Journal of Traditional Chinese Medicine ›› 2023, Vol. 43 ›› Issue (2): 252-264.DOI: 10.19852/j.cnki.jtcm.20230105.003
• Original articles • Previous Articles Next Articles
In vitro assessment of antioxidant, neuroprotective, anti-urease and anti-tyrosinase capacities of Tamarix africana leaves extracts
Esma Anissa Trad Khodja1,2( ), Abd El Hamid Khabtane3, Rabah Arhab4, Djamila Benouchenne5,6, Mohamed Sabri Bensaad7,8, Chawki Bensouici9, Ramazan Erenler10
), Abd El Hamid Khabtane3, Rabah Arhab4, Djamila Benouchenne5,6, Mohamed Sabri Bensaad7,8, Chawki Bensouici9, Ramazan Erenler10
- 1 Biotechnology, Water, Environment and Health Laboratory, Faculty of Natural and Life Sciences, University Abbes Laghrour, Khenchela 40000, Algeria
2 Laboratory of Natural Substances, Biomolecules and Biotechnological Applications, Faculty of Natural and Life Sciences, University Larbi Ben M Hidi, Oum El-Bouaghi 04000, Algeria
3 Biotechnology, Water, Environment and Health Laboratory. Faculty of Natural and Life Sciences, University Abbes Laghrour, Khenchela 40000, Algeria
4 Laboratory of Natural Substances, Biomolecules and Biotechnological Applications, Faculty of Natural and Life Sciences, University Larbi Ben M Hidi, Oum El-Bouaghi 04000, Algeria
5 Genetics, Biochemistry and Plant Biotechnology Laboratory, Faculty of Natural and Life Sciences, University Constantine 1, Constantine 25000, Algeria
6 Pharmaceutical Sciences Research Center, Constantine 25000, Algeria
7 Laboratory of Cellular and Molecular Physio-Toxicology-Pathology and Biomolecules (LPTPCMB), Faculty of Natural and Life Sciences, University Batna 2, Batna 05000, Algeria
8 Laboratory of Biotechnology of Bioactive Molecules and Cellular Physiopathology (LBMBPC), Faculty of Natural and Life Sciences University Batna 2, Batna 05000, Algeria
9 Biotechnology Research Center Ali Mendjli UV 03, Constantine 25000, Algeria
10 Department of Chemistry, Faculty of Science and Literature, University TokatGaziosmanpasa, Tokat 60250, Turkey
-
Received:2022-03-22Accepted:2022-06-18Online:2023-04-15Published:2023-03-14 -
Contact:Esma Anissa Trad Khodja, Biotechnology, Water, Environment and Health Laboratory. Faculty of Natural and Life Sciences, University Abbes Laghrour, Khenchela 40000, Algeria; Laboratory of Natural Substances, Biomolecules and Biotechnological Applications, Faculty of Natural and Life Sciences, University Larbi Ben M Hidi, Oum El-Bouaghi 04000, Algeria. tradkhodja.esma@univ-khenchela.dz. Telephone: +213666712451
Cite this article
Esma Anissa Trad Khodja, Abd El Hamid Khabtane, Rabah Arhab, Djamila Benouchenne, Mohamed Sabri Bensaad, Chawki Bensouici, Ramazan Erenler. In vitro assessment of antioxidant, neuroprotective, anti-urease and anti-tyrosinase capacities of Tamarix africana leaves extracts[J]. Journal of Traditional Chinese Medicine, 2023, 43(2): 252-264.
share this article
| Extracts and fractions | Total phenolic (μg GAE/mg) | Total flavonoids (μg QE/mg) | Total condensed tannin (μg CE/mg) | Total hydrolysable tannin (μg TAE/mg) |
|---|---|---|---|---|
| Methanol | 634.5±3.9 | 188.9±1.6 | 8.8±0.8 | 581.3±4.1 |
| Ethyl acetate | 861.0±2.9 | 334.9±2.5 | 9.7±1.1 | 811.0±4.9 |
| n-butanol | 896.2±3.6 | 399.1±3.0 | 5.9±1.5 | 740.9±3.6 |
| Aqueous | 482.9±2.9 | 117.1±2.1 | 1.0±0.4 | 310.1±2.9 |
Table 1 Phenolic contents of Tamarix africana leaves extracts and fractions ($\bar{x}\pm s$)
| Extracts and fractions | Total phenolic (μg GAE/mg) | Total flavonoids (μg QE/mg) | Total condensed tannin (μg CE/mg) | Total hydrolysable tannin (μg TAE/mg) |
|---|---|---|---|---|
| Methanol | 634.5±3.9 | 188.9±1.6 | 8.8±0.8 | 581.3±4.1 |
| Ethyl acetate | 861.0±2.9 | 334.9±2.5 | 9.7±1.1 | 811.0±4.9 |
| n-butanol | 896.2±3.6 | 399.1±3.0 | 5.9±1.5 | 740.9±3.6 |
| Aqueous | 482.9±2.9 | 117.1±2.1 | 1.0±0.4 | 310.1±2.9 |
| NO | Phenolic substance | Retention Time (min) | MeOH extract (ppb) | Ethyl acetate extract (ppb) | n-BuOH extract (ppb) | Aqueous extract (ppb) |
|---|---|---|---|---|---|---|
| 01 | Tangeretin | 9.088 | ND | ND | ND | ND |
| 02 | Gardenin-B | 9.46 | ND | ND | ND | ND |
| 03 | Gentisic acid | 2.895 | ND | ND | ND | ND |
| 04 | chlorogenic acid | 3.658 | ND | ND | ND | ND |
| 05 | cafic acid | 2.776 | ND | ND | ND | ND |
| 06 | Rutin | 6.512 | ND | ND | 0.3674 | ND |
| 07 | p-Coumaric acid | 4.747 | ND | ND | ND | ND |
| 08 | Ferulic acid | 3.884 | ND | ND | ND | ND |
| 09 | hesperidin | 6.392 | ND | ND | ND | ND |
| 10 | Apigenin 7-O glycoside | 6.796 | ND | ND | 0.1038 | ND |
| 11 | Quercetin | 7.561 | ND | ND | 1.917 | 0.506 |
| 12 | naringenin | 7.441 | ND | ND | ND | ND |
| 13 | kamempferol | 7.943 | ND | ND | ND | ND |
| 14 | protocatechuic acid | 2.855 | ND | ND | ND | ND |
| 15 | gallic acid | 1.149 | ND | ND | ND | ND |
| 16 | 4-OH benzoic acid | 4.403 | ND | ND | ND | ND |
| 17 | 4-OH benzaldehyde | 5.429 | ND | ND | ND | ND |
| 18 | Taxifolin | 5.993 | ND | ND | ND | ND |
| 19 | diosmet | 8.084 | ND | ND | ND | ND |
| 20 | diosmin | 6.734 | 0.0735 | 0.079 | ND | ND |
| 21 | naringin | 6.292 | ND | ND | ND | ND |
| 22 | polydatin | 5.851 | ND | ND | ND | ND |
| 23 | Fisetin | 7.078 | ND | ND | ND | ND |
| 24 | galangin | 8.808 | ND | ND | ND | ND |
| 25 | Quercetin-3-glycoside | 6.573 | ND | 0.037 | 0.9799 | ND |
| 26 | neohesperidin | 6.453 | ND | ND | 0.0608 | ND |
| 27 | apigenin | 8.023 | 0.066 | 0.055 | 0.4448 | 0.09 |
| 28 | Biochanin A | 8.628 | ND | ND | ND | ND |
| 29 | salicylic acid | 4.384 | ND | ND | ND | ND |
| 30 | wogonin | 8.627 | ND | ND | 0.1542 | ND |
| 31 | Sinapic acid | 4.145 | ND | ND | ND | ND |
| 32 | Resveratrol | 6.655 | ND | ND | ND | ND |
| 33 | Cinnamic acid | 5.469 | ND | ND | ND | ND |
| 34 | vanilla acid | 2.223 | ND | ND | ND | ND |
Table 2 Identification and quantification of phenolic compounds in different extracts and fractions of Tamarix africana leaves by LC-MS analysis
| NO | Phenolic substance | Retention Time (min) | MeOH extract (ppb) | Ethyl acetate extract (ppb) | n-BuOH extract (ppb) | Aqueous extract (ppb) |
|---|---|---|---|---|---|---|
| 01 | Tangeretin | 9.088 | ND | ND | ND | ND |
| 02 | Gardenin-B | 9.46 | ND | ND | ND | ND |
| 03 | Gentisic acid | 2.895 | ND | ND | ND | ND |
| 04 | chlorogenic acid | 3.658 | ND | ND | ND | ND |
| 05 | cafic acid | 2.776 | ND | ND | ND | ND |
| 06 | Rutin | 6.512 | ND | ND | 0.3674 | ND |
| 07 | p-Coumaric acid | 4.747 | ND | ND | ND | ND |
| 08 | Ferulic acid | 3.884 | ND | ND | ND | ND |
| 09 | hesperidin | 6.392 | ND | ND | ND | ND |
| 10 | Apigenin 7-O glycoside | 6.796 | ND | ND | 0.1038 | ND |
| 11 | Quercetin | 7.561 | ND | ND | 1.917 | 0.506 |
| 12 | naringenin | 7.441 | ND | ND | ND | ND |
| 13 | kamempferol | 7.943 | ND | ND | ND | ND |
| 14 | protocatechuic acid | 2.855 | ND | ND | ND | ND |
| 15 | gallic acid | 1.149 | ND | ND | ND | ND |
| 16 | 4-OH benzoic acid | 4.403 | ND | ND | ND | ND |
| 17 | 4-OH benzaldehyde | 5.429 | ND | ND | ND | ND |
| 18 | Taxifolin | 5.993 | ND | ND | ND | ND |
| 19 | diosmet | 8.084 | ND | ND | ND | ND |
| 20 | diosmin | 6.734 | 0.0735 | 0.079 | ND | ND |
| 21 | naringin | 6.292 | ND | ND | ND | ND |
| 22 | polydatin | 5.851 | ND | ND | ND | ND |
| 23 | Fisetin | 7.078 | ND | ND | ND | ND |
| 24 | galangin | 8.808 | ND | ND | ND | ND |
| 25 | Quercetin-3-glycoside | 6.573 | ND | 0.037 | 0.9799 | ND |
| 26 | neohesperidin | 6.453 | ND | ND | 0.0608 | ND |
| 27 | apigenin | 8.023 | 0.066 | 0.055 | 0.4448 | 0.09 |
| 28 | Biochanin A | 8.628 | ND | ND | ND | ND |
| 29 | salicylic acid | 4.384 | ND | ND | ND | ND |
| 30 | wogonin | 8.627 | ND | ND | 0.1542 | ND |
| 31 | Sinapic acid | 4.145 | ND | ND | ND | ND |
| 32 | Resveratrol | 6.655 | ND | ND | ND | ND |
| 33 | Cinnamic acid | 5.469 | ND | ND | ND | ND |
| 34 | vanilla acid | 2.223 | ND | ND | ND | ND |

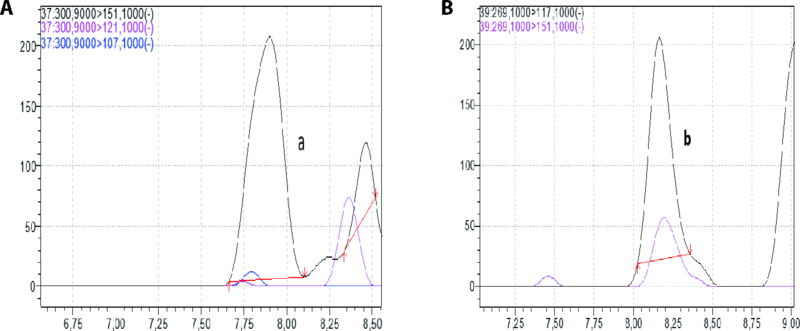
Figure 1 LC-MS chromatograms of Tamarix africana MeOH extract A: apigenin; B: diosmin. LC-MS: liquid chromatography-mass spectrometry; MeOH: methanol extract.
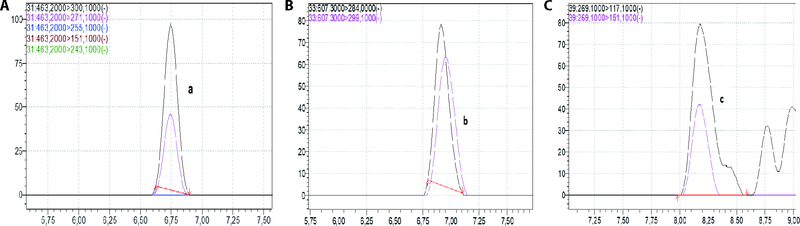
Figure 2 LC-MS chromatograms of Tamarix africana ethyl acetate fraction A: diosmin; B: quercetin-3- glikozit; C: apigenin. LC-MS: liquid chromatography-mass spectrometry.

Figure 3 LC-MS chromatograms of Tamarix africana n-BuOH fraction A: rutin; B: apigenin-7-O-glikozit; C: quercetin-3-glikozit; D: neohesperidin; E: wogonin; F: quercetin; G: apigenin. LC-MS: liquid chromatography-mass spectrometry; n-BuOH: n-butanol fraction.
| Assays | Extracts and Fractions | Dilution (μg/mL) | ||||||
|---|---|---|---|---|---|---|---|---|
| 0.781 | 1.562 | 3.125 | 6.25 | 12.5 | 25 | 50 | ||
| DPPH | MeOH Ethyl acetate n-BuOH Aqueous BHT BHA | 0.16±0.53ab 2.83±0.60cd 16.17±1.09ab 1.95±0.81cd 20.09±2.33 8.52±1.67 | 11.22±0.64ab 15.58±0.18c 32.36±1.02cde 9.31±0.76cde 31.30±1.37 3.97±1.92 | 15.60±0.54a 27.74±0.82d 52.35±1.25ab 15.09±0.08cd 37.71±3.01 12.94±4.21 | 42.27±0.38ce 54.18±0.60ed 87.62±1.26ab 25.76±2.21ce 47.54±0.13 26.68±0.18 | 75.78±0.38a 81.71±1.38ce 88.05±0.83cd 53.42±2.72ce 62.16±2.11 47.12±2.95 | 87.55±0.08a 86.97±0.60b 88.69±0.56b 82.83±0.68a 77.60±0.83 68.69±1.17 | 87.62±0.15a 87.06±0.52a 89.09±1.01ab 87.42±0.51ce 88.33±0.38 83.69±0.54 |
| ABTS | MeOH Ethyl acetate n-BuOH Aqueous BHT BHA | - 9.59±2.75ab 6.65±1.49ce 8.49±0.17cde 35.61±0.75 31.47±1.60 | 1.71±0.11ce 18.62±2.40ab 8.13±0.96cd 13.68±0.43b 58.56±3.22 34.13±1.34 | 55.19±2.71cd 37.41±0.26ab 26.89±1.81ce 26.68±1.57cd 75.57±8.07 40.28±2.88 | 88.59±0.54a 70.36±0.17b 53.58±2.79ns 39.78±0.80a 92.29±1.60 49,71±0.12 | 91.35±0.86b 92.86±0.10ab 90.40±0.63a 87.80±2.20d 93.15±0.19 63.72±2.02 | 92.04±0.57a 93.03±0.49b 91.80±0.97b 90.20±0.17c 94.06±0.63 78.52±0.80 | 92.48±0.00a 93,20±0.10b 93.80±1.09ab 92.37±0.17b 97.31±0.26 96.81±0.37 |
| β-carotene | MeOH Ethyl acetate n-BuOH Aqueous BHT BHA | - 19.00±0.68cd 9.77±0.97ce 0.81±1.95ns 63.76±0.43 65.55±0.80 | 10.76±0.50ab 23.75±0.47cd 16.46±1.20cde 9.31±0.76c 72.52±0.81 74.24±0.24 | 11.62±1.29ab 37.98±1.10c 18.47±0.28ce 15.09±0.08c 81.14±0.84 84.23±1.14 | 20.13±0.46cd 70.88±1.28ce 25.33±1.32ab 25.76±1.21ab 86.0.9±1.04 90.11±0.68 | 26.52±1.02c 88.90±2.09ns 37.80±0.22b 53.42±2.72ns 87.52±4.24 94.59±0.77 | 42.66±0.95c 93.76±1.93ce 61.39±0.01a 82.83±0.68b 91.67±0.52 96.09±0.02 | 71.21±1.95ab 99.67±0.00a 87.27±1.70ab 87.42±0.51a 94.11±0.4 97.35±1.08 |
| GOR | MeOH Ethyl acetate n-BuOH Aqueous BHT BHA | - 2.65±0.58ce 3.23±0.23cd - 27.66±1.62 25.99±2.56 | - 4.73±0.10c 9.82±0.77ce - 39.11±2.34 39.15±0.88 | 7.99±1.03cde 13.89±1.19ab 14.98±0.17c 3.50±1.63cde 58.67±0.94 46.67±0.27 | 30.82±1.61c 45.12±0.45b 40.76±2.06cd 28.58±0.09c 69.65±0.04 62.27±1.40 | 69.21±0.52cd 73.31±0.95ce 67.65±0.86ab 55.67±1.43cd 70.02±0.50 71.46±0.29 | 72.71±0.24a 77.68±1.63b 74.24±1.01ce 76.22±1.22c 72.44±0.23 73.25±0.41 | 74.70±1.20ab 82.49±1.05a 78,01±0.90b 77.71±0,11ce 73.61±0.10 73.78±0.17 |
| CUPRAC | MeOH Ethyl acetate n-BuOH Aqueous BHT BHA | 0.13±0.01ab 0.17±0.01c 0.17±0.01ab 0.13±0.01cd 0.23±0.01 0.33±0.02 | 0.15±0.01ce 0.23±0.03ce 0.20±0.01cd 0.16±0.01ab 0.32±0.01 0.60±0.04 | 0.21±0.03a 0.25±0.02ce 0.25±0.02ab 0.20±0.03cd 0.48±0.03 0.91±0.02 | 0.30±0.01ab 0.35±0.05cde 0.37±0.01b 0.27±0.03cde 0.76±0.02 1.35±0.07 | 0.46±0.05a 0.56±0.08cd 0.57±0.02ce 0.41±0.01ab 1.08±0.05 2.03±0.09 | 0.79±0.05c 0.86±0.04ce 0.86±0.07cde 0.66±0.02ab 1.56±0.08 2.91±0.37 | 1.54±0.16cd 1.37±0.11ab 1.35±0.12ce 1.01±0.05b 2.20±0.03 3.60±0.19 |
| Reducing power | MeOH Ethyl acetate n-BuOH Aqueous BHT BHA Ascorbic acid | 0.17±0.00a 0.14±0.01a 0.18±0.01ab 0.16±0.03ce 0.07±0.00 0.09±0.00 0.09±0.00 | 0.21±0.02c 0.19±0.02ce 0.25±0.05ab 0.17±0.01ce 0.08±0.00 0.11±0.01 0.11±0.00 | 0.28±0.01ce 0.35±0.06 0.40±0.05ab 0.24±0.02ce 0.10±0.01 0.18±0.02 0.16±0.01 | 0.46±0.04ce 0.50±0.03a 0.86±0.03ab 0.29±0.01ab 0.13±0.02 0.36±0.04 0.33±0.04 | 0.69±0.07cd 1.08±0.06 1.00±0.04ce 0.49±0.08ab 0.22±0.04 0.78±0.07 0.76±0.16 | 1.06±0.08ab 1.56±0.05c 1.32±0.09cd 0.89±0.05a 0.28±0.05 1.74±0.07 2.02±0.23 | 1.44±0.23ab 2.63±0.19a 1.82±0.52ce 1.15±0.03c 0.43±0.02 3.53±0.19 3.87±0.27 |
| Phenanthroline | MeOH Ethyl acetate n-BuOH Aqueous BHT BHA | 0.43±0.09ce 0.44±0.03c 0.26±0.01a 0.40±0.01ab 0.47±0.01 0.49±0.01 | 0.51±0.04c 0.46±0.02ce 0.30±0.01a 0.45±0.01a 0.47±0.01 0.59±0.01 | 0.59±0.10ab 0.51±0.02cde 0.37±0.01ab 0.47±0.00a 0.53±0.03 0.73±0.02 | 0.60±0.07b 0.56±0.05c 0.39±0.02ab 0.49±0.00a 1.23±0.02 0.93±0.01 | 0.67±0.04ab 0.62±0.08ce 0.39±0.01ab 0.61±0.04ce 1.84±0.01 1.25±0.04 | 0.97±0.14b 0.79±0.08b 0.41±0.00a 0.84±0.07b 3.48±0.03 2.10±0.05 | 1.28±0.12ab 1.20±0.11ce 0.54±0.00a 1.25±0.22ab 4.84±0.01 4.89±0.06 |
Table 3 Antioxidant capacity (inhibition percentage %) of Tamarix africana extracts and fractions ($\bar{x}\pm s$)
| Assays | Extracts and Fractions | Dilution (μg/mL) | ||||||
|---|---|---|---|---|---|---|---|---|
| 0.781 | 1.562 | 3.125 | 6.25 | 12.5 | 25 | 50 | ||
| DPPH | MeOH Ethyl acetate n-BuOH Aqueous BHT BHA | 0.16±0.53ab 2.83±0.60cd 16.17±1.09ab 1.95±0.81cd 20.09±2.33 8.52±1.67 | 11.22±0.64ab 15.58±0.18c 32.36±1.02cde 9.31±0.76cde 31.30±1.37 3.97±1.92 | 15.60±0.54a 27.74±0.82d 52.35±1.25ab 15.09±0.08cd 37.71±3.01 12.94±4.21 | 42.27±0.38ce 54.18±0.60ed 87.62±1.26ab 25.76±2.21ce 47.54±0.13 26.68±0.18 | 75.78±0.38a 81.71±1.38ce 88.05±0.83cd 53.42±2.72ce 62.16±2.11 47.12±2.95 | 87.55±0.08a 86.97±0.60b 88.69±0.56b 82.83±0.68a 77.60±0.83 68.69±1.17 | 87.62±0.15a 87.06±0.52a 89.09±1.01ab 87.42±0.51ce 88.33±0.38 83.69±0.54 |
| ABTS | MeOH Ethyl acetate n-BuOH Aqueous BHT BHA | - 9.59±2.75ab 6.65±1.49ce 8.49±0.17cde 35.61±0.75 31.47±1.60 | 1.71±0.11ce 18.62±2.40ab 8.13±0.96cd 13.68±0.43b 58.56±3.22 34.13±1.34 | 55.19±2.71cd 37.41±0.26ab 26.89±1.81ce 26.68±1.57cd 75.57±8.07 40.28±2.88 | 88.59±0.54a 70.36±0.17b 53.58±2.79ns 39.78±0.80a 92.29±1.60 49,71±0.12 | 91.35±0.86b 92.86±0.10ab 90.40±0.63a 87.80±2.20d 93.15±0.19 63.72±2.02 | 92.04±0.57a 93.03±0.49b 91.80±0.97b 90.20±0.17c 94.06±0.63 78.52±0.80 | 92.48±0.00a 93,20±0.10b 93.80±1.09ab 92.37±0.17b 97.31±0.26 96.81±0.37 |
| β-carotene | MeOH Ethyl acetate n-BuOH Aqueous BHT BHA | - 19.00±0.68cd 9.77±0.97ce 0.81±1.95ns 63.76±0.43 65.55±0.80 | 10.76±0.50ab 23.75±0.47cd 16.46±1.20cde 9.31±0.76c 72.52±0.81 74.24±0.24 | 11.62±1.29ab 37.98±1.10c 18.47±0.28ce 15.09±0.08c 81.14±0.84 84.23±1.14 | 20.13±0.46cd 70.88±1.28ce 25.33±1.32ab 25.76±1.21ab 86.0.9±1.04 90.11±0.68 | 26.52±1.02c 88.90±2.09ns 37.80±0.22b 53.42±2.72ns 87.52±4.24 94.59±0.77 | 42.66±0.95c 93.76±1.93ce 61.39±0.01a 82.83±0.68b 91.67±0.52 96.09±0.02 | 71.21±1.95ab 99.67±0.00a 87.27±1.70ab 87.42±0.51a 94.11±0.4 97.35±1.08 |
| GOR | MeOH Ethyl acetate n-BuOH Aqueous BHT BHA | - 2.65±0.58ce 3.23±0.23cd - 27.66±1.62 25.99±2.56 | - 4.73±0.10c 9.82±0.77ce - 39.11±2.34 39.15±0.88 | 7.99±1.03cde 13.89±1.19ab 14.98±0.17c 3.50±1.63cde 58.67±0.94 46.67±0.27 | 30.82±1.61c 45.12±0.45b 40.76±2.06cd 28.58±0.09c 69.65±0.04 62.27±1.40 | 69.21±0.52cd 73.31±0.95ce 67.65±0.86ab 55.67±1.43cd 70.02±0.50 71.46±0.29 | 72.71±0.24a 77.68±1.63b 74.24±1.01ce 76.22±1.22c 72.44±0.23 73.25±0.41 | 74.70±1.20ab 82.49±1.05a 78,01±0.90b 77.71±0,11ce 73.61±0.10 73.78±0.17 |
| CUPRAC | MeOH Ethyl acetate n-BuOH Aqueous BHT BHA | 0.13±0.01ab 0.17±0.01c 0.17±0.01ab 0.13±0.01cd 0.23±0.01 0.33±0.02 | 0.15±0.01ce 0.23±0.03ce 0.20±0.01cd 0.16±0.01ab 0.32±0.01 0.60±0.04 | 0.21±0.03a 0.25±0.02ce 0.25±0.02ab 0.20±0.03cd 0.48±0.03 0.91±0.02 | 0.30±0.01ab 0.35±0.05cde 0.37±0.01b 0.27±0.03cde 0.76±0.02 1.35±0.07 | 0.46±0.05a 0.56±0.08cd 0.57±0.02ce 0.41±0.01ab 1.08±0.05 2.03±0.09 | 0.79±0.05c 0.86±0.04ce 0.86±0.07cde 0.66±0.02ab 1.56±0.08 2.91±0.37 | 1.54±0.16cd 1.37±0.11ab 1.35±0.12ce 1.01±0.05b 2.20±0.03 3.60±0.19 |
| Reducing power | MeOH Ethyl acetate n-BuOH Aqueous BHT BHA Ascorbic acid | 0.17±0.00a 0.14±0.01a 0.18±0.01ab 0.16±0.03ce 0.07±0.00 0.09±0.00 0.09±0.00 | 0.21±0.02c 0.19±0.02ce 0.25±0.05ab 0.17±0.01ce 0.08±0.00 0.11±0.01 0.11±0.00 | 0.28±0.01ce 0.35±0.06 0.40±0.05ab 0.24±0.02ce 0.10±0.01 0.18±0.02 0.16±0.01 | 0.46±0.04ce 0.50±0.03a 0.86±0.03ab 0.29±0.01ab 0.13±0.02 0.36±0.04 0.33±0.04 | 0.69±0.07cd 1.08±0.06 1.00±0.04ce 0.49±0.08ab 0.22±0.04 0.78±0.07 0.76±0.16 | 1.06±0.08ab 1.56±0.05c 1.32±0.09cd 0.89±0.05a 0.28±0.05 1.74±0.07 2.02±0.23 | 1.44±0.23ab 2.63±0.19a 1.82±0.52ce 1.15±0.03c 0.43±0.02 3.53±0.19 3.87±0.27 |
| Phenanthroline | MeOH Ethyl acetate n-BuOH Aqueous BHT BHA | 0.43±0.09ce 0.44±0.03c 0.26±0.01a 0.40±0.01ab 0.47±0.01 0.49±0.01 | 0.51±0.04c 0.46±0.02ce 0.30±0.01a 0.45±0.01a 0.47±0.01 0.59±0.01 | 0.59±0.10ab 0.51±0.02cde 0.37±0.01ab 0.47±0.00a 0.53±0.03 0.73±0.02 | 0.60±0.07b 0.56±0.05c 0.39±0.02ab 0.49±0.00a 1.23±0.02 0.93±0.01 | 0.67±0.04ab 0.62±0.08ce 0.39±0.01ab 0.61±0.04ce 1.84±0.01 1.25±0.04 | 0.97±0.14b 0.79±0.08b 0.41±0.00a 0.84±0.07b 3.48±0.03 2.10±0.05 | 1.28±0.12ab 1.20±0.11ce 0.54±0.00a 1.25±0.22ab 4.84±0.01 4.89±0.06 |
| Extracts/Fractions/standards | Antioxydant capacities | ||||||
|---|---|---|---|---|---|---|---|
| DPPH assay IC50 (μg/mL) | ABTS assay IC50 (μg/mL) | β-carotene assay IC50 (μg/mL) | GOR assay IC50 (μg/mL) | Reducing power assay A0.50 (μg/mL) | CUPRAC assay A0.50 (μg/mL) | Phenanthroline assay A0.50 (μg/mL) | |
| Ethylacetate | 5.68±0.06a | 4.34±0.03a | 4.22±0.02a | 7.84±0.20a | 2.73±0.16c | 11.13±2.65a | 0.75±0.23 |
| n-BuOH | 2.91±0.08a | 4.73±0.56a | 19.01±0.12a | 8.92±0.11a | 1.72±0.05c | 10.41±0.43a | 10.44±0.20a |
| MeOH | 7.80±0.11a | 2.01±0.11ns | 31.90±0.71a | 5.99±0.22b | 3.93±0.22c | 13.94±0.72a | 0.20±0.08a |
| Aqueous | 11.49±0.83a | 7.08±0.21a | 21.87±1.28a | 7.44±0.75b | 6.44±0.32c | 17.19±0.92a | 1.52±0.13ns |
| BHA | 6.14±0.41 | 1.81±0.10 | 1.05±0.03 | 5.38±0.06 | 8.41±0.67 | 5.35±0.71 | 0.93±0.07 |
| BHT | 12.99±0.41 | 1.29±0.30 | 0.91±0.01 | 3.32±0.18 | >50 | 8.97±3.94 | 2.24±0.17 |
| Ascorbic acid | NT | NT | NT | NT | 9.01±1.46 | NT | NT |
Table 4 Antioxidant capacities (IC50 and A0.50 μg/mL) of standards and leaves plant extracts and fractions ($\bar{x}\pm s$)
| Extracts/Fractions/standards | Antioxydant capacities | ||||||
|---|---|---|---|---|---|---|---|
| DPPH assay IC50 (μg/mL) | ABTS assay IC50 (μg/mL) | β-carotene assay IC50 (μg/mL) | GOR assay IC50 (μg/mL) | Reducing power assay A0.50 (μg/mL) | CUPRAC assay A0.50 (μg/mL) | Phenanthroline assay A0.50 (μg/mL) | |
| Ethylacetate | 5.68±0.06a | 4.34±0.03a | 4.22±0.02a | 7.84±0.20a | 2.73±0.16c | 11.13±2.65a | 0.75±0.23 |
| n-BuOH | 2.91±0.08a | 4.73±0.56a | 19.01±0.12a | 8.92±0.11a | 1.72±0.05c | 10.41±0.43a | 10.44±0.20a |
| MeOH | 7.80±0.11a | 2.01±0.11ns | 31.90±0.71a | 5.99±0.22b | 3.93±0.22c | 13.94±0.72a | 0.20±0.08a |
| Aqueous | 11.49±0.83a | 7.08±0.21a | 21.87±1.28a | 7.44±0.75b | 6.44±0.32c | 17.19±0.92a | 1.52±0.13ns |
| BHA | 6.14±0.41 | 1.81±0.10 | 1.05±0.03 | 5.38±0.06 | 8.41±0.67 | 5.35±0.71 | 0.93±0.07 |
| BHT | 12.99±0.41 | 1.29±0.30 | 0.91±0.01 | 3.32±0.18 | >50 | 8.97±3.94 | 2.24±0.17 |
| Ascorbic acid | NT | NT | NT | NT | 9.01±1.46 | NT | NT |
| Assays | Extracts/ Fractions/ Standard | Dilution (μg/mL) | ||||||
|---|---|---|---|---|---|---|---|---|
| 3.125 μg | 6.25μg | 12.5 μg | 25 μg | 50 μg | 100 μg | 200 μg | ||
| AChE | Galantamine | 35.93±2.28 | 43.77±0.00 | 68.50±0.31 | 80.69±0.41 | 85.78±1.63 | 91.80±0.20 | 94.77±0.34 |
| MeOH | 33.21±0.72a | 37.96±0.04c | 43.43±2.12b | 47.12±1.31b | 60.52±0.72b | 69.85±1.48b | 76.25±0.17b | |
| AE | NA | 9.05±0.50b | 21.71±1.69b | 25.67±0.18b | 26.68±0.28b | 51.40±0.63b | 64.37±0.76b | |
| n-BuOH | 13.09±0.27b | 23.49±0.27b | 28.09±0.07c | 32.35±0.32b | 35.35±0.72b | 46.45±0.53b | 77.88±0.19b | |
| Aqueous | 30.78±0.17c | 38.48±0.16c | 81.89±0.68c | 57.92±1.91b | 67.09±0.13c | 74.27±0.08b | 74.57±0.38c | |
| BChE | Galantamine | 3.26±0.62 | 6.93±0.62 | 24.03±2.94 | 45.13± 2.60 | 63.87± 2.85 | 73.57± 0.77 | 78.95± 0.58 |
| MeOH | 40.21±0.02b | 47.06±0.14b | 62.97±1.82b | 71.22±0.83b | 76.84±0.96c | 82.76±0.55a | 82.76±0.55c | |
| AE | 2.89±0.0b | 5.09±0.02ns | 10.77±1.77c | 15.50±0.00b | 23.61±1.37b | 54.58±0.60ac | 79.22±0.99a | |
| n-BuOH | 43.09±0.27b | 50.49±0.17b | 58.19±1.07c | 65.35±1.32b | 71.69±0.12c | 81.51±2.02ac | 89.17±0.43b | |
| Aqueous | 10.78±0.16b | 29.48±0.76b | 36.34±0.23c | 49.95±1.90a | 68.08±0.25a | 78.60±0.08b | 81.94±0.75a | |
Table 5 Neuroprotective activity of Tamarix africana extracts and fractions ($\bar{x}\pm s$)
| Assays | Extracts/ Fractions/ Standard | Dilution (μg/mL) | ||||||
|---|---|---|---|---|---|---|---|---|
| 3.125 μg | 6.25μg | 12.5 μg | 25 μg | 50 μg | 100 μg | 200 μg | ||
| AChE | Galantamine | 35.93±2.28 | 43.77±0.00 | 68.50±0.31 | 80.69±0.41 | 85.78±1.63 | 91.80±0.20 | 94.77±0.34 |
| MeOH | 33.21±0.72a | 37.96±0.04c | 43.43±2.12b | 47.12±1.31b | 60.52±0.72b | 69.85±1.48b | 76.25±0.17b | |
| AE | NA | 9.05±0.50b | 21.71±1.69b | 25.67±0.18b | 26.68±0.28b | 51.40±0.63b | 64.37±0.76b | |
| n-BuOH | 13.09±0.27b | 23.49±0.27b | 28.09±0.07c | 32.35±0.32b | 35.35±0.72b | 46.45±0.53b | 77.88±0.19b | |
| Aqueous | 30.78±0.17c | 38.48±0.16c | 81.89±0.68c | 57.92±1.91b | 67.09±0.13c | 74.27±0.08b | 74.57±0.38c | |
| BChE | Galantamine | 3.26±0.62 | 6.93±0.62 | 24.03±2.94 | 45.13± 2.60 | 63.87± 2.85 | 73.57± 0.77 | 78.95± 0.58 |
| MeOH | 40.21±0.02b | 47.06±0.14b | 62.97±1.82b | 71.22±0.83b | 76.84±0.96c | 82.76±0.55a | 82.76±0.55c | |
| AE | 2.89±0.0b | 5.09±0.02ns | 10.77±1.77c | 15.50±0.00b | 23.61±1.37b | 54.58±0.60ac | 79.22±0.99a | |
| n-BuOH | 43.09±0.27b | 50.49±0.17b | 58.19±1.07c | 65.35±1.32b | 71.69±0.12c | 81.51±2.02ac | 89.17±0.43b | |
| Aqueous | 10.78±0.16b | 29.48±0.76b | 36.34±0.23c | 49.95±1.90a | 68.08±0.25a | 78.60±0.08b | 81.94±0.75a | |
| Extracts/Fractions/ standard | Acetylcholinestease Inhibitory Activity | Butyrylcholinesterase inhibitory activity |
|---|---|---|
| Ethylacetate | 100.64±1.77a | 85.42±2.06a |
| n-BuOH | 124.95±0.58a | 7.01±0.28a |
| MeOH | 9.46±0.12b | 8.54±0.32a |
| Aqueous | 28.39±0.62a | 27.14±0.84c |
| Galantamine | 6.27±1.15 | 34.75±1.99 |
Table 6 Neuroprotective activities (IC50 μg/mL) of standard and leaves plant extracts and fractions ($\bar{x}\pm s$)
| Extracts/Fractions/ standard | Acetylcholinestease Inhibitory Activity | Butyrylcholinesterase inhibitory activity |
|---|---|---|
| Ethylacetate | 100.64±1.77a | 85.42±2.06a |
| n-BuOH | 124.95±0.58a | 7.01±0.28a |
| MeOH | 9.46±0.12b | 8.54±0.32a |
| Aqueous | 28.39±0.62a | 27.14±0.84c |
| Galantamine | 6.27±1.15 | 34.75±1.99 |
| Extracts/ Fractions/ Standard | Dilution (μg/mL) | ||||||
|---|---|---|---|---|---|---|---|
| 3.125 μg | 6.25 μg | 12.5 μg | 25 μg | 50 μg | 100 μg | 200 μg | |
| Thiourea | 4.49±0.78 | 19.85±2.74 | 55.64±4.24 | 94.17±0.15 | 98.42±0.19 | 98.49±0.41 | 98.90±0.05 |
| MeOH | 45.01±0.12a | 48.06±0.44a | 52.89±0.85a | 60.89±0.67a | 67.60±1.19b | 85.11±0.09a | 86.33±0.96b |
| AE | 24.15±0.56a | 30.56±0.00a | 36.89±1.03a | 49.90±0.10a | 55.01±0.37a | 63.76±0.04b | 66.89±1.15a |
| n-BuOH | 43.09±0.27a | 49.49±0.10b | 57.10±0.07ns | 60.71±0.08a | 66.02±1.33a | 74.82±1.53b | 82.40±2.71b |
| Aqueous | 18.90±0.06b | 25.89±0.16b | 31.23±0.66c | 38.30±0.49a | 43.17±0.97a | 50.10±1.24a | 57.89±1.59a |
Table 7 Urease inhibitory activity of Tamarix africana extracts and fractions ($\bar{x}\pm s$)
| Extracts/ Fractions/ Standard | Dilution (μg/mL) | ||||||
|---|---|---|---|---|---|---|---|
| 3.125 μg | 6.25 μg | 12.5 μg | 25 μg | 50 μg | 100 μg | 200 μg | |
| Thiourea | 4.49±0.78 | 19.85±2.74 | 55.64±4.24 | 94.17±0.15 | 98.42±0.19 | 98.49±0.41 | 98.90±0.05 |
| MeOH | 45.01±0.12a | 48.06±0.44a | 52.89±0.85a | 60.89±0.67a | 67.60±1.19b | 85.11±0.09a | 86.33±0.96b |
| AE | 24.15±0.56a | 30.56±0.00a | 36.89±1.03a | 49.90±0.10a | 55.01±0.37a | 63.76±0.04b | 66.89±1.15a |
| n-BuOH | 43.09±0.27a | 49.49±0.10b | 57.10±0.07ns | 60.71±0.08a | 66.02±1.33a | 74.82±1.53b | 82.40±2.71b |
| Aqueous | 18.90±0.06b | 25.89±0.16b | 31.23±0.66c | 38.30±0.49a | 43.17±0.97a | 50.10±1.24a | 57.89±1.59a |
| Extracts/ Fractions/ Standard | Dilution (μg/mL) | ||||||
|---|---|---|---|---|---|---|---|
| 3.125 μg | 6.25 μg | 12.5 μg | 25 μg | 50 μg | 100 μg | 200 μg | |
| Kojic acid | 6.91±0.75 | 19.22±0.75 | 36.29±2.92 | 49.46±2.24 | 58.32±0.37 | 64.36±0.65 | 66.95±2.24 |
| MeOH | 37.96±0.54a | 40.99±0.89a | 46.80±1.18a | 48.22±1.79a | 49.52±0.81c | 51.19±0.67b | 59.85±1.79c |
| AE | 42.53±0.00a | 47.11±0.57a | 48.92±1.70a | 49.08±0.57b | 50.50±0.65b | 51.86±0.49b | 52.84±0.65a |
| n-BuOH | 46.52±0.82a | 49.63±0.41a | 50.03±0.94a | 57.47±0.00a | 58.54±0.61b | 61.58±0.25c | 68.69±0.20b |
| Aqueous | 43.66±0.56a | 44.95±0.43a | 45.52±0.49a | 46.51±0.99b | 48.93±0.25c | 50.92±1.23a | 52.35±0.00a |
Table 8 Tyrosinase inhibitory activity of Tamarix africana extracts and fractions ($\bar{x}\pm s$)
| Extracts/ Fractions/ Standard | Dilution (μg/mL) | ||||||
|---|---|---|---|---|---|---|---|
| 3.125 μg | 6.25 μg | 12.5 μg | 25 μg | 50 μg | 100 μg | 200 μg | |
| Kojic acid | 6.91±0.75 | 19.22±0.75 | 36.29±2.92 | 49.46±2.24 | 58.32±0.37 | 64.36±0.65 | 66.95±2.24 |
| MeOH | 37.96±0.54a | 40.99±0.89a | 46.80±1.18a | 48.22±1.79a | 49.52±0.81c | 51.19±0.67b | 59.85±1.79c |
| AE | 42.53±0.00a | 47.11±0.57a | 48.92±1.70a | 49.08±0.57b | 50.50±0.65b | 51.86±0.49b | 52.84±0.65a |
| n-BuOH | 46.52±0.82a | 49.63±0.41a | 50.03±0.94a | 57.47±0.00a | 58.54±0.61b | 61.58±0.25c | 68.69±0.20b |
| Aqueous | 43.66±0.56a | 44.95±0.43a | 45.52±0.49a | 46.51±0.99b | 48.93±0.25c | 50.92±1.23a | 52.35±0.00a |
| 1. |
Dalar A, Mukemre M, Unal M, et al. Traditional medicinal plants of Ağrı province, turkey. J Ethnopharmacol 2018; 226: 56-72.
DOI PMID |
| 2. | Villaverde JJ, Sandín-España P, Sevilla-Morán B, et al. Biopesticides from natural products: current development, legislative framework, and future trends. Bioresources 2016; 11: 5618-40. |
| 3. |
Mahomoodally MF, Vlaisavljevic S, Berezni S, et al. Lotus aegaeus (gris.) boiss and iberis sempervirens l.: chemical fingerprints, antioxidant potential, and inhibition activities and docking on key enzymes linked to global health problems. Ind Crops Prod 2018; 120: 271-8.
DOI URL |
| 4. |
GülçinI, Gören AC, Taslimi P, et al. Anticholinergic, antidiabetic and antioxidant activities of anatolian pennyroyal (menthapulegium)-analysis of its polyphenol contents by lc-ms/ms. Biocatal Agric Biotechnol 2020; 23: 101441.
DOI URL |
| 5. |
Nikousaleh A, Prakash J. Antioxidant components and properties of dry heat treated clove in different extraction solvents. J Food Sci Technol 2016; 53: 1993-2000.
DOI PMID |
| 6. |
Khennouf S, Benabdallah H, Gharzouli K, et al. Effect of tannins from quercussuber and quercuscoccifera leaves on ethanol-induced gastric lesions in mice. J Agric Food Chem 2003; 51: 1469-73.
DOI URL |
| 7. | Benabdallah H, Gharzouli K, Khennouf S, et al. Phytochemical analysis and anti-lipid peroxidation activity of Tamarix africana l. extracts. Glob J Med Plant Res 2014; 3: 278-85. |
| 8. |
Mahfoudhi A, Grosso C, Gonçalves RF, et al. Evaluation of antioxidant, anticholinesterase, and antidiabetic potential of dry leaves and stems in tamarixaphylla growing wild in Tunisia. Chem Biodivers 2016; 13: 1747-55.
DOI PMID |
| 9. |
Rasouli H, Farzaei MH, Khodarahmi R. Polyphenols and them benefits: review. Int J Food Prop 2017; 20: 1700-41.
DOI URL |
| 10. | Bahramsoltani R, Kalkhorani M, Zaidi SM, et al. The genus Tamarix: traditional uses, phytochemistry, and pharmacology. J Ethnopharmaco 2020; 246:112245. |
| 11. | Quattrocchi U. CRC world dictionary of medicinal and poisonous plants: common names, scientificnames, eponyms, synonyms, and etymology (5 volume set). CRC press 2016. |
| 12. | Benabdallah H, Gharzouli K, Khennouf S, et al. Phytochemical analysis and anti-lipid peroxidation activity of Tamarix africana l. extracts. Glob J Med Plant Res 2014; 3: 278-85. |
| 13. |
Lekouaghet A, Boutefnouchet A, Bensuici C, et al. In vitro evaluation of antioxidant and anti-inflammatory activities of the hydroalcoholic extract and its fractions from leuzeaconifera l. roots. S Afr J Bot 2020; 132: 103-7.
DOI URL |
| 14. | Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic 1965; 16: 144-58. |
| 15. |
Müller L, Gnoyke S, Popken AM, et al. Antioxidant capacity and related parameters of different fruit formulations. LWT - Food Sci Technol 2010; 43: 992-23.
DOI URL |
| 16. |
El Aanachi S, Gali L, Nacer SN, et al. Phenolic contents and in vitro investigation of the antioxidant, enzyme inhibitory, photoprotective, and antimicrobial effects of the organic extracts of pelargonium graveolens growing in morocco. Biocatal Agric Biotechnol 2020; 29: 101819.
DOI URL |
| 17. |
Topçu G, Ay M, Bilici A, et al. A new flavone from antioxidant extracts of pistaciaterebinthus. Food Chem 2007; 103: 816-22.
DOI URL |
| 18. |
Saci F, Louaileche H, Gali L, et al. Changes in anticholinesterase, antioxidant activities and related bioactive compounds of carob pulp (ceratonia siliqua l.) during ripening stages. J Food Meas Charact 2020; 14: 937-45.
DOI |
| 19. |
Pierson JT, Curry MC, Shaw PN, et al. Polyphenolic contents and the effects of methanol extracts from mango varieties on breast cancer cells. Food Sci Biotechnol 2015; 24: 265-71.
DOI URL |
| 20. | Karan YB, Balkan T, Erenler R. Phenolic contents of different potato genotypes grown in the central northern region in turkey. Turk J Agri Food Sci Tech 2021; 9: 1606-11. |
| 21. |
Blois MS. Antioxidant determinations by the use of a stable free radical. Nature 1958; 181: 1199-200.
DOI |
| 22. |
Re R, Pellegrini N, Proteggente A, et al. Antioxidant activity applying an improved abts radical cation decolorization assay. Free Radic Biol Med 1999; 26: 1231-7.
DOI URL |
| 23. | Shi H, Noguchi N, Niki E. Galvinoxyl method for standardizing electron and proton donation activity. Methods Enzymol 2001; 335: 157-66. |
| 24. |
Apak R, Güçlü k, Özyürek M, et al. Novel total antioxidant capacity index for dietary polyphenols and vitamins c and e, using their cupric ion reducing capability in the presence of neocuproine: cuprac method. J Agric Food Chem 2004; 52: 7970-81.
DOI URL |
| 25. | Oyaizu M. Studies on products of browning reaction antioxidative activities of products of browning reaction prepared from glucosamine. Jap J Nutri Diet 1986; 44: 307-15. |
| 26. |
Marco GJ. A rapid method for evaluation of antioxidants. J Am Oil Chem Soc 1968; 45: 594-8.
DOI URL |
| 27. |
Szydłowska-Czerniak A, Dianoczki C, Recseg K, et al. Determination of antioxidant capacities of vegetable oils by ferric-ion spectrophotometric methods. Talanta 2008; 76: 899-905.
DOI PMID |
| 28. |
Ellman GL, Courtney KD, Andres V, et al. A new and rapid colorimetric determination of acetylcholinesterase activity. Bioch Pharm 1961; 7: 88-95.
DOI URL |
| 29. |
Taha M, Ullah H, Al Muqarrabun LMR, et al. Bisindolylmethane thiosemicarbazides as potential inhibitors of urease: synthesis and molecular modeling studies. Bioorg Med Chem 2018; 26: 152-160.
DOI URL |
| 30. |
Deveci E, Tel-Çayan G, Duru M E. Phenolic profile, antioxidant, anticholinesterase, and anti-tyrosinase activities of the various extracts of ferulaelaeochytris and sideritis stricta. Int J Food Prop 2018; 21: 771-83.
DOI URL |
| 31. |
Suwal S, Marciniak A. Technologies for the extraction, separation and purification of polyphenols-a review. Nepal J Biotechnol 2018; 6: 74-91.
DOI URL |
| 32. |
Maisetta G, Batoni G, Caboni P, et al. Tannin profile, antioxidant properties, and antimicrobial activity of extracts from two mediterranean species of parasitic plant cytinus. BMC Complement Altern Med 2019; 19: 82.
DOI |
| 33. |
Zou TB, Xia E Q, He TP, et al. Ultrasound-assisted extraction of mangiferin from mango (mangifera indica l.) leaves using response surface methodology. Molecules 2014; 19: 1411-21.
DOI URL |
| 34. |
Jayaprakasha G, Girennavar B, Patil BS. Radical scavenging activities of rio red grapefruits and sour orange fruit extracts in different in vitro model systems. Bioresour Technol 2008; 99: 4484-94.
DOI URL |
| 35. |
Prasad KN, Yang E, Yi C, et al. Effects of high pressure extraction on the extraction yield, total phenolic content and antioxidant activity of longan fruit pericarp. Innov Food Sci Emerg Technol 2009; 10: 155-9.
DOI URL |
| 36. |
Thavamoney N, Sivanadian L, Tee LH. et al. Extraction and recovery of phytochemical components and antioxidative properties in fruit parts of Dacryodes rostrata influenced by different solvents. J Food Sci Technol 2018; 55: 2523-32.
DOI PMID |
| 37. | Benabdallah H, Gharzouli K, Khennouf S, et al. Phytochemical analysis and anti-lipid peroxidation activity of Tamarix africana l. extracts. Glob J Med Plant Res 2014; 3: 278-85. |
| 38. | Halliwell B. Antioxidant characterization: methodology and mechanism. Bioch Pharma 1995; 49: 1341-8. |
| 39. |
Carmona-jiménez Y, García-moreno MV, Igartuburu JM, et al. Simplification of the dpph assay for estimating the antioxidant activity of wine and wine by-products. Food Chem 2014; 165: 198-204.
DOI PMID |
| 40. |
Khabtane A, Zeraib A, Aouidane L, et al. In vitro evaluation of the anti-microbial activity and the anti-oxidant activity of the flavonoids extracted from the flowers of the Tamarix africana Poir. Int J Biosci 2017; 11: 417-26.
DOI URL |
| 41. | Ueno H, Yamakura S, Arastoo RS, et al. Systematic evaluation and mechanistic investigation of antioxidant activity of fullerenols using β-carotene bleaching assay. J Nanomater 2014; 14: 1-7. |
| 42. |
Shahidi F, Zhong Y. Measurement of antioxidant activity. J Funct Foods 2015; 18: 757-81.
DOI URL |
| 43. |
Chekroun-bechlaghem N, Belyagoubi-benhammou N, BelyagoubiL, et al. Phytochemical analysis and antioxidant activity of Tamarix africana, arthrocnemum macrostachyum and suaeda fruticosa, three halophyte species from algeria. Plant Biosyst 2019; 153: 843-52.
DOI |
| 44. |
Wink M. Modes of action of herbal medicines and plant secondary metabolites. Medicines 2015; 2: 251-86.
DOI PMID |
| 45. | langyanai S, Chaniad P, Puripattanavong J. Acetylcholinesterase inhibitoryand antioxidant properties of thai vegetables. Int J Pharm Med Biol Sci 2017; 6: 1-6. |
| 46. |
Bettaib J, Talarmin H, Droguet M, et al. Tamarix gallica phenolics protect IEC-6 cells against H2O2 induced stress by restricting oxidative injuries and MAPKs signaling pathways. Biomed Pharmacother 2017; 89: 490-8.
DOI PMID |
| 47. | Goedert M. Neurodegeneration. alzheimer's and parkinson's 644 diseases: the prion concept in relation to assembled abeta, tau, and alpha-645 synuclein. Science 2015; 349: 646. |
| 48. |
Yakoubi R, Megateli S, Sadok TH. A synergistic interactions of Algerian essential oils of Laurus nobilis L., Lavandula stoechas L. and Mentha pulegium L. on anticholinesterase and antioxidant activities. Biocatal Agric Biotechnol 2021; 31: 101891
DOI URL |
| 49. |
Phan HT, Samarat K, Takamura Y et al. Polyphenols modulate alzheimer’s amyloid beta aggregation in a structure-dependent manner. Nutrients 2019; 11: 756.
DOI URL |
| 50. |
Djermane N, Gali L, Arhab R, et al. Chemical composition and in vitro evaluation of antioxidant, antimicrobial, and enzyme inhibitory activities of erucaria uncata and thymeleae hirsuta. Biocatal Agric Biotechnol 2020; 29: 101834.
DOI URL |
| 51. | Bensaad MS, Dassamiour S, Hambaba L, et al. In vitro assessment of antioxidant, anti-inflammatory, neuroprotective and antimicrobial activities of Centaurea tougourensis Boiss. & Reut. J Pharm Pharmacogn Res 2021; 9: 790-802. |
| 52. |
Murray AP, Faraoni MB, Castro MJ, Alza NP, Cavallaro V. Natural AChE inhibitors from plants and their contribution to Alzheimer’s disease therapy. Curr Neuropharmacol 2013; 11: 388-413.
DOI PMID |
| 53. |
Dyrks T, Dyrks E, Hartmann T, et al. Amyloidogenicity beta a4 and beta a4-bearing amyloid protein precursor fragments by metal-catalyzed oxidation. J Bio Chem 1992; 267: 18210-17.
DOI URL |
| 54. |
Thomas T, Thomas G, Mclendon C, et al. β-amyloid-mediated vasoactivity and vascular endothelial damage. Nature 1996; 380: 168-71.
DOI |
| 55. |
Christen Y. Oxidative stress and alzheimer disease. Am J Clin Nutr 2000; 71: 621s-29s.
PMID |
| 56. |
Kosikowska P, Berlicki L. Urease inhibitors as potential drugs for gastric and urinary tract infections: a patent review. Expert Opin Ther Pat 2011; 21: 945-57.
DOI PMID |
| 57. |
Shabana S, Kawai A, Kai K, et al. Inhibitory activity against urease of quercetin glycosides isolated from allium cepa and psidium guajava. Biosci Biotechnol Biochem 2010; 74: 878-80.
DOI URL |
| 58. |
Hassan STS, Zemlicka M. Plant-derived urease inhibitors as alternative chemotherapeutic agents. Arch Pharm 2016; 349: 507-22.
DOI PMID |
| 59. |
Mahfoudhi A, Grosso C, Gonçalves RF. et al. Evaluation of Antioxidant, Anticholinesterase, and Antidiabetic Potential of Dry Leaves and Stems in Tamarix aphylla Growing Wild in Tunisia. Chem. Biodivers 2016; 13: 1747-55.
DOI PMID |
| 60. |
Iraji A, Panahi Z, Edraki N, et al. Design, synthesis, in vitro and in silico studies of novel schiff base derivative 2-hydroxy-4-methoxybenzamide as tyrosinase inhibitors. Drug Dev Res 2021; 82: 533-42.
DOI URL |
| 61. |
Zaidi SF, Muhammad JS, Shahryar S. Anti-inflammatory and cytoprotective effects of selected Pakistani medicinal plants in Helicobacter pylori-infected gastric epithelial cells. J. Ethnopharmacol 2012; 141: 403-10.
DOI PMID |
| 62. |
Sepehri N, Iraji A, Yavari A, et al. The natural-based optimization of kojic acid conjugated to different thio-quinazolinones as potential anti-melanogenesis agents with tyrosinase inhibitory activity. Bioorg Med Chem 2021; 36: 116044.
DOI URL |
| 63. |
Han H, Yilmaz H, Gulcin I. Antioxidant activity of flaxseed (linum usitatissimum l.) shell and analysis of its polyphenol contents by lc-ms/ms. Rec Nat Prod 2018; 12: 397-402.
DOI URL |
| 64. |
Xu D, Hu MJ, Wang YQ, et al. Antioxidant activities of quercetin and its complexes for medicinal application. Molecules 2019; 24: 1123.
DOI URL |
| 65. |
Tian C, Liu X, Chang Y, et al. Investigation of the anti-inflammatory and antioxidant activities of luteolin, kaempferol, apigenin and quercetin. S Afr J Bot 2021; 137: 257-64.
DOI URL |
| 66. |
Camarda L, Distefano V, Delbosco SF, et al. Antiproliferative activity of citrus juices and hplc evaluation of their flavonoid composition. Fitoterapia 2007; 78: 426-9.
PMID |
| 67. | Zhang H, Tsao R. Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Curr Opin Food Sci 2016; 8: 33-42. |
| 68. | Sharma S, Ali A, Ali J, et al. Rutin: therapeutic potential and recent advances in drug delivery. exp. opinion on inves. Drugs 2013; 22: 1063-79. |
| 69. |
Wang J, Yuan Y, Zhang P, et al. Neohesperidin prevents aβ 25-35-induced apoptosis in primary cultured hippocampal neurons by blocking the s-nitrosylation of protein-disulphide isomerase. Neuroch Res 2018; 43: 1736-44.
DOI |
| 70. |
Hong KB, Han SH, Park Y. Romaine lettuce/skullcap mixture improves sleep behavior in vertebrate models. Biol Pharm Bull 2018; 41: 1269-76.
DOI URL |
| 71. |
Zhang R, Guo L, Ji Z, et al. Radix scutellariae attenuates cums-induced depressive-like behavior by promoting neurogenesis via camp/pka pathway. Neurochem Res 2018; 43: 2111-20.
DOI PMID |
| 72. |
Sharma S, Ali A, Ali J, et al. Rutin: therapeutic potential and recent advances in drug delivery. Expert Opin Investig Drugs 2013; 22: 1063-79.
DOI URL |
| 73. |
Wang J, Yuan Y, Zhang P. et al. Neohesperidin prevents Aβ 25-35-induced apoptosis in primary cultured hippocampal neurons by blocking the S-nitrosylation of protein-disulphide isomerase. Neurochem Res 2018; 43: 1736-44.
DOI |
| 74. |
Hong K B, Han S H, Park Y. et al. Romaine lettuce/skullcap mixture improves sleep behavior in vertebrate models. Biol Pharm Bull 2018; 41: 1269-76.
DOI URL |
| 75. |
Chekroun-Bechlaghem N, Belyagoubi-Benhammou N, Belyagoubi L. et al. Antimicrobial and anti-inflammatory activities of three halophyte plants from Algeria and detection of some biomolecules by HPLC-DAD. Nat Prod Res 2019; 35: 2107-11.
DOI URL |
| [1] | XIE Le, MAO Guo, XIE Yao, CAO Sijia, ZHOU Shen, JIANG Junlin, YAO Ting, FAN Jianhu, LIU Dong, KANG Fuliang, WU Dahua, GE Jinwen. Efficacy of Baishao Luoshi decoction (白芍络石方) on synaptic plasticity in rats with post stroke spasticity [J]. Journal of Traditional Chinese Medicine, 2023, 43(2): 295-302. |
| [2] | Naser Mirazi, Sheida Hesami, Alireza Nourian, Abdolkarim Hosseini. Protective efficacy of dark chocolate in letrozole-induced ovary toxicity model rats: hormonal, biochemical, and histopathological investigation [J]. Journal of Traditional Chinese Medicine, 2022, 42(5): 741-748. |
| [3] | Kamal Dawood, Roohullah, Rabbi Fazle, Naz Attiqa, Bilal Muhammad. In-vitro and in-vivo pharmacological screening of Iris albicans [J]. Journal of Traditional Chinese Medicine, 2022, 42(1): 9-16. |
| [4] | Elham A.Abd-Allah, Nouf S.Al-Abbas, Mona M.Atia, Fawzia Alzahrani, El-Mokhtar M.Ahmed, Soad S.Ali, Soad K.Al Jaouni. Can Fig and Olive Ameliorate the toxicity Induced by 2-nitropropane in some organs of mice? role of inflammatory versus anti-inflammatory genes [J]. Journal of Traditional Chinese Medicine, 2021, 41(6): 891-899. |
| [5] | XIA Xichao, LI Bin, QIU Ju, TIAN Gang, CHEN Changdong, LA Ming, ZHANG Ke, QI Jinxu, LI Yanyan, GAO Huashan, SHAO Xiangyang, SU Congying, WANG Mengqi, OUYANG Jingfeng. Antioxidative and immunological effects of Cyclocarya paliurus polysaccharides on the spleen injury of diabetic rats [J]. Journal of Traditional Chinese Medicine, 2021, 41(5): 739-746. |
| [6] | TANG Chengfang, GAO Yang, Gulibairemu Yusuyin, MAO Yan, LI Yujun, WANG Yandong, GU Zhengyi. Anti-cataract effects of Dajizhi(Euphorbium) eye drops on selenite-induced cataracts in rats [J]. Journal of Traditional Chinese Medicine, 2021, 41(5): 747-752. |
| [7] | Mohsen Akbaribazm, Fatemeh Khazaei, Leila Naseri, Mona Pazhouhi, Mohammad Zamanian, Mozafar Khazaei. Pharmacological and therapeutic properties of the Red Clover(Trifolium pratense L.): an overview of the new findings [J]. Journal of Traditional Chinese Medicine, 2021, 41(4): 642-649. |
| [8] | ?nder Aybast?er;. Efficacy of methanol-water extract of Inula helenium root against oxidative DNA damage [J]. Journal of Traditional Chinese Medicine, 2021, 41(2): 293-300. |
| [9] | KONG Deyan, LUO Jiefeng, SHI Shengliang, HUANG Zhenhua. Efficacy of tanshinone ⅡA and mesenchymal stem cell treatment of learning and memory impairment in a rat model of vascular dementia [J]. Journal of Traditional Chinese Medicine, 2021, 41(1): 133-139. |
| [10] | Hou Jiguang, Fang Fang, Kang Shunai, Wang Zhicheng, Yang Yanming. Curcumin from Jianghuang (Rhizoma Curcumae Longae) protects against exposure to ultraviolet B by antioxidation and attenuating mitochondrion-dependent apoptosis [J]. Journal of Traditional Chinese Medicine, 2020, 40(5): 782-791. |
| [11] | Emel Akta?, Hilal Yildiran. Antioxidant and ntiinflammatory efficacy of curcumin on lung tissue in rats with sepsis [J]. Journal of Traditional Chinese Medicine, 2020, 40(5): 820-826. |
| [12] | Seval Yilmaz, Emre Kaya, Erhan Yilmaz, Ahmet Kavakli, Suleyman Gurbuz, Mustafa Ozkaraca. Effect of acupuncture therapy on fracture healing in rats with femur fractures [J]. Journal of Traditional Chinese Medicine, 2020, 40(2): 275-283. |
| [13] | Yu Lijun, Cao Xinyuan, Tao Wendi, Li Maoxing, Li Xiaolin, Chen Liping. Antioxidant activity and potential ameliorating effective ingredients for high altitude-induced fatigue from Gansu Maxianhao(Pedicularis Kansuensis Maxim.) [J]. Journal of Traditional Chinese Medicine, 2020, 40(1): 83-93. |
| [14] | Ghosia Lutfullah, Asma Shah, Kafeel Ahmad, Jamila Haider. Phytochemical screening, antioxidant and antibacterial properties of daphne mucronata [J]. Journal of Traditional Chinese Medicine, 2019, 39(06): 764-771. |
| [15] | Xia Xichao, Yu Ruixue, Wang Xiaowei, Wei Mengwei, Yi Li, Wang Aimei, Ma Yuhong, Zhang Junfeng, Ji Zhaohui, Li Yuan, Wang Qiong. Role of Eclipta prostrata extract in improving spatial learning and memory deficits in D-galactose-induced aging in rats [J]. Journal of Traditional Chinese Medicine, 2019, 39(05): 649-657. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||