Journal of Traditional Chinese Medicine ›› 2024, Vol. 44 ›› Issue (5): 934-943.DOI: 10.19852/j.cnki.jtcm.20231231.001
Previous Articles Next Articles
Effect of phosphatase and tensin homolog-induced putative kinase 1/ E3 ubiquitin ligase Parkin mediated mitochondrial autophagy on chronic kidney disease myocardial injury and the intervention mechanism of Shenshuai recipe (肾衰方)
ZHANG Gedi1, LIU Gengxin1, GUO Min1, LUO Fuli2, YAN Ziyou2( ), GE Wei3(
), GE Wei3( )
)
- 1 School of Graduate, Jiangxi University of Chinese Medicine, Nanchang 330000, China
2 Department of Nephrology, Affiliated Hospital of Jiangxi University of Chinese Medicine, Nanchang 330000, China
3 Department of Anorectal, Affiliated Hospital of Jiangxi University of Chinese Medicine, Nanchang 330000, China
-
Received:2023-06-22Accepted:2023-10-10Online:2024-10-15Published:2023-12-31 -
Contact:Prof. YAN Ziyou, Department of Nephrology, Affiliated Hospital of Jiangxi University of Chinese Medicine, Nanchang, 330000, China. 13970025368@163.com; Prof. GE Wei, Department of Anorectal, Affiliated Hospital of Jiangxi University of Chinese Medicine, Nanchang 330000, China. 303860796@qq.com -
Supported by:Effect of Phosphatase and Tensin Homolog-induced Putative Kinase 1/Parkin Mediated Mitochondrial Autophagy on Myocardial Injury in Chronic Kidney Disease and the Intervention Mechanism of Shenshuai Recipe(81960843);Training Plan for Young and Middle-aged Key Talents Project of Traditional Chinese Medicine of Jiangxi Province (No. [2022] 7)
Cite this article
ZHANG Gedi, LIU Gengxin, GUO Min, LUO Fuli, YAN Ziyou, GE Wei. Effect of phosphatase and tensin homolog-induced putative kinase 1/ E3 ubiquitin ligase Parkin mediated mitochondrial autophagy on chronic kidney disease myocardial injury and the intervention mechanism of Shenshuai recipe (肾衰方)[J]. Journal of Traditional Chinese Medicine, 2024, 44(5): 934-943.
share this article
| Group | n | BUN | SCr |
|---|---|---|---|
| Sham operated | 6 | 8.1±0.6 | 56.9±12.1 |
| Model | 5 | 38.2±11.8a | 122.7±28.9a |
| Benazepril | 6 | 36.1±11.3 | 118.8±28.5 |
| Low dose | 6 | 31.2±9.8 | 102.2±28.7 |
| Medium dose | 6 | 29.1±10.3 | 98.1±26.7 |
| High dose | 6 | 27.3±10.0 | 93.8±27.8 |
Table 1 Comparison of the relative expression of BUN and SCr in serum of rats in each group (
| Group | n | BUN | SCr |
|---|---|---|---|
| Sham operated | 6 | 8.1±0.6 | 56.9±12.1 |
| Model | 5 | 38.2±11.8a | 122.7±28.9a |
| Benazepril | 6 | 36.1±11.3 | 118.8±28.5 |
| Low dose | 6 | 31.2±9.8 | 102.2±28.7 |
| Medium dose | 6 | 29.1±10.3 | 98.1±26.7 |
| High dose | 6 | 27.3±10.0 | 93.8±27.8 |
| Group | n | CK-MB | hs-cTnI |
|---|---|---|---|
| Sham operated | 6 | 1.00±0.14 | 1.00±0.08 |
| Model | 5 | 1.47±0.13a | 1.61±0.17a |
| Benazepril | 6 | 1.13±0.05b | 1.06±0.18b |
| Low dose | 6 | 1.09±0.11b | 1.02±0.14b |
| Medium dose | 6 | 1.05±0.11b | 1.16±0.11b |
| High dose | 6 | 1.11±0.13b | 1.31±0.07b |
Table 2 Comparison of the relative expression of CM-MB and hs-cTnI in serum of rats in each group (ratio,
| Group | n | CK-MB | hs-cTnI |
|---|---|---|---|
| Sham operated | 6 | 1.00±0.14 | 1.00±0.08 |
| Model | 5 | 1.47±0.13a | 1.61±0.17a |
| Benazepril | 6 | 1.13±0.05b | 1.06±0.18b |
| Low dose | 6 | 1.09±0.11b | 1.02±0.14b |
| Medium dose | 6 | 1.05±0.11b | 1.16±0.11b |
| High dose | 6 | 1.11±0.13b | 1.31±0.07b |
| Group | n | EF% | FS% | LVEDD (mm) | LVESD (mm) |
|---|---|---|---|---|---|
| Sham-operated | 6 | 80.1±10.6 | 56.9±13.1 | 7.8±2.0 | 5.2±1.1 |
| Model | 5 | 38.2±3.8a | 30.7±5.9a | 23.5±7.7a | 6.1±1.3 |
| Benazepril | 6 | 49.1±4.3b | 39.8±8.5 | 13.9±3.6b | 5.5±1.2 |
| Low dose | 6 | 43.2±2.8 | 33.2±7.7 | 18.1±4.9 | 5.9±1.1 |
| Medium dose | 6 | 48.1±3.3b | 38.1±6.7 | 16.6±4.6 | 5.1±1.0 |
| High dose | 6 | 50.3±4.3b | 40.8±6.8 | 14.8±3.1b | 5.3±1.1 |
Table 3 Comparison of the cardiac function in each group (
| Group | n | EF% | FS% | LVEDD (mm) | LVESD (mm) |
|---|---|---|---|---|---|
| Sham-operated | 6 | 80.1±10.6 | 56.9±13.1 | 7.8±2.0 | 5.2±1.1 |
| Model | 5 | 38.2±3.8a | 30.7±5.9a | 23.5±7.7a | 6.1±1.3 |
| Benazepril | 6 | 49.1±4.3b | 39.8±8.5 | 13.9±3.6b | 5.5±1.2 |
| Low dose | 6 | 43.2±2.8 | 33.2±7.7 | 18.1±4.9 | 5.9±1.1 |
| Medium dose | 6 | 48.1±3.3b | 38.1±6.7 | 16.6±4.6 | 5.1±1.0 |
| High dose | 6 | 50.3±4.3b | 40.8±6.8 | 14.8±3.1b | 5.3±1.1 |
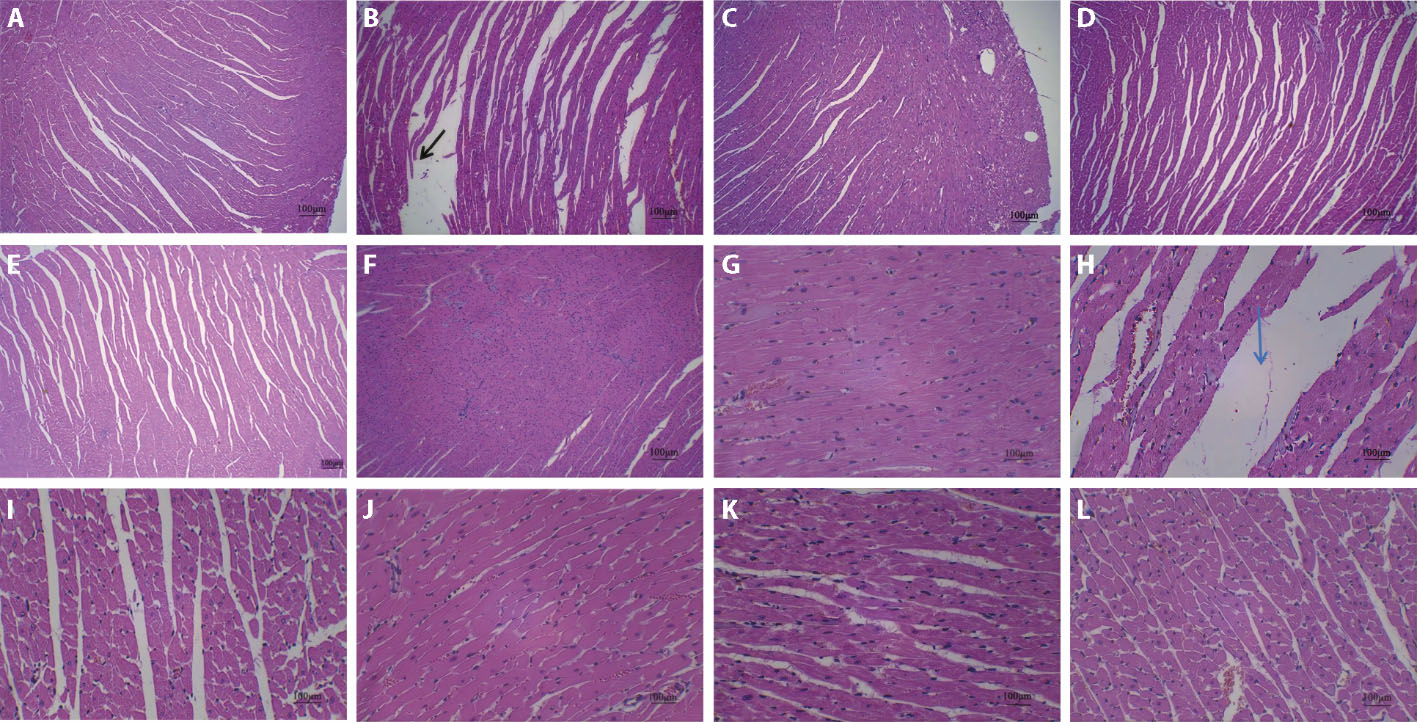
Figure 1 Pathology of rats in each group after HE staining A: sham-operated group (× 40); B: Model group (× 40); C: Benazepril group (× 40); D: Low dose group (× 40); E: Meduim dose group (× 40); F: High dose group (× 40);G: sham-operated group (× 200); H: Model group (× 200); I: Benazepril group (× 200); J: Low dose group (× 200); K: Meduim dose group (× 200); L: High dose group (× 200). Sham-operated groups and Model group were given normal saline 2 mL per day for 6 weeks; Benazepril group was given atorvastatin at 1.5 mg·kg-1·d-1 by gavage for 6 weeks; Low dose group (9.75 g·kg-1·d-1), Medium dose group (19.5 g·kg-1·d-1) and High dose group (39 g·kg-1·d-1) were given the corresponding dose by gavage for 6 weeks. Black arrow: myocardial fibers are loose and broken; Blue arrow:fibrous stroma is widened.
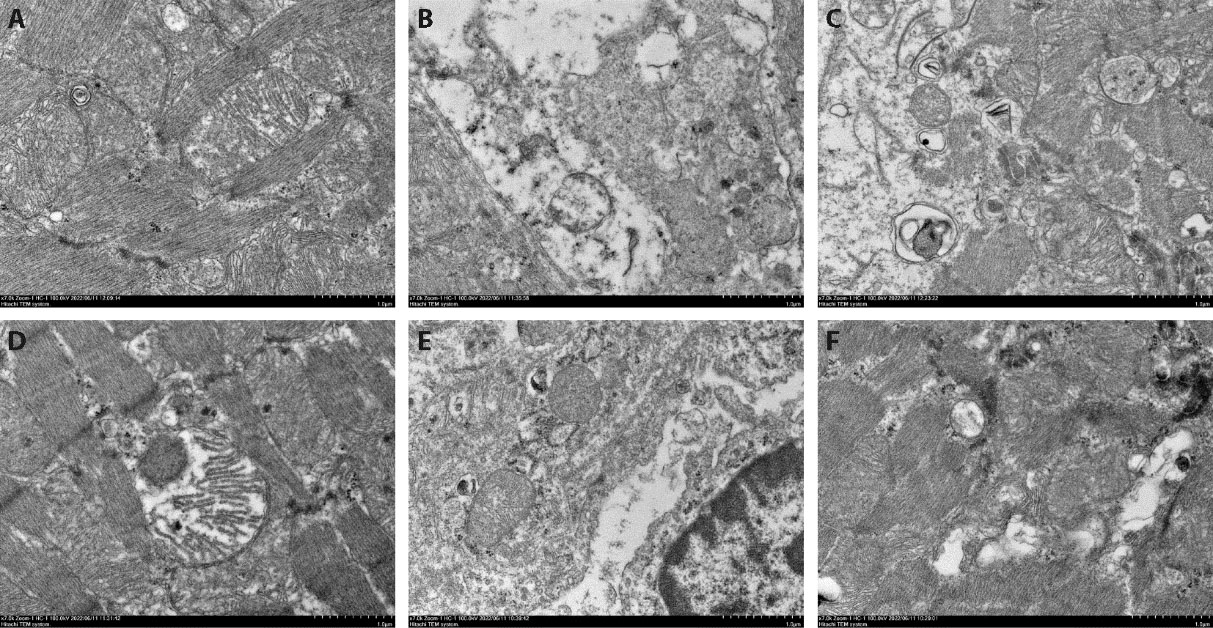
Figure 2 Autophagosomes and autophagiclysosomes of rats in each group under electron microscope A: sham-operated group (× 7000); B: Model group (× 7000); C: Benazepril group (× 7000); D: Low dose group (× 7000); E: Meduim dose group (× 7000); F: High dose group (× 7000). Sham-operated groups and Model group were given normal saline 2 mL per day for 6 weeks; Benazepril group was given atorvastatin at 1.5 mg·kg-1·d-1 by gavage for 6 weeks; Low dose group (9.75 g·kg-1·d-1), Medium dose group (19.5 g·kg-1·d-1) and High dose group (39 g·kg-1·d-1) were given the corresponding dose by gavage for 6 weeks. Red arrow: autophagosomes; Blue arrow: autophagiclysosomes.
| Group | n | Autophagosome | Autophagolysosome |
|---|---|---|---|
| Sham-operated | 6 | 13.6±4.9 | 3.0±1.0 |
| Model | 5 | 11.0±3.0 | 3.0±2.0 |
| Benazepril | 6 | 23.0±4.3a | 12.6±3.2a |
| Low dose | 6 | 14.3±7.2 | 10.6±1.1a |
| Medium dose | 6 | 24.6±1.53a | 18.0±3.0a |
| High dose | 6 | 31.3±5.0a | 16.6±2.3a |
Table 4 Comparison of the number of autophagic bodies and autophagiclysosomes of rats in each group (
| Group | n | Autophagosome | Autophagolysosome |
|---|---|---|---|
| Sham-operated | 6 | 13.6±4.9 | 3.0±1.0 |
| Model | 5 | 11.0±3.0 | 3.0±2.0 |
| Benazepril | 6 | 23.0±4.3a | 12.6±3.2a |
| Low dose | 6 | 14.3±7.2 | 10.6±1.1a |
| Medium dose | 6 | 24.6±1.53a | 18.0±3.0a |
| High dose | 6 | 31.3±5.0a | 16.6±2.3a |
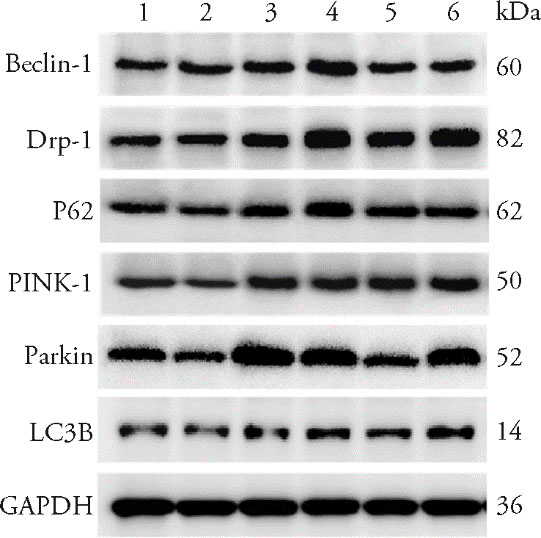
Figure 3 Western blot strips of proteins in each group 1 : Sham-operated group, 2; Model group, 3: Benazepril group, 4: Low dose group, 5: Medium group, 6; High dose group. Sham-operated groups and Model group were given normal saline 2 mL per day for 6 weeks; Benazepril group was given atorvastatin at 1.5 mg·kg-1·d-1 by gavage for 6 weeks; Low dose group (9.75 g·kg-1·d-1), Medium dose group (19.5 g·kg-1·d-1) and High dose group (39 g·kg-1·d-1) were given the corresponding dose by gavage for 6 weeks.

Figure 4 Immunohistochemical expression pictures of PINK1, Parkin, LC3B and P62 A: PINK1, B: Parkin, C: LC3B, D: P62. A1, B1, C1, D1: Sham-operated group; A2, B2, C2, D2: Model group; A3, B3, C3, D3: Benazepril group; A4, B4, C4, D4: Low dose group; A5, B5, C5, D5: Medium group; A6, B6, C6, D6: High dose group. Sham-operated groups and Model group were given normal saline 2 mL per day for 6 weeks; Benazepril group was given atorvastatin at 1.5 mg·kg-1·d-1 by gavage for 6 weeks; Low dose group (9.75 g·kg-1·d-1), Medium dose group (19.5 g·kg-1·d-1) and High dose group (39 g·kg-1·d-1) were given the corresponding dose by gavage for 6 weeks. PINK1: phosphatase and tensin homolog-induced putative kinase 1; Parkin: E3 ubiquitin ligase Parkin; LC3B: microtubule-associated protein1 light chain 3 Ⅱ; P62: sequestosome 1.
| Group | n | PINK1 | Parkin | LC3B | P62 | Beclin-1 | Drp-1 |
|---|---|---|---|---|---|---|---|
| Sham-operated | 6 | 1.04±0.33 | 1.11±0.22 | 1.11±0.20 | 0.86±0.15 | 0.76±0.09 | 1.00±0.23 |
| Model | 5 | 0.58±0.08a | 0.41±0.23a | 0.19±0.04a | 1.49±0.43a | 1.33±0.32a | 1.61±0.08a |
| Benazepril | 6 | 0.99±0.05b | 1.00±0.35b | 0.70±0.05b | 0.59±0.10b | 0.52±0.11b | 0.84±0.05b |
| Low dose | 6 | 1.00±0.17b | 1.30±0.41b | 0.57±0.24b | 0.64±0.04b | 0.67±0.08b | 1.34±0.04b |
| Medium dose | 6 | 0.98±0.13b | 1.40±0.41b | 0.70±0.05b | 0.62±0.16b | 0.22±0.06b | 1.02±0.22b |
| High dose | 6 | 1.06±0.24b | 1.17±0.43b | 0.84±0.10b | 0.24±0.09b | 0.74±0.17b | 1.07±0.16b |
Table 5 Comparison of the relative expression of PINK1, Parkin, LC3B, P62, Beclin-1, and Drp-1 mRNA in myocardial tissue of rats in each group (
| Group | n | PINK1 | Parkin | LC3B | P62 | Beclin-1 | Drp-1 |
|---|---|---|---|---|---|---|---|
| Sham-operated | 6 | 1.04±0.33 | 1.11±0.22 | 1.11±0.20 | 0.86±0.15 | 0.76±0.09 | 1.00±0.23 |
| Model | 5 | 0.58±0.08a | 0.41±0.23a | 0.19±0.04a | 1.49±0.43a | 1.33±0.32a | 1.61±0.08a |
| Benazepril | 6 | 0.99±0.05b | 1.00±0.35b | 0.70±0.05b | 0.59±0.10b | 0.52±0.11b | 0.84±0.05b |
| Low dose | 6 | 1.00±0.17b | 1.30±0.41b | 0.57±0.24b | 0.64±0.04b | 0.67±0.08b | 1.34±0.04b |
| Medium dose | 6 | 0.98±0.13b | 1.40±0.41b | 0.70±0.05b | 0.62±0.16b | 0.22±0.06b | 1.02±0.22b |
| High dose | 6 | 1.06±0.24b | 1.17±0.43b | 0.84±0.10b | 0.24±0.09b | 0.74±0.17b | 1.07±0.16b |
| Group | n | PINK1 | Parkin | LC3B | P62 | Beclin-1 | Drp-1 |
|---|---|---|---|---|---|---|---|
| Sham-operated | 6 | 0.66±0.12 | 0.91±0.16 | 0.99±0.15 | 0.72±0.14 | 0.96±0.19 | 0.62±0.06 |
| Model | 5 | 0.40±0.05a | 0.51±0.01a | 0.42±0.06a | 1.13±0.20a | 1.32±0.22a | 1.04±0.25a |
| Benazepril | 6 | 0.89±0.12b | 0.78±0.04b | 0.77±0.09b | 0.68±0.14b | 0.72±0.19b | 0.50±0.27b |
| Low dose | 6 | 0.66±0.15b | 0.81±0.07b | 0.67±0.19b | 0.54±0.15b | 0.88±0.22b | 0.58±0.05b |
| Medium dose group | 6 | 0.68±0.11b | 0.86±0.08b | 0.76±0.10b | 0.79±0.11b | 0.60±0.11bb | 0.51±0.23b |
| High dose | 6 | 0.64±0.11b | 0.85±0.09b | 0.92±0.12b | 0.39±0.10b | 0.89±0.16bb | 0.34±0.24b |
Table 6 Comparison of the relative expression of PINK1, Parkin, LC3B, P62, Beclin-1, and Drp-1 protein in myocardial tissue of rats in each group (WB,
| Group | n | PINK1 | Parkin | LC3B | P62 | Beclin-1 | Drp-1 |
|---|---|---|---|---|---|---|---|
| Sham-operated | 6 | 0.66±0.12 | 0.91±0.16 | 0.99±0.15 | 0.72±0.14 | 0.96±0.19 | 0.62±0.06 |
| Model | 5 | 0.40±0.05a | 0.51±0.01a | 0.42±0.06a | 1.13±0.20a | 1.32±0.22a | 1.04±0.25a |
| Benazepril | 6 | 0.89±0.12b | 0.78±0.04b | 0.77±0.09b | 0.68±0.14b | 0.72±0.19b | 0.50±0.27b |
| Low dose | 6 | 0.66±0.15b | 0.81±0.07b | 0.67±0.19b | 0.54±0.15b | 0.88±0.22b | 0.58±0.05b |
| Medium dose group | 6 | 0.68±0.11b | 0.86±0.08b | 0.76±0.10b | 0.79±0.11b | 0.60±0.11bb | 0.51±0.23b |
| High dose | 6 | 0.64±0.11b | 0.85±0.09b | 0.92±0.12b | 0.39±0.10b | 0.89±0.16bb | 0.34±0.24b |
| Group | n | PINK1 | Parkin | LC3B | P62 |
|---|---|---|---|---|---|
| Sham-operated | 6 | 183079±20650 | 265533±26591 | 267318±8622 | 38781±5733 |
| Model | 5 | 70026±11298a | 91809±14756a | 67988±10397a | 71151±13587a |
| Benazepril | 6 | 113379±30689b | 161869±23557b | 160131±27944b | 46035±10737b |
| Low dose | 6 | 102011±139b | 141255±13478b | 120706±14396b | 40491±5294b |
| Medium dose | 6 | 127373±18125b | 152109±20738b | 163192±20119b | 32060±9663b |
| High dose | 6 | 140242±22569b | 177205±20136b | 188348±12890b | 21825±7940b |
Table 7 Comparison of the area value of PINK1, Parkin, LC3B and P62 protein in myocardial tissue of rats in each group (IHC,
| Group | n | PINK1 | Parkin | LC3B | P62 |
|---|---|---|---|---|---|
| Sham-operated | 6 | 183079±20650 | 265533±26591 | 267318±8622 | 38781±5733 |
| Model | 5 | 70026±11298a | 91809±14756a | 67988±10397a | 71151±13587a |
| Benazepril | 6 | 113379±30689b | 161869±23557b | 160131±27944b | 46035±10737b |
| Low dose | 6 | 102011±139b | 141255±13478b | 120706±14396b | 40491±5294b |
| Medium dose | 6 | 127373±18125b | 152109±20738b | 163192±20119b | 32060±9663b |
| High dose | 6 | 140242±22569b | 177205±20136b | 188348±12890b | 21825±7940b |
| 1. | Yuan TT, Yao SF. Research progress in the relationship between intestinal metabolite trimethylamine oxide and heart and kidney diseases. Jie Fang Jun Yi Xue Yuan Xue Bao 2021; 42: 978-82. |
| 2. |
Jankowski J, Floege J, Fliser D, Böhm M, Marx N. Cardiovascular disease in chronic kidney disease: pathophysiological insights and therapeutic options. Circulation 2021; 143: 1157-72.
DOI PMID |
| 3. | Li HL, Lip GYH, Feng Q, et al. Sodium-glucose cotransporter 2 inhibitors (SGLT2i) and cardiac arrhythmias: a systematic review and Meta-analysis. Cardiovasc Diabetol 2021; 20: 100. |
| 4. | Chen D, Yu W, Zhong C, et al. Elabela ameliorates doxorubicin-induced cardiotoxicity by promoting autophagic flux through TFEB pathway. Pharmacol Res 2022; 178: 106186. |
| 5. |
Nah J, Zablocki D, Sadoshima J. The role of autophagic cell death in cardiac disease. J Mol Cell Cardiol 2022; 173: 16-24.
DOI PMID |
| 6. | Ikeda S, Zablocki D, Sadoshima J. The role of autophagy in death of cardiomyocytes. J Mol Cell Cardiol 2022; 165: 1-8. |
| 7. | Yan ZY, Yang YQ, Shen JF, et al. Effects of Shenshuai recipe and its disassembled formulas on VEGF, VEGFR2, α-SMA in renal interstitial fibrosis. Zhong Hua Zhong Yi Yao Xue Kan 2020; 38: 10-5, 260-1. |
| 8. | Shen JF, Luo FL, Huang W, et al. Shenshuai recipe and its disassembled prescriptions can prevent renal interstitial fibrosis Wnt4, β-effect of catenin protein expression. Shi Yong Yi Xue Za Zhi 2018; 34: 4050-55. |
| 9. | Yan ZY, He ZY, Wang T, et al. Effect of Shenshuai recipe on the structure and function of hypersensitive C-reactive protein and left heart in patients with chronic kidney disease. Zhong Guo Zhong Xi Yi Jie He Shen Bing Za Zhi 2016; 17: 1084-6. |
| 10. | Jie Z, Li W, Ai-Li C, et al. Huangqi decoction attenuates renal interstitial fibrosis transforming growth factor-β1/mitogen-activated protein kinase signaling pathways in 5/6 nephrectomy mice. J Tradit Chin Med 2022; 42: 723-31. |
| 11. | Zhang XX, Xin X, Wu ZH, et al. Effect of Dahuang Xiezhuo formula on renal protection and PERK/ATF4/CHOP pathway in Rats with 5/6 nephrectomy. Shi Zhen Guo Yi Guo Yao 202; 33: 2908-13. |
| 12. | Zhou J, Wang Z, He Y, et al. Qiliqiangxin reduced cardiomyocytes apotosis and improved heart function in infarcted heart through Pink1/Parkin-mediated mitochondrial autophagy. BMC Complement Med Ther 2020; 20: 203. |
| 13. | Ding XQ, Jian TY, Gai YN, et al. Chicoric acid attenuated renal tubular injury in HFD-induced chronic kidney disease mice through the promotion of mitophagy via the Nrf2/PINK/parkin pathway. J Agric Food Chem 2022; 70: 2923-35. |
| 14. | Xin GJ, Fu JH, Han X, et al. Salvianolic acid B regulates NIX-mediated mitochondrial autophagy to protect H9c2 cardiomyocytes from hypoxia/reoxygenation injury. Zhong Guo Zhong Yao Za Zhi 2022; 45: 2960-5. |
| 15. | Liu H, Zang C, Yuan F, et al. The role of FUNDC 1 in mitophagy, mitochondrial dynamics and human diseases. Biochem Pharmacol 2022; 197: 114891. |
| 16. |
Poole LP, Macleod KF. Mitophagy in tumorigenesis and metastasis. Cell Mol Life Sci 2021; 78: 3817-51.
DOI PMID |
| 17. | Malpartida AB, Williamson M, Narendra DP, et al. Mitochondrial dysfunction and mitophagy in parkinson's disease: from mechanism to therapy. Trends Biochem Sci 2021; 46: 329-43. |
| 18. | Lazarou M, Sliter DA, Kane LA, et al. The ubiquitin kinase PINK1 recruits autophagy receptors to induce mitophagy. Nature 2015; 524: 309-14. |
| 19. | Cao YX, Zheng FR, Wang H, et al. Effect of Shenfu Yixin granule on mitochondrial autophagy of myocardial cells in rats with heart failure after acute myocardial infarction. Zhong Guo Yao Yang 2022; 33: 1183-8. |
| 20. | Li TT, Guo YJ, Ren J, et al. Danggui Buxue decoction improves cardiac function in chronic intermittent hypoxia mice by promoting mitochondrial autophagy and inhibiting cardiomyocyte apoptosis. Zhong Guo Zhong Yao Za Zhi 2022; 47: 3066-72. |
| 21. | Tanki A, Gultekin N, Kucukates E. Evaluation of autophagy and microtubules inhibition through blood beclin-1 levels in subgroups with heart failure reduced ejection fraction. Clin Interventional Cardiol 2021; 1-11. |
| 22. | Tan Y, Huang Y, Mei R, et al. HucMSC-derived exosomes delivered BECN1 induces ferroptosis of hepatic stellate cells via regulating the xCT/GPX4 axis. Cell Death Dis 2022; 13: 319. |
| 23. | Givvimani S, Pushpakumar S, Veeranki S, et al. Dysregulation of Mfn2 and Drp-1 proteins in heart failure. Can J Physiol Pharmacol 2014; 92: 583-91. |
| 24. | Shaoqing L, Ting Z, Hao L, et al. Nicorandil, an ATP-sensitive potassium channel activation, attenuates myocardial injury in rats with ischemic cardiomyopathy. Med Mol Morphol 2022; 55: 41-6. |
| 25. | Wang P, Zheng Q, Hu T, et al. The effect of total saponins of Panax notoginseng on ischemia-reperfusion injury in rats and its effect on myocardial autophagy level. Zhong Guo Zhong Yi Ji Chu Yi Xue Za Zhi 2021; 27: 1249-52+85. |
| 26. | Sun J, Liang XJ. Discussion on the relationship between mitochondrial autophagy and heart failure based on the theory of blood stasis in Traditional Chinese Medicine. Shi Zhen Guo Yi Guo Yao 2022; 33: 2718-20. |
| 27. | Zhang WW. Study on Realgar induced disorder of cortical lipid mass spectrometry and disturbance of autophagy mediated P62-Keap1-Nrf2 axis regulation neurotoxic action pathway. Shenyang: Chinese Medical University, 2021: 1-99. |
| Viewed | ||||||||||||||||||||||||||||||||||||||||||||||||||
|
Full text 67
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||
|
Abstract 53
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||

