Journal of Traditional Chinese Medicine ›› 2025, Vol. 45 ›› Issue (6): 1295-1306.DOI: 10.19852/j.cnki.jtcm.2025.06.009
• Original Articles • Previous Articles Next Articles
Wenshen Zhuanggu Fang (温肾壮骨方) reduces breast cancer bone metastasis by regulating macrophage polarization: a bioinformatics analysis combined with experimental validation
ZHANG Yang1, SHI Youyang1, LIU Xiaofei2, YANG Rui3, LI Qiong4, LI Feifei1, YANG Xiaojuan1, WANG Yi1, SUN Chenping1, HAN Xianghui1( ), LIU Sheng1(
), LIU Sheng1( )
)
- 1 Institute of Chinese Traditional Surgery, Longhua Hospital Affiliated to Shanghai University of Traditional Chinese Medicine, Shanghai 200032, China
2 Department of breast surgery, Affiliated Hospital of Shandong University of Traditional Chinese Medicine, Jinan 250014, China
3 Department of breast surgery, Shanxi Provincial Cancer Hospital, Taiyuan 030000, China
4 Department of breast surgery, Yueyang Hospital of Traditional Chinese and Western Medicine Affiliated to Shanghai University of Traditional Chinese Medicine, Shanghai 200000, China
-
Received:2024-03-12Accepted:2024-11-23Online:2025-12-15Published:2025-11-24 -
Contact:LIU Sheng, Institute of Chinese Traditional Surgery, Longhua Hospital Affiliated to Shanghai University of Traditional Chinese Medicine, Shanghai 200032, China, lshtcm@163.com, Telephone: +86-531-68616031;
HAN Xianghui, Institute of Chinese Traditional Surgery, Longhua Hospital Affiliated to Shanghai University of Traditional Chinese Medicine, Shanghai 200032, China, hanxianghui1106@163.com -
About author:ZHANG Yang and SHI Youyang are co-first authors and contributed equally to this work -
Supported by:Scientific Connotation of Traditional Chinese Medicine "Conghua Theory" by Regulating Metastatic Site-specific Polarization of Macrophages in Breast Cancer Lung and Bone Metastases(81774308);Pharmacodynamic Substances and Mechanism of Wenshen Zhuanggu Recipe Regulating Exocrine Integrin/Sarcoma/Mitogen-activated protein kinase Signal Axis in Intervention of Bone Metastasis Niche Formation in Breast Cancer(82174016)
Cite this article
ZHANG Yang, SHI Youyang, LIU Xiaofei, YANG Rui, LI Qiong, LI Feifei, YANG Xiaojuan, WANG Yi, SUN Chenping, HAN Xianghui, LIU Sheng. Wenshen Zhuanggu Fang (温肾壮骨方) reduces breast cancer bone metastasis by regulating macrophage polarization: a bioinformatics analysis combined with experimental validation[J]. Journal of Traditional Chinese Medicine, 2025, 45(6): 1295-1306.
share this article
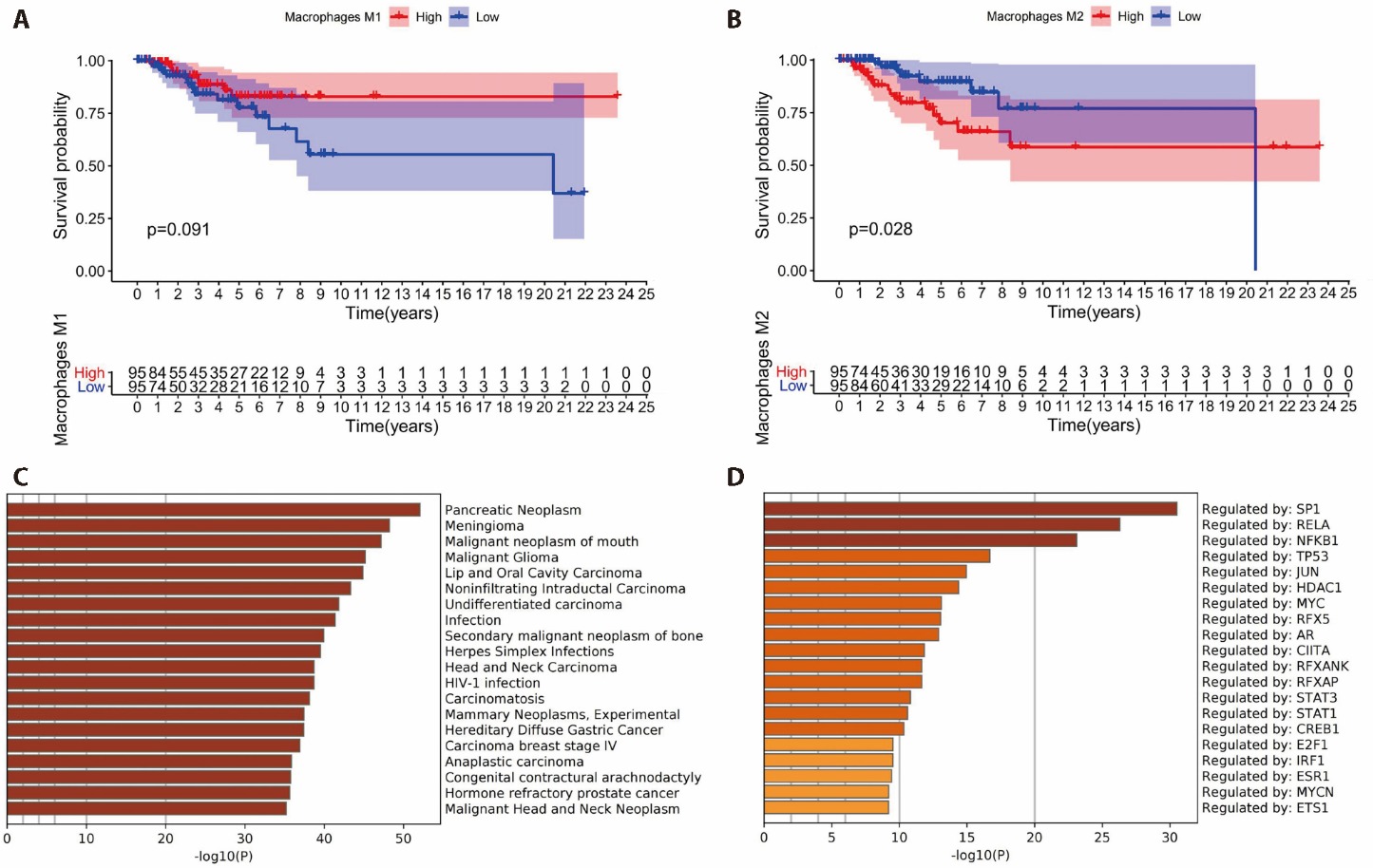
Figure 1 Correlation between macrophages and TNBC A: survival analysis of patients with M1 infiltration; B: survival analysis of patients with M1 infiltration; C: results of differential gene enrichment analysis of high M2 macrophage infiltration for related diseases; D: results of differential gene enrichment analysis of high M2 macrophage infiltration for related signaling pathways. SP1: specificity protein 1; RELA: RELA proto-oncogene; NFκB1: nuclear factor kappa-light-chain-enhancer of activated B cells 1; TP53: tumor protein p53; JUN: jun proto-oncogene; HDAC1: histone deacetylase 1; MYC: MYC proto-oncogene; RFX5: regulatory factor X 5; AR: androgen receptor; CⅡTA: class Ⅱ major histocompatibility complex transactivator; RFXANK: regulatory factor X-associated ankyrin-containing protein; RFXAP: regulatory factor X-associated protein; STAT3: signal transducer and activator of transcription 3; STAT1: signal transducer and activator of transcription 1; CREB1: cAMP responsive element binding protein 1; E2F1: E2F transcription factor 1; IRF2: interferon regulatory factor 2; ESR1: estrogen receptor 1; MYCN: MYCN proto-oncogene; ETS1: ETS proto-oncogene 1.
| Molecule ID | Active compounds | OB (%) | DL |
|---|---|---|---|
| MOL000448 MOL001950 MOL005530 MOL003590 MOL001941 MOL001942 MOL000614 MOL004576 MOL000422 MOL001953 MOL001945 MOL004759 MOL000006 MOL000173 MOL003244 | Isobavachin Psoralen Hydroxygenkwanin Isopsoralen Imperatorin Isoimperatorin Osthole Dihydroquercetin Kaempferol Xanthotoxin Bergapten Napelline Luteolin Wogonin Triptonide | 36.57 33.06 36.47 19.60 34.55 45.46 38.75 57.84 41.88 35.30 42.21 34.48 36.16 30.68 68.45 | 0.32 0.10 0.27 0.10 0.22 0.23 0.13 0.27 0.24 0.13 0.13 0.72 0.25 0.23 0.68 |
Table 1 Information of the active compounds of WSZG
| Molecule ID | Active compounds | OB (%) | DL |
|---|---|---|---|
| MOL000448 MOL001950 MOL005530 MOL003590 MOL001941 MOL001942 MOL000614 MOL004576 MOL000422 MOL001953 MOL001945 MOL004759 MOL000006 MOL000173 MOL003244 | Isobavachin Psoralen Hydroxygenkwanin Isopsoralen Imperatorin Isoimperatorin Osthole Dihydroquercetin Kaempferol Xanthotoxin Bergapten Napelline Luteolin Wogonin Triptonide | 36.57 33.06 36.47 19.60 34.55 45.46 38.75 57.84 41.88 35.30 42.21 34.48 36.16 30.68 68.45 | 0.32 0.10 0.27 0.10 0.22 0.23 0.13 0.27 0.24 0.13 0.13 0.72 0.25 0.23 0.68 |
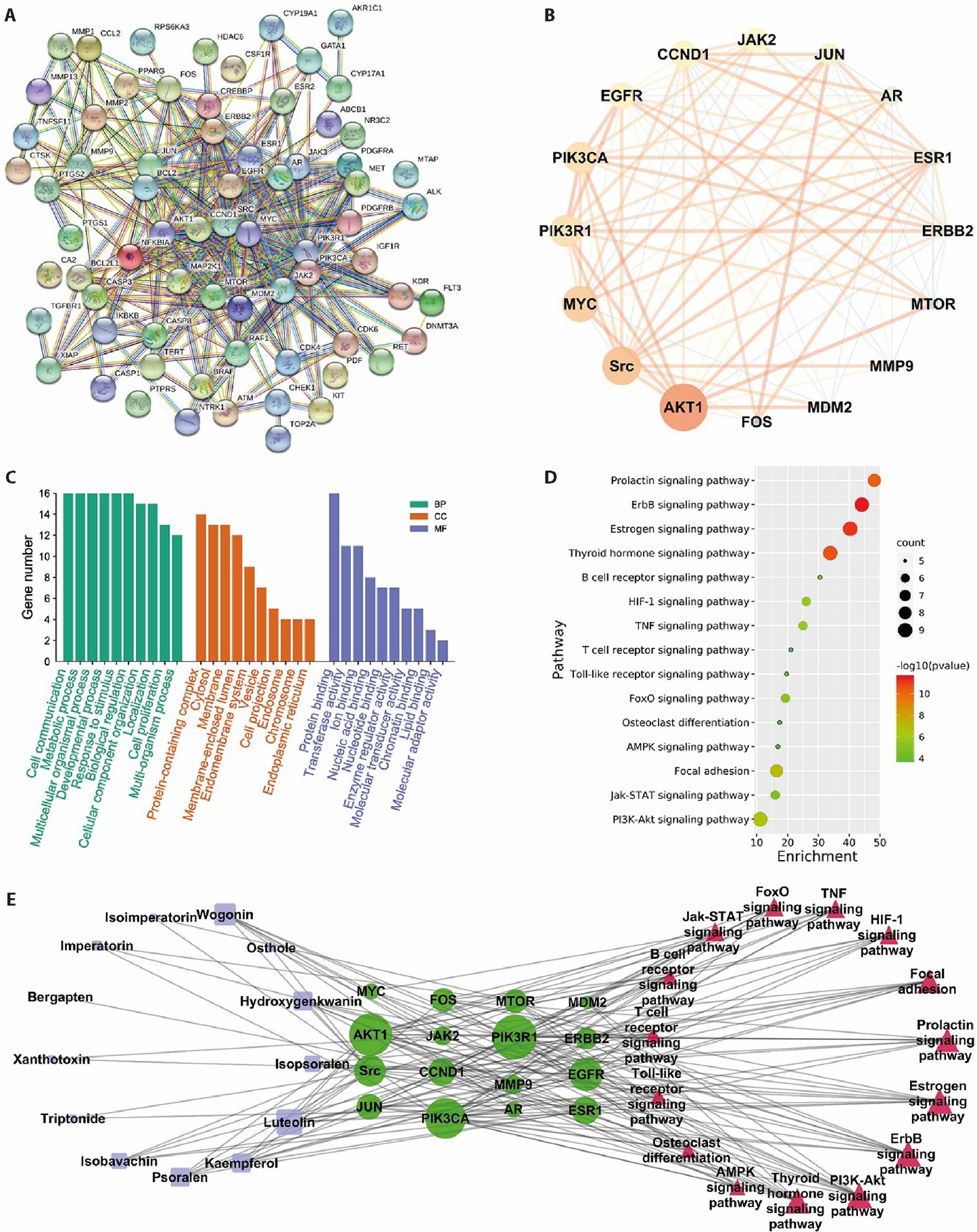
Figure 2 Prediction of the mechanism of WSZG in the treatment of breast cancer bone metastasis by network pharmacology A: ion flow diagram of WSZG in negative ion mode; B: ion flow diagram of WSZG in positive ion mode; C: PPI network of 70 targets; D: 16 hub targets in the network; E: the GO functional analysis of 16 putative targets; F: KEGG pathway enrichment analysis of 16 putative targets using dot plot; G: a network map of compounds-targets-pathways of WSZG against breast cancer bone metastasis. WSZG: Wenshen Zhuanggu Fang; PPI: protein-protein interaction; GO: gene ontology; KEGG: kyoto encyclopedia of genes and genomes pathway enrichment.

Figure 3 Impact of TAMs with different polarization states on the malignant behavior of breast cancer cells and the intervention effect of WSZG A: migration effects of M0, M1, and M2 supernatants on MDA-MB-231BO cells; A1, A2, A3: BO + M0, BO + M1, BO + M2 for 0 h; A4, A5, A6: BO + M0, BO + M1, BO + M2 for 24 h; A7, A8, A9: BO + M0, BO + M1, BO + M2 for 48 h; B: antagonized migration effects of WSZG on MDA-MB-231BO cells. MDA-MB-231BO cells were treated with the supernatants of M0, M1, or M2 together with WSZG ( 20 μg/mL) for 24 or 48 h; B1, B2, B3: BO + M0, BO + M1, BO + M2 treated with WSZG (20 μg/mL) for 0 h; B4, B5, B6: BO + M0, BO + M1, BO + M2 treated with WSZG (20 μg/mL) for 24 h; B7, B8, B9: BO + M0, BO + M1, BO + M2 treated with WSZG (20 μg/mL) for 48 h; C: antagonized migration effects of WSZG on MDA-MB-231BO cells. MDA-MB-231BO cells were treated with the supernatants of M0, M1, or M2 together with WSZG ( 40 μg/mL) for 24 or 48 h. C1, C2, C3: BO + M0, BO + M1, BO + M2 treated with WSZG (40 μg/mL) for 0 h; C4, C5, C6: BO + M0, BO + M1, BO + M2 treated with WSZG (40 μg/mL) for 24 h; C7, C8, C9: BO + M0, BO + M1, BO + M2 treated with WSZG (40 μg/mL) for 48 h; D: inhibitory effect of WSZG extract on the M2-induced invasion of MDA-MB-231BO cells for 24 h through crystalline purple dyeing; D1, D2, D3: control group of BO + M0, BO + M1, BO + M2 for 24 h; D4, D5, D6: BO + M0, BO + M1, BO + M2 treated with WSZG (10 μg/mL) for 24 h; D7, D8, D9: BO + M0, BO + M1, BO + M2 treated with WSZG (20 μg/mL) for 24 h; E: inhibitory effect of WSZG extract on the M2-induced invasion of MDA-MB-231BO cells for 48 h through crystalline purple dyeing; E1, E2, E3: Control group of BO + M0, BO + M1, BO + M2 for 48 h; E4, E5, E6: BO + M0, BO + M1, BO + M2 treated with WSZG (10 μg/mL) for 48 h; E7, E8, E9: BO + M0, BO + M1, BO + M2 treated with WSZG (20μg/mL) for 48 h; F: expression of proteins specific to the malignant phenotype of MDA-MB-231BO cells; G: expression of proteins specific to the malignant phenotype of MDA-MB-231BO cells; H: expression of M1 and M2 macrophage-specific proteins; I: ELISA of cytokine content in co-culture systems; I1: ELISA of IFN-γcontent in co-culture systems; I2: ELISA of IL-4 content in co-culture systems; I3: ELISA of IL-12 content in co-culture systems; I4: ELISA of IL-10 content in co-culture systems. WSZG: Wenshen Zhuanggu Fang; TAMs: tumor-associated macrophages; ELISA: enzyme-linked immunosorbent assay; IFN-γ: recombinant murine interferon-gamma; IL: interleukin. The t-test was applied for comparisons between two groups, data are presented as mean ± standard error of the mean (n = 3). aP < 0.01 vs M0 + BO 24 h group; bP < 0.01 vs M0 + BO + WSZG (20 μg/mL) 24 h group; cP < 0.05 vs M0 + BO 24 h group; dP < 0.01 vs M0 + BO 48 h group; eP < 0.01 vs M0 + BO (40 μg/mL) 24 h group; f P < 0.01 vs M0 + BO + WSZG (20 μg/mL) 48 h group.

Figure 4 WSZG extract regulated macrophage polarization via STAT signaling A: FCM analysis of the M2/M1 ratio in the bone tissues; B: IHC index of macrophage-specific protein markers F4/80; C: IHC index of macrophage-specific protein markers CD206 expression; D: confocal immunofluorescence analysis of the effect on DAPI, STAT1 and STAT6; A1, B1, C1, D1-D4: model group (injection of cancer cells only and oral administration of saline for 35 d); A2, B2, C2, D5-D8: zoledronic acid group (i.p zoledronic acid, 100 μg/kg per week for 35 d); A3, B3, C3, D9-D12: WSZG (6.5 g·kg?1·d?1) group (oral administration of WSZG for 35 d); A4, B4, C4, D13-D16: WSZG (13 g·kg?1·d?1) group (oral administration of WSZG for 35 d). WSZG: Wenshen Zhuanggu Fang; STAT: signal transducer and activator of transcription; FCM: flow cytometry; IHC: immunohistochemistry; DAPI: 4',6-diamidino-2-phenylindole.
| 1. |
Liang YR, Zhang HW, Song XJ, Yang QF. Metastatic heterogeneity of breast cancer: molecular mechanism and potential therapeutic targets. Semin Cancer Biol 2020; 60: 14-27.
DOI PMID |
| 2. |
Kang Y. Dissecting Tumor-stromal Interactions in breast cancer bone metastasis. Endocrinol Metab (Seoul) 2016; 31: 206-12.
DOI URL |
| 3. | Padzińska-Pruszyńska IB, Taciak B, Kiraga Ł, et al. Targeting cancer: microenvironment and immunotherapy innovations. Int J Mol Sci 2024; 25: 13569. |
| 4. | Yang HN, Kim C, Zou WP. Metabolism and macrophages in the tumor microenvironment. Curr Opin Immunol 2024; 91: 102491. |
| 5. |
Wu KY, Lin KJ, Li XY, et al. Redefining tumor-associated macrophage subpopulations and functions in the tumor microenvironment. Front Immunol 2020; 11: 1731.
DOI PMID |
| 6. |
Koul B, Taak P, Kumar A, Kumar A, Sanyal I. Genus Psoralea: a review of the traditional and modern uses, phytochemistry and pharmacology. J Ethnopharmacol 2019; 232: 201-26.
DOI PMID |
| 7. |
Gao YB, Fan H, Nie AZ, et al. Aconitine: a review of its pharmacokinetics, pharmacology, toxicology and detoxification. J Ethnopharmacol 2022; 293: 115270.
DOI URL |
| 8. | Sun Y, Yang AWH, Lenon GB. Phytochemistry, ethnopharmacology, pharmacokinetics and toxicology of cnidium monnieri (L.) Cusson. Int J Mol Sci 2020; 21: 1006. |
| 9. |
Ma Y, Wang LN, Zheng SY, et al. Osthole inhibits osteoclasts formation and bone resorption by regulating NF-κB signaling and NFATc1 activations stimulated by RANKL. J Cell Biochem 2019; 120: 16052-61.
DOI PMID |
| 10. |
Zeng XZ, He LG, Wang S, et al. Aconine inhibits RANKL-induced osteoclast differentiation in RAW264.7 cells by suppressing NF-κB and NFATc1 activation and DC-STAMP expression. Acta Pharmacol Sin 2016; 37: 255-63.
DOI |
| 11. |
Zhang T, Han WQ, Zhao KX, et al. Psoralen accelerates bone fracture healing by activating both osteoclasts and osteoblasts. FASEB J 2019; 33: 5399-410.
DOI PMID |
| 12. |
Han XH, Wang CL, Xie Y, et al. Anti-metastatic effect and mechanisms of Wenshen Zhuanggu formula in human breast cancer cells. J Ethnopharmacol 2015; 162: 39-46.
DOI URL |
| 13. | Li JJ, Chen WL, Wang JY, et al. Wenshen Zhuanggu formula effectively suppresses breast cancer bone metastases in a mouse Xenograft model. Acta Pharmacol Sin 2017; 38: 1369-80. |
| 14. |
Ma J, Li J, Wang Y, et al. WSZG inhibits BMSC-induced EMT and bone metastasis in breast cancer by regulating TGF-β1/Smads signaling. Biomed Pharmacother 2020; 121: 109617.
DOI PMID |
| 15. |
Wu CY, Chen MC, Sun ZP, et al. Wenshen Zhuanggu formula mitigates breast cancer bone metastasis through the signaling crosstalk among the Jagged1/Notch, TGF-β and IL-6 signaling pathways. J Ethnopharmacol 2019; 232: 145-54.
DOI PMID |
| 16. |
Chen WL, Li JJ, Sun ZP, et al. Comparative pharmacokinetics of six coumarins in normal and breast cancer bone-metastatic mice after oral administration of Wenshen Zhuanggu formula. J Ethnopharmacol 2018; 224: 36-44.
DOI PMID |
| 17. |
Xu X, Zhang WX, Huang C, et al. A novel chemometric method for the prediction of human oral bioavailability. Int J Mol Sci 2012; 13: 6964-82.
DOI PMID |
| 18. | Ru JL, Li P, Wang JN, et al. TCMSP: a database of systems pharmacology for drug discovery from herbal medicines. J Cheminform 2014; 6: 13. |
| 19. |
Szklarczyk D, Gable AL, Lyon D, et al. STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res 2019; 47: D607-13.
DOI |
| 20. |
Vats D, Mukundan L, Odegaard JI, et al. Oxidative metabolism and PGC-1beta attenuate macrophage-mediated inflammation. Cell Metab 2006; 4: 13-24.
DOI PMID |
| 21. |
Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, et al. Macrophage-specific PPAR gamma controls alternative activation and improves insulin resistance. Nature 2007; 447: 1116-20.
DOI |
| 22. |
Farooqi AA, Shepetov AM, Rakhmetova V, et al. Interplay between JAK/STAT pathway and non-coding RNAs in different cancers. Noncoding RNA Res 2024; 9: 1009-22.
DOI PMID |
| 23. |
Sanpaolo ER, Rotondo C, Cici D, Corrado A, Cantatore FP. JAK/STAT pathway and molecular mechanism in bone remodeling. Mol Biol Rep 2020; 47: 9087-96.
DOI PMID |
| 24. | Yuan HD, Ma QQ, Cui HY, et al. How can synergism of traditional medicines benefit from network pharmacology? Molecules 2017; 22: 1135. |
| 25. |
Zhou DX, Huang C, Lin Z, et al. Macrophage polarization and function with emphasis on the evolving roles of coordinated regulation of cellular signaling pathways. Cell Signal 2014; 26: 192-7.
DOI PMID |
| 26. | Massagué J, Obenauf AC. Metastatic colonization by circulating tumour cells. Nature 2016; 529: 298-306. |
| 27. | Wilczyński JR, Nowak M. Cancer immunoediting: elimination, equilibrium, and immune escape in solid tumors. Exp Suppl 2022; 113: 1-57. |
| 28. |
Hinshaw DC, Shevde LA. The tumor microenvironment innately modulates cancer progression. Cancer Res 2019; 79: 4557-66.
DOI PMID |
| 29. |
Chen PW, Zuo H, Xiong H, et al. Gpr 132 sensing of lactate mediates tumor-macrophage interplay to promote breast cancer metastasis. Proc Natl Acad Sci USA 2017; 114: 580-5.
DOI URL |
| 30. | Erin N, Grahovac J, Brozovic A, Efferth T. Tumor microenvironment and epithelial mesenchymal transition as targets to overcome tumor multidrug resistance. Drug Resist Updat 2020; 53: 100715. |
| 31. |
Yang R, Xie Y, Li Q, et al. Ruyiping extract reduces lung metastasis in triple negative breast cancer by regulating macrophage polarization. Biomed Pharmacother 2021; 141: 111883.
DOI PMID |
| 32. |
Locati M, Curtale G, Mantovani A. Diversity, mechanisms, and significance of macrophage plasticity. Annu Rev Pathol 2020; 15: 123-47.
DOI PMID |
| 33. |
Timperi E, Croizer H, Khantakova D, et al. At the interface of tumor-associated macrophages and fibroblasts: immune-suppressive networks and emerging exploitable targets. Clin Cancer Res 2024; 30: 5242-51.
DOI URL |
| 34. |
Petty AJ, Yang Y. Tumor-associated macrophages: implications in cancer immunotherapy. Immunotherapy 2017; 9: 289-302.
DOI PMID |
| 35. |
Luo K, Zhong YM, Guo YD, et al. Moxibustion inhibits the macrophage M1 polarization toll-like receptor 4/myeloid differentiation factor 88/nuclear factor kappa B signaling pathway by regulating T-cell immunoglobulin and mucin-containing protein-3 in rheumatoid arthritis. J Tradit Chin Med 2024; 44: 1227-35.
DOI |
| 36. | Tian XR, Huo RS, Liu XG, Zhao P, Tian YG, LI JS. Yangqing Chenfei formula alleviates crystalline silica induced pulmonary inflammation and fibrosis by suppressing macrophage polarization. J Tradit Chin Med 2023; 43: 1126-39. |
| 37. |
Hughes R, Qian BZ, Rowan C, et al. Perivascular M2 macrophages stimulate tumor relapse after chemotherapy. Cancer Res 2015; 75: 3479-91.
DOI PMID |
| 38. |
Wang D, Wang XH, Si MH, et al. Exosome-encapsulated miRNAs contribute to CXCL12/CXCR4-induced liver metastasis of colorectal cancer by enhancing M2 polarization of macrophages. Cancer Lett 2020; 474: 36-52.
DOI PMID |
| 39. |
Lee S, Lee E, Ko E, et al. Tumor-associated macrophages secrete CCL2 and induce the invasive phenotype of human breast epithelial cells through upregulation of ERO1-α and MMP-9. Cancer Lett 2018; 437: 25-34.
DOI PMID |
| 40. |
D'Oronzo S, Brown J, Coleman R. The role of biomarkers in the management of bone-homing malignancies. J Bone Oncol 2017; 9: 1-9.
DOI PMID |
| 41. | Colombo G, Pessolano E, Talmon M, Genazzani AA, Kunderfranco P. Getting everyone to agree on gene signatures for murine macrophage polarization in vitro. PLoS One 2024; 19: e0297872. |
| 42. |
Zhu LW, Li Z, Dong XH, et al. Ficolin-A induces macrophage polarization to a novel pro-inflammatory phenotype distinct from classical M1. Cell Commun Signal 2024; 22: 271.
DOI PMID |
| 43. |
Yang L, Han PS, Cui T, et al. M2 macrophage inhibits the antitumor effects of Lenvatinib on intrahepatic cholangiocarcinoma. Front Immunol 2023; 14: 1251648.
DOI URL |
| 44. |
Zhuang XQ, Zhang H, Hu GH. Cancer and microenvironment plasticity: double-edged swords in metastasis. Trends Pharmacol Sci 2019; 40: 419-29.
DOI PMID |
| [1] | TIAN Yuan, BU He, WANG Tieshan, YANG Dongliang, ZHANG Wei, LIU Tong, ZHANG Li, HUO Zejun. Efficacy of electro-acupuncture at “Weizhong” (BL40) on macrophage polarization in rats with injured lumbar multifidus [J]. Journal of Traditional Chinese Medicine, 2025, 45(2): 335-347. |
| [2] | ZHANG Pengqiang, FENG Qi, HUANG Weiyan, OU Shan. Protective effect of lappaconitine on Freund's complete adjuvant-induced arthritis exerted through P2X7 receptor-mediated regulation of M1/M2 balance in rats [J]. Journal of Traditional Chinese Medicine, 2025, 45(1): 39-48. |
| [3] | LUO Kun, ZHONG Yumei, GUO Yanding, ZHANG Linlin, HU Danhui, MA Wenbin, YANG Xin, ZHOU Haiyan. Moxibustion inhibits the macrophage M1 polarization toll-like receptor 4/myeloid differentiation factor 88/nuclear factor kappa B signaling pathway by regulating T-cell immunoglobulin and mucin-containing protein-3 in rheumatoid arthritis [J]. Journal of Traditional Chinese Medicine, 2024, 44(6): 1227-1235. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||

