Chin Med 2019; 47: 1223-35.
DOI URL |
| 25. |
Jiang M, Lu C, Zhang C, et al. syndrome differentiation in modern research of Traditional Chinese Medicine. J Ethnopharmacol 2012; 140: 634-42.
DOI PMID |
| 26. | Wang WJ, Zhang T. Integration of Traditional Chinese Medicine and Western medicine in the era of precision medicine. J Integr Med 2017; 15: 1-7. |
| 27. | Zhou LY, Wang YJ, Shi Q. Discuss the unity of opposites between precision medicine and Traditional Chinese Medicine. Zhong Hua Yi Xue Za Zhi 2017; 97: 3281-2. |
| 28. | Liu Y, Zhang C, Li C, Bai C, Shang H. Marked reduction in 28-d mortality among elderly patients with severe community-acquired pneumonia: post hoc analysis of a large randomized controlled trial. Clin Interv Aging 2020; 15: 2109-15. |
| 29. |
Qi F, Liang ZX, She DY, Yan GT, Chen LA. A clinical study on the effects and mechanism of Xuebijing injection in severe pneumonia patients. J Tradit Chin Med 2011; 31: 46-9.
DOI PMID |
| 30. | Li Y, Tian WM, Wu Q, Zhang R. Combined Traditional and Western Medicine in the treatment of 100 elderly patients suffering from severe pneumonia: an analysis of clinical results. Zhong Guo Wei Zhong Bing Ji Jiu Yi Xue 2011; 23: 44-5. |
| 31. |
Menendez R, Torres A. Treatment failure in community-acquired pneumonia. Chest 2007; 132: 1348-55.
PMID |
| 32. | Dinh A, Duran C, Ropers J, et al. Factors associated with treatment failure in moderately severe community-acquired pneumonia: a secondary analysis of a randomized clinical trial. JAMA Netw Open 2021; 4: e2129566. |
| 33. | Ceccato A, Cilloniz C, Ranzani OT, et al. Treatment with macrolides and glucocorticosteroids in severe community-acquired pneumonia: a post-hoc exploratory analysis of a randomized controlled trial. PLoS One 2017; 12: e0178022. |
| 34. |
Garcia-Vidal C, Carratala J. Early and late treatment failure in community-acquired pneumonia. Semin Respir Crit Care Med 2009; 30: 154-60.
DOI PMID |
| 35. | Walden AP, Clarke GM, McKechnie S, et al. Patients with community acquired pneumonia admitted to European intensive care units: an epidemiological survey of the GenOSept cohort. Crit Care 2014; 18: R58. |
| 36. |
Li Y, Guo C, Chen Q, et al. Improvement of pneumonia by curcumin-loaded bionanosystems based on platycodon grandiflorum polysaccharides via calming cytokine storm. Int J Biol Macromol 2022; 202: 691-706.
DOI URL |
| 37. | Yang D, Chen C, Zhang Q, Gong J. Network pharmacology predicts targets and pathways of herbal components for the treatment of pneumonia: a review. Medicine (Baltimore) 2025; 104: e41372. |
| 38. |
Liang Y, Li Y, Zhang K, et al. Qingfei Jiedu Huatan formula inhibits NLRP 3 inflammasome activation to attenuates inflammation and pyroptosis in severe pneumonia: integrating experimental verification, network pharmacology and transcriptomics. J Ethnopharmacol 2025; 343: 119449.
DOI URL |
| 39. |
Niu L, Xiao L, Zhang X, et al. Comparative efficacy of Chinese herbal injections for treating severe pneumonia: a systematic review and bayesian network Meta-analysis of randomized controlled trials. Front Pharmacol 2021; 12: 743486.
DOI URL |
| 40. |
Luo W, Liu Y, Zhang Q, Zhong H, Deng J. Effect of Traditional Chinese Medicine injections on severe pneumonia: a protocol for systematic review and Meta-analysis. Medicine (Baltimore) 2020; 99: e22012.
DOI URL |
| 41. |
Lodise TP, Anzueto AR, Weber DJ, et al. Assessment of time to clinical response, a proxy for discharge readiness, among hospitalized patients with community-acquired pneumonia who received either ceftaroline fosamil or ceftriaxone in two phase III FOCUS trials. Antimicrob Agents Chemother 2015; 59: 1119-26.
DOI PMID |
| 42. |
Blasi F, Ostermann H, Racketa J, Medina J, McBride K, Garau J. Early versus later response to treatment in patients with community-acquired pneumonia: analysis of the REACH study. Respir Res 2014; 15: 6.
DOI URL |
| 43. |
Takada K, Matsumoto S, Kojima E, et al. Predictors and impact of time to clinical stability in community-acquired pneumococcal pneumonia. Respir Med 2014; 108: 806-12.
DOI PMID |
| 44. |
Blum CA, Nigro N, Briel M, et al. Adjunct prednisone therapy for patients with community-acquired pneumonia: a multicentre, double-blind, randomised, placebo-controlled trial. Lancet 2015; 385: 1511-8.
DOI PMID |
| [1] | ZHANG Yibao, CHEN Feng, SUN Meng, WANG Zhenwei, TANG Binqing, QIAN Yechang, JIA Wei, BAO Yufang, LI Wenjie, LI Shanqun, ZHANG Wei. Dongtian Changchun ointment (洞天长春膏) for moderate-to-severe chronic obstructive pulmonary disease: a multicenter, prospective, open-label, randomized controlled trial [J]. Journal of Traditional Chinese Medicine, 2025, 45(6): 1395-1404. |
| [2] | ZHANG Xiaosi, ZHANG Shuangyuan, CHEN Hanqing, LIN Zhengdao, XIE Chune, LI Junxiang, LI Xiaohong. Hewei Jiangni recipe (和胃降逆方) improved the quality of life in patients with cold-heat mixed nonerosive reflux disease: a randomized, double-blinded study [J]. Journal of Traditional Chinese Medicine, 2025, 45(5): 1106-1118. |
| [3] | HAO Shulan, NAN Peng, LIU Likun, LI Xiaoli, ZHONG Qiming, GAO Yu, WANG Xixing, NIE Yingfang. Effectiveness of Yiqi Chupi powder (益气除疲散) for alleviating cancer-related fatigue in patients following colorectal cancer surgery: a randomized controlled trial [J]. Journal of Traditional Chinese Medicine, 2025, 45(5): 1119-1126. |
| [4] | GUO Jixing, JI Changchun, XIE Chaoju, RAO Xiang, SUN Zhangyin, XING Yu, ZHANG Rongni, QU Qiangqiang, DONG Youpeng, YANG Jinsheng. Various acupuncture therapies for managing nonspecific low back pain: a network Meta-analysis [J]. Journal of Traditional Chinese Medicine, 2025, 45(5): 954-962. |
| [5] | CHEN Ziying, ZHAO Xiaoping, FAN Xiaoxuan, TANG Didi, SUN Wen, LYU Jing, HUANG Lan, QI Fan. Seven Traditional Chinese Medicine external treatments combined with rehabilitation training on the functional recovery of limbs in patients with cerebral hemorrhage: a network Meta-analysis [J]. Journal of Traditional Chinese Medicine, 2025, 45(4): 711-719. |
| [6] | ZHAO Weibo, WANG Yaqi, KONG Lingyao, WANG Tianyi, ZHAO Haihong, ZHANG Ying, LUO Bin, WANG Ji, WANG Qi. Efficacy and safety of Tuomin Zhiti decoction (脱敏止嚏汤) on patients with seasonal allergic rhinitis: a randomized, double-blind, placebo-controlled trial [J]. Journal of Traditional Chinese Medicine, 2025, 45(4): 829-835. |
| [7] | LI Yuxuan, LI Yan, WANG Wujiao, CUI Xiaoyun, WAN Jie, ZHOU Kun, LU Jinjin, LIU Jing, LIN Qian, LI Dong. Clinical study of Yiqi Liangxue Shengji prescription (益气凉血生肌方) for improving cardiac function after myocardial ischemia reperfusion injury in patients with acute myocardial infarction: a randomized, double-blind, placebo-controlled trial [J]. Journal of Traditional Chinese Medicine, 2025, 45(4): 836-844. |
| [8] | ZHENG Ruwen, DONG Xu, WANG Tianyi, FENG Liyuan, ZHANG Hongyan, HUO Hong, ZHANG Ying, ZHANG Qianshi, ZHU Xingyan, WANG Dongyan. Electroacupuncture versus conventional acupuncture of scalp motor area for post-stroke wrist dyskinesia and its effect on muscle function: a randomized, controlled clinical trial [J]. Journal of Traditional Chinese Medicine, 2025, 45(4): 852-859. |
| [9] | XIAO Jing, SONG Danlei, LIANG Caiming, HE Yinuo, ZHENG Weifang, WU Xiaqiu. Efficacy of Jianpi formulas (健脾剂) in reducing the recurrence of colorectal adenoma after polypectomy: a systematic review and Meta-analysis [J]. Journal of Traditional Chinese Medicine, 2025, 45(2): 225-233. |
| [10] | LAI Xiaolei, SHANG Juju, LIU Hongxu, HU Jing, LI Xiang, ZHANG Zhenmin, XING Wenlong. Clinical efficacy of Angong Jiangya pill (安宫降压丸) for grade 2 hypertension with liver-fire hyperactivity syndrome: a randomized, double-blind, placebo-controlled, multicenter trial [J]. Journal of Traditional Chinese Medicine, 2025, 45(2): 422-429. |
| [11] | CHENG Jianping, FAN Chanjuan, ZHAI Lili, WANG Hui, XIE Dongling, CAI Yong, LI Zhen, HUANG Kun, BAI Qixuan. Efficacy and safety of Qingwei Zhitong pellets (清胃止痛微丸)-containing quadruple therapy for Helicobacter pylori eradication: a prospective, single-center, randomized trial [J]. Journal of Traditional Chinese Medicine, 2025, 45(2): 430-436. |
| [12] | YE Wujie, YANG Yawei, ZHANG Da, TANG Ling, CUI Minying, FU Bin, ZHANG Meng, HU Xingang, ZHAO Yan. Effectiveness of combining Qingyanyin formulated granules (轻燕饮配方颗粒) with press needles in treating abdominal obesity: a multicenter randomized controlled trial [J]. Journal of Traditional Chinese Medicine, 2025, 45(1): 107-114. |
| [13] | LANG Jiawang, JIN Lingqing, LUO Jianchang, LANG Boxu. Effects of acupuncture combined with bone-setting therapy to treat tourette syndrome: a three-arm randomized controlled trial [J]. Journal of Traditional Chinese Medicine, 2025, 45(1): 176-183. |
| [14] | Emre Bulut, Didem Özkal Eminoğlu, Yasemin Çayır. Effect of electroacupuncture on pain after periodontal flap surgery: a randomized controlled trial [J]. Journal of Traditional Chinese Medicine, 2025, 45(1): 184-191. |
| [15] | WU Qiaomin, GUAN Xuanke, LIU Jinfeng, WANG Yanli, CHANG Xing, LIU Zhiming, LIU Ruxiu. Compound Tongyang Fumai decoction (通阳复脉方) improves quality of life in sick sinus syndrome: a randomized controlled study [J]. Journal of Traditional Chinese Medicine, 2024, 44(6): 1247-1253. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||