Journal of Traditional Chinese Medicine ›› 2025, Vol. 45 ›› Issue (1): 39-48.DOI: 10.19852/j.cnki.jtcm.2025.01.004
Previous Articles Next Articles
Protective effect of lappaconitine on Freund's complete adjuvant-induced arthritis exerted through P2X7 receptor-mediated regulation of M1/M2 balance in rats
ZHANG Pengqiang1, FENG Qi2, HUANG Weiyan2, OU Shan2( )
)
- 1 School of Medical and Life Sciences, Chengdu University of Traditional Chinese Medicine, Chengdu 610075, China
2 Department of Anesthesiology, Chengdu First People's Hospital, Chengdu 610095, China
-
Received:2023-11-11Accepted:2024-04-15Online:2025-02-15Published:2025-01-10 -
Contact:Prof. OU Shan, Department of Anesthesiology, Chengdu First People's Hospital, Chengdu 610095, China.313577313@qq.com Telephone: +86-18011390162
Cite this article
ZHANG Pengqiang, FENG Qi, HUANG Weiyan, OU Shan. Protective effect of lappaconitine on Freund's complete adjuvant-induced arthritis exerted through P2X7 receptor-mediated regulation of M1/M2 balance in rats[J]. Journal of Traditional Chinese Medicine, 2025, 45(1): 39-48.
share this article
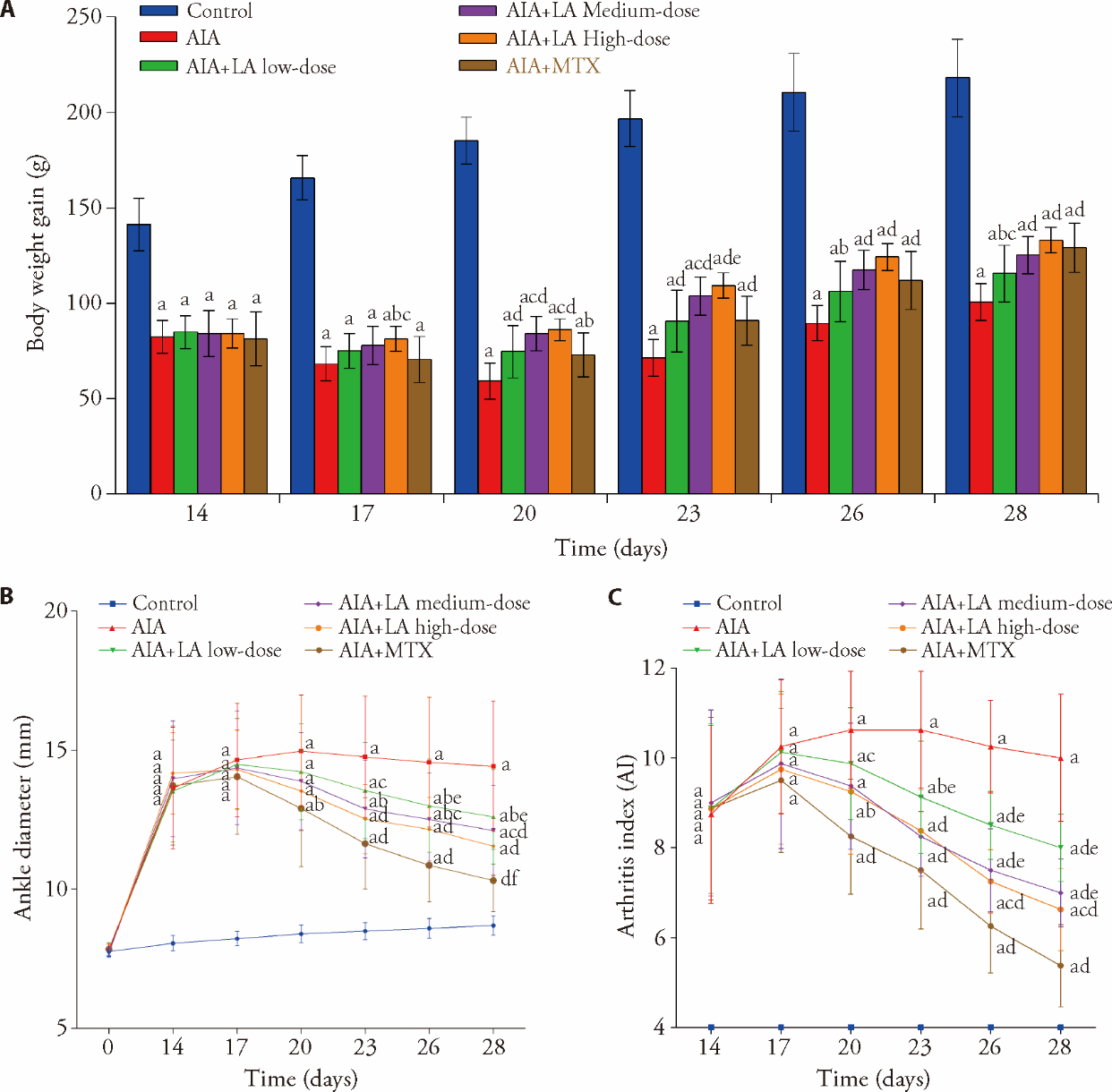
Figure 1 Effects of LA on macroscopic features of AIA rats A: body weight gain was observed every 3 d from days 14 to 28; B: ankle diameter was observed at day 0 and every 3 d from days 14 to 28; C: AI was observed every 3 d from days 14 to 28. Control: normal group and treated with 4 mL·kg-1·d-1 of NS for 14 d; AIA: treated with 4 mL·kg-1·d-1 of NS for 14 d; AIA + LA low-dose: treated with 2 mg·kg-1·d-1 of LA for 14 d; AIA + LA medium-dose: treated with 4 mg·kg-1·d-1 of LA for 14 d; AIA + LA high-dose: treated with 8 mg·kg-1·d-1 of LA for 14 d; AIA + MTX: treated with 0.5 mg/kg per 3 d of MTX for 14 d. LA: lappaconitine; AIA: adjuvant induced arthritis; MTX: methotrexate; AI: arthritis index; NS: normal saline. Data are represented as mean ± standard deviation (n = 8). The groups were compared using a one-way analysis of variance followed by Tukey's multiple comparisons test. aP < 0.01 and fP < 0.05, compared with the control group rats; bP < 0.05 and dP < 0.01, compared with the AIA group rats; cP < 0.05 and eP < 0.01, compared with the AIA + MTX group rats.
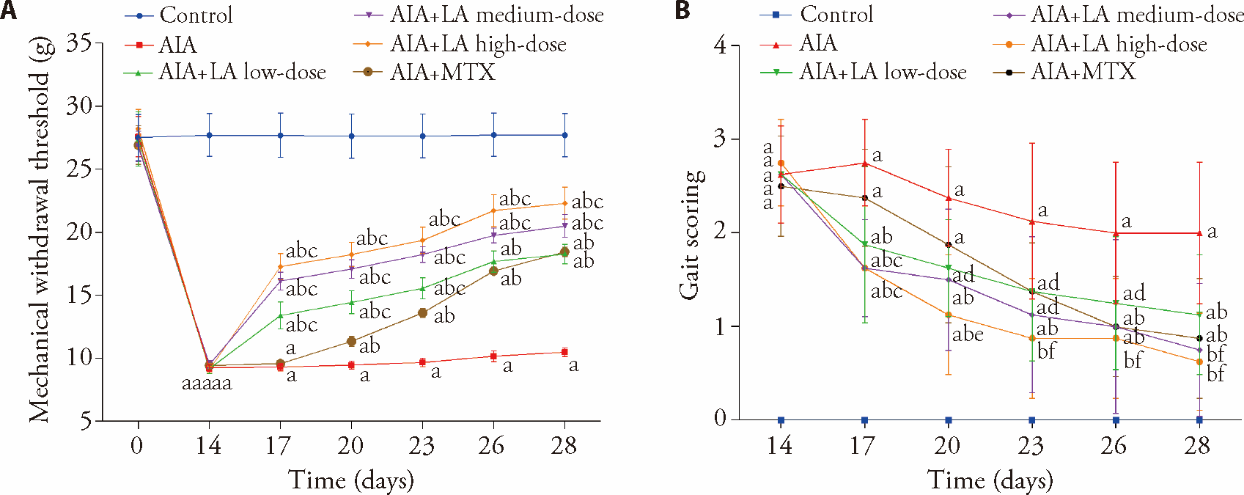
Figure 2 Effect of LA on the antinociceptive activity of AIA rats A: measurement of hyperalgesia in rats by mechanical withdrawal threshold; B: semi-quantitative assessment of arthritis pain in rats by gait soring. Control: normal group and treated with 4 mL·kg-1·d-1 of NS for 14 d; AIA: treated with 4 mL·kg-1·d-1 of NS for 14 d; AIA + LA low-dose: treated with 2 mg·kg-1·d-1 of LA for 14 d; AIA + LA medium-dose: treated with 4 mg·kg-1·d-1 of LA for 14 d; AIA + LA high-dose: treated with 8 mg·kg-1·d-1 of LA for 14 d; AIA + MTX: treated with 0.5 mg/kg per 3 d of MTX for 14 d. LA: lappaconitine; AIA: adjuvant induced arthritis; MTX: methotrexate; NS: normal saline. Data are represented as mean ± standard deviation (n = 8). The groups were compared using a one-way analysis of variance followed by Tukey's multiple comparisons test. aP < 0.01 and fP < 0.05, compared with the control group rats; bP < 0.01 and dP < 0.05, compared with the AIA group rats; cP < 0.01 and eP < 0.05, compared with the AIA + MTX group rats.

Figure 3 Effects of LA on the histopathology of AIA joints by HE staining A-F: representative histological changes of hematoxylin and eosin-stained sections of the right hind ankle joints (scale bar: 200 μm). A: control group; B: AIA group; C: AIA + LA low-dose group; D: AIA + LA medium-dose group; E: AIA + LA high-dose group; F: AIA + MTX group. Red arrow: pannus formation; black arrow: bone erosion; golden arrow: inflammatory cell infiltration; blue arrow: cartilage destruction; JS: joint space; HP: synovial proliferation. G: histopathological scores of right hind ankle joints. Control: normal group and treated with 4 mL·kg-1·d-1 of NS for 14 d; AIA: treated with 4 mL·kg-1·d-1 of NS for 14 d; AIA + LA low-dose: treated with 2 mg·kg-1·d-1 of LA for 14 d; AIA + LA medium-dose: treated with 4 mg·kg-1·d-1 of LA for 14 d; AIA + LA high-dose: treated with 8 mg·kg-1·d-1 of LA for 14 d; AIA + MTX: treated with 0.5 mg/kg per 3 d of MTX for 14 d. LA: lappaconitine; AIA: adjuvant induced arthritis; MTX: methotrexate; NS: normal saline; HE: hematoxylin and eosin. Data are expressed as the mean ± standard deviation (n = 5). The groups were compared using a one-way analysis of variance followed by Tukey's multiple comparisons test. aP < 0.01, compared with the control group rats; bP < 0.01, compared with the AIA group rats.
| Group | n | IL-1β mMRA relative expression | iNOS mMRA relative expression | Arg1mMRA relative expression |
|---|---|---|---|---|
| Control | 5 | 1.27±0.92 | 1.72±1.32 | 1.41±1.22 |
| AIA | 5 | 15.78±4.06a | 15.85±4.25a | 1.94±0.68 |
| AIA+LA low-dose | 5 | 7.16±0.84ab | 4.29±1.76b | 2.21±0.39 |
| AIA+LA medium-dose | 5 | 5.16±1.18ab | 2.71±0.28b | 4.71±0.37ab |
| AIA+LA high-dose | 5 | 3.36±0.29b | 2.21±0.64b | 12.28±1.09ab |
| AIA+MTX | 5 | 1.93±0.14b | 1.55±0.50b | 24.96±0.96ab |
Table 1 Effect of LA on the expression of macrophage M1 and M2 biomarkers in synovial tissues ($\bar{x}±s$)
| Group | n | IL-1β mMRA relative expression | iNOS mMRA relative expression | Arg1mMRA relative expression |
|---|---|---|---|---|
| Control | 5 | 1.27±0.92 | 1.72±1.32 | 1.41±1.22 |
| AIA | 5 | 15.78±4.06a | 15.85±4.25a | 1.94±0.68 |
| AIA+LA low-dose | 5 | 7.16±0.84ab | 4.29±1.76b | 2.21±0.39 |
| AIA+LA medium-dose | 5 | 5.16±1.18ab | 2.71±0.28b | 4.71±0.37ab |
| AIA+LA high-dose | 5 | 3.36±0.29b | 2.21±0.64b | 12.28±1.09ab |
| AIA+MTX | 5 | 1.93±0.14b | 1.55±0.50b | 24.96±0.96ab |
| Group | n | Serum TNF-α | Serum IL-1β | Serum IL-10 | Serum IL-18 |
|---|---|---|---|---|---|
| Control | 6 | 16.1±2.4 | 10.0±1.2 | 235.1±13.4 | 50.0±3.9 |
| AIA | 6 | 192.7±10.3a | 48.2±4.2a | 97.6±11.8a | 165.5±13.1a |
| AIA+LA low-dose | 6 | 146.7±9.9abc | 33.9±3.3abc | 141.3±10.12abc | 132.8±8.1abc |
| AIA+LA medium-dose | 6 | 126.6±10.8abc | 26.1±3.7abc | 162.5±12.7abc | 117.7±8.19abc |
| AIA+LA high-dose | 6 | 114.1±9.5ab | 21.97±2.43ab | 176.4±12.4abc | 105.3±7.7ab |
| AIA+MTX | 6 | 99.17±8.11ab | 21.01±2.52ab | 197.3±14.9ab | 100.9±8.5ab |
Table 2 Effects of LA on inflammatory cytokines in the serum of the rat AIA model (pg/mL, $\bar{x}±s$)
| Group | n | Serum TNF-α | Serum IL-1β | Serum IL-10 | Serum IL-18 |
|---|---|---|---|---|---|
| Control | 6 | 16.1±2.4 | 10.0±1.2 | 235.1±13.4 | 50.0±3.9 |
| AIA | 6 | 192.7±10.3a | 48.2±4.2a | 97.6±11.8a | 165.5±13.1a |
| AIA+LA low-dose | 6 | 146.7±9.9abc | 33.9±3.3abc | 141.3±10.12abc | 132.8±8.1abc |
| AIA+LA medium-dose | 6 | 126.6±10.8abc | 26.1±3.7abc | 162.5±12.7abc | 117.7±8.19abc |
| AIA+LA high-dose | 6 | 114.1±9.5ab | 21.97±2.43ab | 176.4±12.4abc | 105.3±7.7ab |
| AIA+MTX | 6 | 99.17±8.11ab | 21.01±2.52ab | 197.3±14.9ab | 100.9±8.5ab |
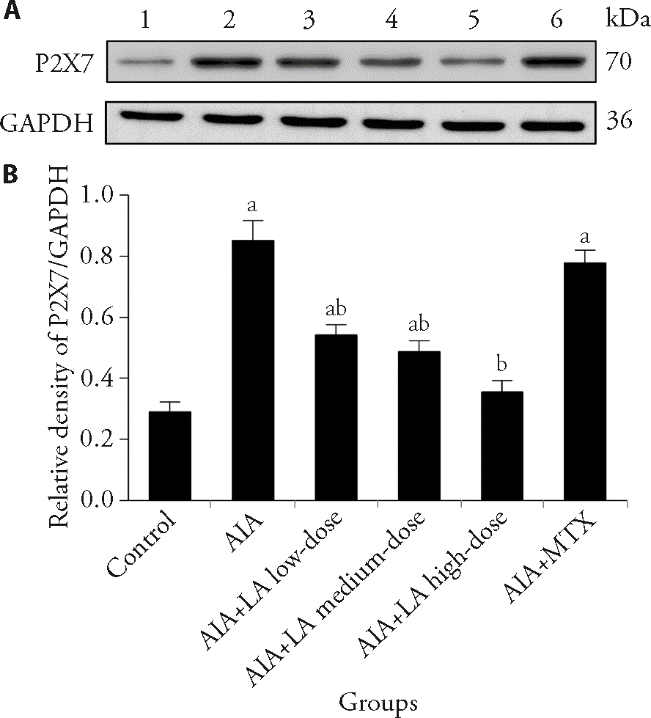
Figure 4 Effect of LA on the protein expression of P2X7r in synovial tissues by Western blotting A: Western blotting representative images of P2X7 in each group; B: relative protein expression levels of P2X7 in each group. 1: Control group; 2: AIA group; 3: AIA + LA low-dose group; 4: AIA + LA medium-dose group; 5: AIA + LA high-dose group; 6: AIA + MTX group. Control: normal group and treated with 4 mL·kg-1·d-1 of NS for 14 d; AIA: treated with 4 mL·kg-1·d-1 of NS for 14 d; AIA + LA low-dose: treated with 2 mg·kg-1·d-1 of LA for 14 d; AIA + LA medium-dose: treated with 4 mg·kg-1·d-1 of LA for 14 d; AIA + LA high-dose: treated with 8 mg·kg-1·d-1 of LA for 14 d; AIA + MTX: treated with 0.5 mg/kg per 3 d of MTX for 14 d. LA: lappaconitine; AIA: adjuvant induced arthritis; MTX: methotrexate; NS: normal saline; GAPDH: glyceraldehyde-3-phosphate dehydrogenase. Data are expressed as the mean ± standard deviation (n = 6). The groups were compared using a one-way analysis of variance followed by Tukey's multiple comparisons test. Compared with the control group, aP < 0.01; compared with the AIA group, bP < 0.01.
| 1. |
Finckh A, Gilbert B, Hodkinson B, et al. Global epidemiology of rheumatoid arthritis. Nat Rev Rheumatol 2022; 18: 591-602.
DOI PMID |
| 2. | Christenson K, Björkman L, Tängemo C, Bylund J. Serum amyloid A inhibits apoptosis of human neutrophils via a P2X7-sensitive pathway independent of formyl peptide receptor-like 1. J Leukoc Biol 2008; 83: 139-48. |
| 3. | Dudics S, Langan D, Meka RR, et al. Natural products for the treatment of autoimmune arthritis: their mechanisms of action, targeted delivery, and interplay with the host microbiome. Int J Mol Sci 2018; 19: 2508. |
| 4. | Radner H, Yoshida K, Mjaavatten MD, et al. Development of a multimorbidity index: impact on quality of life using a rheumatoid arthritis cohort. Semin Arthritis Rheum 2015; 45: 167-73. |
| 5. | Krane SM, Simon LS. Rheumatoid arthritis: clinical features and pathogenetic mechanisms. Med Clin North Am 1986; 70: 263-84. |
| 6. |
Akhtari M, Jalal Zargar S, Javinani A, et al. Prototypic P2X7 receptor agonist, BzATP, induced the expression of unfolded protein response genes in human M1 macrophages. Iran J Allergy Asthma Immunol 2022; 21: 73-80.
DOI PMID |
| 7. |
Gui X, Wang H, Wu L, et al. Botulinum toxin type A promotes microglial M2 polarization and suppresses chronic constriction injury-induced neuropathic pain through the P2X7 receptor. Cell Biosci 2020; 10: 45.
DOI PMID |
| 8. | Nieto FR, Clark AK, Grist J, Hathway GJ, Chapman V, Malcangio M. Neuron-immune mechanisms contribute to pain in early stages of arthritis. J Neuroinflammation 2016; 13: 96. |
| 9. |
Guo Q, Wang Y, Xu D, Nossent J, Pavlos NJ, Xu J. Rheumatoid arthritis: pathological mechanisms and modern pharmacologic therapies. Bone Res 2018; 6: 15.
DOI PMID |
| 10. | Tang Y, Xie D, Gong W, Wu H, Qiang Y. Pentahydroxy flavonoid isolated from madhuca indica ameliorated adjuvant-induced arthritis via modulation of inflammatory pathways. Sci Rep 2021; 11: 17971. |
| 11. |
Smolen JS, Landewé RBM, Bijlsma JWJ, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis 2020; 79: 685-99.
DOI PMID |
| 12. |
Tong L, Nanjundaiah SM, Venkatesha SH, Astry B, Yu H, Moudgil KD. Pristimerin, a naturally occurring triterpenoid, protects against autoimmune arthritis by modulating the cellular and soluble immune mediators of inflammation and tissue damage. Clin Immunol 2014; 155: 220-30.
DOI PMID |
| 13. | Venkatesha SH, Berman BM, Moudgil KD. Herbal medicinal products target defined biochemical and molecular mediators of inflammatory autoimmune arthritis. Bioorg Med Chem 2011; 19: 21-9. |
| 14. |
Pang L, Liu CY, Gong GH, Quan ZS. Synthesis, in vitro and in vivo biological evaluation of novel lappaconitine derivatives as potential anti-inflammatory agents. Acta Pharm Sin B 2020; 10: 628-45.
DOI PMID |
| 15. |
Singhuber J, Zhu M, Prinz S, Kopp B. Aconitum in Traditional Chinese Medicine: a valuable drug or an unpredictable risk? J Ethnopharmacol 2009; 126: 18-30.
DOI PMID |
| 16. | Gutser UT, Friese J, Heubach JF, et al. Mode of antinociceptive and toxic action of alkaloids of aconitum spec. Naunyn Schmiedebergs Arch Pharmacol 1998; 357: 39-48. |
| 17. | Tang M, Zhao W, Xing M, et al. Resource allocation strategies among vegetative growth, sexual reproduction, asexual reproduction and defense during growing season of aconitum kusnezoffii Reichb. Plant J 2021; 105: 957-77. |
| 18. |
Shaheen F, Ahmad M, Khan MT, et al. Alkaloids of aconitum laeve and their anti-inflammatory antioxidant and tyrosinase inhibition activities. Phytochemistry 2005; 66: 935-40.
PMID |
| 19. | Yu JZ, Zhang BJ, Jiang XT. Clinical application of lappaconitine hydrobromide. Di Er Jun Yi Da Xue Xue Bao 2005; 26: 822-4. |
| 20. |
Ameri A. The effects of aconitum alkaloids on the central nervous system. Prog Neurobiol 1998; 56: 211-35.
PMID |
| 21. | Zeng W, Shen C, Mo S, et al. The effective treatment of purpurin on inflammation and adjuvant-induced arthritis. Molecules 2023; 28: 366. |
| 22. | Arab HH, Salama SA, Abdelghany TM, et al. Camel milk attenuates rheumatoid arthritis via inhibition of mitogen activated protein kinase pathway. Cell Physiol Biochem 2017; 43: 540-52. |
| 23. |
Ou S, Zhao YD, Xiao Z, Wen HZ, Cui J, Ruan HZ. Effect of lappaconitine on neuropathic pain mediated by P2X3 receptor in rat dorsal root ganglion. Neurochem Int 2011; 58: 564-73.
DOI PMID |
| 24. | Chen F, Shen X, Huang P, Fu H, Jin Y, Wen C. Quantification of lappaconitine in mouse blood by UPLC-MS/MS and its application to a pharmacokinetic study. Biomed Res Int 2019; 6: 6262105. |
| 25. | Lei B, Wang SH, Wang ZM. Lappaconitine inhibits inflammatory pain by inhibiting microglial activation. Yanan Da Xue Xue Bao 2019; 17: 7-10 + 22. |
| 26. | Abdel-Maged AE, Gad AM, Wahdan SA, Azab SS. Efficacy and safety of ramucirumab and methotrexate co-therapy in rheumatoid arthritis experimental model: involvement of angiogenic and immunomodulatory signaling. Toxicol Appl Pharmacol 2019; 380: 114702. |
| 27. |
Abdel Jaleel GA, Azab SS, El-Bakly WM, Hassan A. 'Methyl palmitate attenuates adjuvant induced arthritis in rats by decrease of CD68 synovial macrophages. Biomed Pharmacother 2021; 137: 111347.
DOI PMID |
| 28. | Mathias K, Amarnani A, Pal N, et al. Chronic pain in patients with rheumatoid arthritis. Curr Pain Headache Rep 2021; 25: 59. |
| 29. |
Walsh DA, McWilliams DF. Mechanisms, impact and management of pain in rheumatoid arthritis. Nat Rev Rheumatol 2014; 10: 581-92.
DOI PMID |
| 30. |
Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol 2008; 8: 958-69.
DOI PMID |
| 31. |
Abeles AM, Pillinger MH. The role of the synovial fibroblast in rheumatoid arthritis: cartilage destruction and the regulation of matrix metalloproteinases. Bull NYU Hosp Jt Dis 2006; 64: 20-4.
PMID |
| 32. | Yu Y, Cai W, Zhou J, et al. Anti-arthritis effect of berberine associated with regulating energy metabolism of macrophages through AMPK/ HIF-1α pathway. Int Immunopharmacol 2020; 87: 106830. |
| 33. | Ma Y, Di R, Zhao H, Song R, Zou H, Liu Z. P2X7 receptor knockdown suppresses osteoclast differentiation by inhibiting autophagy and Ca2+/calcineurin signaling. Int Immunopharmacol 2022; 25: 160. |
| 34. |
Chen Z, Bozec A, Ramming A, Schett G. Anti-inflammatory and immune-regulatory cytokines in rheumatoid arthritis. Nat Rev Rheumatol 2019; 15: 9-17.
DOI PMID |
| 35. |
Udalova IA, Mantovani A, Feldmann M. Macrophage heterogeneity in the context of rheumatoid arthritis. Nat Rev Rheumatol 2016; 12: 472-85.
DOI PMID |
| 36. | Liu JH, Zhu YX, Tang XC. Anti-inflammatory and analgesic activities of N-deacetyllappaconitine and lappaconitine. Zhong Guo Yao Li Xue Bao 1987; 8: 301-5. |
| 37. | Li X, Wang X, Li Z, et al. A metabolomic study of the analgesic effect of lappaconitine hydrobromide (LAH) on inflammatory pain. Metabolites 2022; 12: 923. |
| 38. | Ren W, Rubini P, Tang Y, Engel T, Illes P. Inherent P2X7 receptors regulate macrophage functions during inflammatory diseases. Int J Mol Sci 2021; 23: 232. |
| 39. |
Qin J, Zhang X, Tan B, et al. Blocking P2X7-mediated macrophage polarization overcomes treatment resistance in lung cancer. Cancer Immunol Res 2020; 8: 1426-39.
DOI PMID |
| 40. | Wu P, Zhou G, Wu X, Lyu R, Yao J, Wen Q. P2X7 receptor induces microglia polarization to the M1 phenotype in cancer-induced bone pain rat models. Mol Pain 2022; 18: 1-11. |
| 41. |
Aeschlimann D, Knäuper V. P2X7 receptor-mediated TG2 externalization: a link to inflammatory arthritis? Amino Acids 2017; 49: 453-60.
DOI PMID |
| 42. | Baroja-Mazo A, Pelegrín P. Modulating P2X7 receptor signaling during rheumatoid arthritis: new therapeutic approaches for bisphosphonates. J Osteoporos 2012; 3: 408242. |
| 43. | Bevaart L, Vervoordeldonk MJ, Tak PP. Evaluation of therapeutic targets in animal models of arthritis: how does it relate to rheumatoid arthritis? Arthritis Rheum 2010; 62: 2192-205. |
| 44. |
Wiley JS, Sluyter R, Gu BJ, Stokes L, Fuller SJ. The human P2X7 receptor and its role in innate immunity. Tissue Antigens 2011; 78: 321-32.
DOI PMID |
| 45. |
Portales-Cervantes L, Niño-Moreno P, Doníz-Padilla L, et al. Expression and function of the P2X(7) purinergic receptor in patients with systemic lupus erythematosus and rheumatoid arthritis. Hum Immunol 2010; 71: 818-25.
DOI PMID |
| 46. |
Surprenant A, North RA. Signaling at purinergic P2X receptors. Annu Rev Physiol 2009; 71: 333-59.
DOI PMID |
| 47. | Shen HH, Yang YX, Meng X, et al. NLRP3: a promising therapeutic target for autoimmune diseases. Autoimmun Rev 2018; 17: 694-702. |
| 48. |
Di Virgilio F. Liaisons dangereuses: P2X(7) and the inflammasome. Trends Pharmacol Sci 2007; 28: 465-72.
DOI PMID |
| 49. |
Omenetti S, Pizarro TT. The Treg/Th17 axis: a dynamic balance regulated by the gut microbiome. Front Immunol 2015; 6: 639.
DOI PMID |
| 50. | Fasching P, Stradner M, Graninger W, Dejaco C, Fessler J. Therapeutic potential of targeting the Th17/Treg axis in autoimmune disorders. Molecules 2017; 22: 134. |
| 51. | Lopez-Castejon G, Brough D. Understanding the mechanism of IL-1β secretion. Cytokine Growth Factor Rev 2011; 22: 189-95. |
| 52. |
Miossec P. The role of interleukin 1 in the pathogenesis of rheumatoid arthritis. Clin Exp Rheumatol 1987; 5: 305-8.
PMID |
| 53. |
Lopez-Castejon G, Theaker J, Pelegrin P, Clifton AD, Braddock M, Surprenant A. P2X(7) receptor-mediated release of cathepsins from macrophages is a cytokine-independent mechanism potentially involved in joint diseases. J Immunol 2010; 185: 2611-9.
DOI PMID |
| 54. | Wang D, Brömme D. Drug delivery strategies for cathepsin inhibitors in joint diseases. Expert Opin Drug Deliv 2005; 2: 1015-28. |
| 55. |
Adamczyk M, Griffiths R, Dewitt S, Knäuper V, Aeschlimann D. P2X7 receptor activation regulates rapid unconventional export of transglutaminase-2. J Cell Sci. 2015; 128: 4615-28.
DOI PMID |
| 56. | Mousseau M, Burma NE, Lee KY, et al. Microglial pannexin-1 channel activation is a spinal determinant of joint pain. Sci Adv 2018; 4: eaas9846. |
| 57. | Cutolo M, Campitiello R, Gotelli E, Soldano S. The role of M1/M2 macrophage polarization in rheumatoid arthritis synovitis. Front Immunol 2022; 13: 867260. |
| 58. | Fukui S, Iwamoto N, Takatani A, et al. M1 and M2 monocytes in rheumatoid arthritis: a contribution of imbalance of M1/M2 monocytes to osteoclastogenesis. Front Immunol 2018; 8: 1958. |
| 59. | Ambarus CA, Noordenbos T, de Hair MJ, Tak PP, Baeten DL. Intimal lining layer macrophages but not synovial sublining macrophages display an IL-10 polarized-like phenotype in chronic synovitis. Arthritis Res Ther 2012; 14: R74. |
| 60. |
Wang Y, Han CC, Cui D, Li Y, Ma Y, Wei W. Is macrophage polarization important in rheumatoid arthritis? Int Immunopharmacol 2017; 50: 345-52.
DOI PMID |
| 61. | MacKay K, Milicic A, Lee D, et al. Rheumatoid arthritis susceptibility and interleukin 10: a study of two ethnically diverse populations. Rheumatology (Oxford) 2003; 42: 149-53. |
| 62. | Mocellin S, Marincola F, Rossi CR, Nitti D, Lise M. The multifaceted relationship between IL-10 and adaptive immunity: putting together the pieces of a puzzle. Cytokine Growth Factor Rev 2004; 15: 61-76. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||

