Journal of Traditional Chinese Medicine ›› 2025, Vol. 45 ›› Issue (4): 720-729.DOI: 10.19852/j.cnki.jtcm.2025.04.002
• Original Articles • Previous Articles Next Articles
Dujieqing decoction (毒结清复方) suppresses multiple myeloma growth by inhibiting the Wnt/β-catenin pathway
XU Jiawei1, LU Haisong2, SHI Yushi1, LEI Yu2, LI Xueping2, CHENG Weimin2( )
)
- 1 Graduate School of Guangxi University of Chinese Medicine, Nanning 530200, China
2 Department of Hematology, the First Affiliated Hospital of Guangxi University of Chinese Medicine, Nanning 530200, China
-
Received:2024-06-12Accepted:2024-11-18Online:2025-08-15Published:2025-07-25 -
Contact:CHENG Weimin -
About author:Prof. CHENG Weimin, Department of Hematology, the First Affiliated Hospital of Guangxi University of Chinese Medicine, Nanning 530200, China. cheng5min@126.com,Telephone: +86-771-5848504
-
Supported by:National Science Foundation-Funded Project: based on the Connection Sclerostin with Wnt/β-catenin Pathway to Explored the Effect Mechanism of Dujieqing Oral Liquid on the Inhibit Tumor and Promote Bone Formation in Multiple Myeloma(81960839);Innovation Project of Guangxi Graduate Education-Funded Project: Sclerostin Regulates Wnt/β-catenin Pathway to Explore the Mechanism of Dujieqing Decoction in Inhibiting Tumor Growth and Promoting Osteogenesis of Multiple Myeloma(YCSW2021224);To Investigate the Effect and Mechanism of Dujiangqing Oral Liquid on Osteogenic Differentiation of Mesenchymal Stem Cells in Multiple Myeloma Mice based on Wnt/β-catenin Signaling Pathway(YCSW2023386)
Cite this article
XU Jiawei, LU Haisong, SHI Yushi, LEI Yu, LI Xueping, CHENG Weimin. Dujieqing decoction (毒结清复方) suppresses multiple myeloma growth by inhibiting the Wnt/β-catenin pathway[J]. Journal of Traditional Chinese Medicine, 2025, 45(4): 720-729.
share this article
| Number | Quasi-molecular ion | RT (min) | Calc. MW | Group area | Formula | Identification |
|---|---|---|---|---|---|---|
| 1 | [M+H]+1 | 1.249 | 161.1052 | 7.08E+08 | C7 H15 N O3 | L(-)-Carnitine |
| 2 | [M+H]+1 | 1.462 | 143.0946 | 1.89E+09 | C7 H13 N O2 | DL-Stachydrine |
| 3 | [M+H]+1 | 1.638 | 203.1158 | 1.55E+08 | C9 H17 N O4 | Acetyl-L-carnitine |
| 4 | [M+H]+1 | 2.233 | 122.0483 | 1.27E+09 | C6 H6 N2 O | Nicotinamide |
| 5 | [M-H]-1 | 2.715 | 129.0415 | 5.93E+08 | C5 H7 N O3 | L-Phenylalanine |
| 6 | [M+H]+1 | 5.135 | 165.079 | 5.8E+09 | C9 H11 N O2 | Scopoletin |
| 7 | [M+H]+1 | 11.373 | 192.0425 | 8.53E+08 | C10 H8 O4 | Quercetin |
| 8 | [M+H]+1 | 12.14 | 302.0426 | 4.45E+08 | C15 H10 O7 | Taxifolin |
| 9 | [M+H]+1 | 12.792 | 304.0581 | 7.43E+08 | C15 H12 O7 | Emodin |
| 10 | [M+H]+1 | 13.084 | 270.0528 | 2.98E+08 | C15 H10 O5 | Daidzein |
| 11 | [M+H]+1 | 14.011 | 254.0577 | 6.54E+08 | C15 H10 O4 | Bis(4-ethylbenzylidene)sorbitol |
| 12 | [M+H]+1 | 17.537 | 414.2039 | 2.43E+08 | C24 H30 O6 | Glycodeoxycholic acid |
| 13 | [M-H]-1 | 17.66 | 449.3146 | 18731659 | C26 H43 N O5 | Dipropyleneglycol dibenzoate |
| 14 | [M+Na]+1 | 18.141 | 342.1463 | 2.54E+09 | C20 H22 O5 | Dibutyl phthalate |
| 15 | [M+H]+1 | 18.494 | 278.1516 | 3.17E+09 | C16 H22 O4 | Deoxycholic acid |
| 16 | [M-H]-1 | 19.95 | 392.2927 | 3.53E+08 | C24 H40 O4 | Palmitoleic acid |
| 17 | [M-H]-1 | 21.676 | 254.2244 | 62206233 | C16 H30 O2 | Palmitic acid |
| 18 | [M-H]-1 | 22.599 | 256.2403 | 2.05E+08 | C16 H32 O2 | Di(2-ethylhexyl) phthalate |
| 19 | [M+H]+1 | 23.022 | 390.2765 | 6.99E+08 | C24 H38 O4 | Stearic acid |
| 20 | [M-H]-1 | 23.902 | 284.2717 | 2.01E+08 | C18 H36 O2 | 4-oxoproline |
Table 1 Key components of DJQ-containing serum
| Number | Quasi-molecular ion | RT (min) | Calc. MW | Group area | Formula | Identification |
|---|---|---|---|---|---|---|
| 1 | [M+H]+1 | 1.249 | 161.1052 | 7.08E+08 | C7 H15 N O3 | L(-)-Carnitine |
| 2 | [M+H]+1 | 1.462 | 143.0946 | 1.89E+09 | C7 H13 N O2 | DL-Stachydrine |
| 3 | [M+H]+1 | 1.638 | 203.1158 | 1.55E+08 | C9 H17 N O4 | Acetyl-L-carnitine |
| 4 | [M+H]+1 | 2.233 | 122.0483 | 1.27E+09 | C6 H6 N2 O | Nicotinamide |
| 5 | [M-H]-1 | 2.715 | 129.0415 | 5.93E+08 | C5 H7 N O3 | L-Phenylalanine |
| 6 | [M+H]+1 | 5.135 | 165.079 | 5.8E+09 | C9 H11 N O2 | Scopoletin |
| 7 | [M+H]+1 | 11.373 | 192.0425 | 8.53E+08 | C10 H8 O4 | Quercetin |
| 8 | [M+H]+1 | 12.14 | 302.0426 | 4.45E+08 | C15 H10 O7 | Taxifolin |
| 9 | [M+H]+1 | 12.792 | 304.0581 | 7.43E+08 | C15 H12 O7 | Emodin |
| 10 | [M+H]+1 | 13.084 | 270.0528 | 2.98E+08 | C15 H10 O5 | Daidzein |
| 11 | [M+H]+1 | 14.011 | 254.0577 | 6.54E+08 | C15 H10 O4 | Bis(4-ethylbenzylidene)sorbitol |
| 12 | [M+H]+1 | 17.537 | 414.2039 | 2.43E+08 | C24 H30 O6 | Glycodeoxycholic acid |
| 13 | [M-H]-1 | 17.66 | 449.3146 | 18731659 | C26 H43 N O5 | Dipropyleneglycol dibenzoate |
| 14 | [M+Na]+1 | 18.141 | 342.1463 | 2.54E+09 | C20 H22 O5 | Dibutyl phthalate |
| 15 | [M+H]+1 | 18.494 | 278.1516 | 3.17E+09 | C16 H22 O4 | Deoxycholic acid |
| 16 | [M-H]-1 | 19.95 | 392.2927 | 3.53E+08 | C24 H40 O4 | Palmitoleic acid |
| 17 | [M-H]-1 | 21.676 | 254.2244 | 62206233 | C16 H30 O2 | Palmitic acid |
| 18 | [M-H]-1 | 22.599 | 256.2403 | 2.05E+08 | C16 H32 O2 | Di(2-ethylhexyl) phthalate |
| 19 | [M+H]+1 | 23.022 | 390.2765 | 6.99E+08 | C24 H38 O4 | Stearic acid |
| 20 | [M-H]-1 | 23.902 | 284.2717 | 2.01E+08 | C18 H36 O2 | 4-oxoproline |

Figure 1 Cell viability of RPMI8226 cells following various treatments assessed by CCK-8 assays A: cells treated with FBS (1% and 10%) and RS (5%, 10%, and 20%) for 24 h; B: cells treated with DJQ-CS (0%, 2%, 4%, 8%, 16%, and 20%) for 24 h; C: cells treated with 20% DJQ-CS for 24 h, assessed at different time points (0, 6, 12, 18, and 24 h); D: cells treated with DJQ-CS (0%, 5%, 10%, and 20%) for 24 h; E: cells treated with XAV-939 (0, 1, 2, 4, and 8 μmol/mL) for 24 h; F: cells treated with 2 μmol/mL XAV-939 for 24 h. 1%FBS group: treated with 1%FBS for 24 h; 10%FBS group: treated with 10%FBS for 24 h; 5%RS group: treated with 5%RS for 24 h; 10%RS group: treated with 10%RS for 24 h; 20%RS group: treated with 20%RS for 24 h; Control group: treated with 10%FBS for 24 h; 5%DJQ-CS group: treated with 5%DJQ-CS for 24 h; 10%DJQ-CS group: treated with 10%DJQ-CS for 24 h; 20%DJQ-CS group: treated with 20%DJQ-CS for 24 h; DMSO group: treated with DMSO for 24 h; XAV-939 group: 2 μmol/mL XAV-939 for 24 h. FBS: fetal bovine serum; RS: rat serum; DJQ-CS: Dujieqing-containing serum; DMSO: dimethyl sulfoxide; CCK8: cell counting kit-8. One-way analysis of variance was used to compare more than two groups, followed by the least significant difference test to detect differences between groups. The data are presented as the mean ± standard deviation (n = 3). Compared with 0% DJQ-CS treatment group (control), aP < 0.05; compared with control group; bP < 0.05.
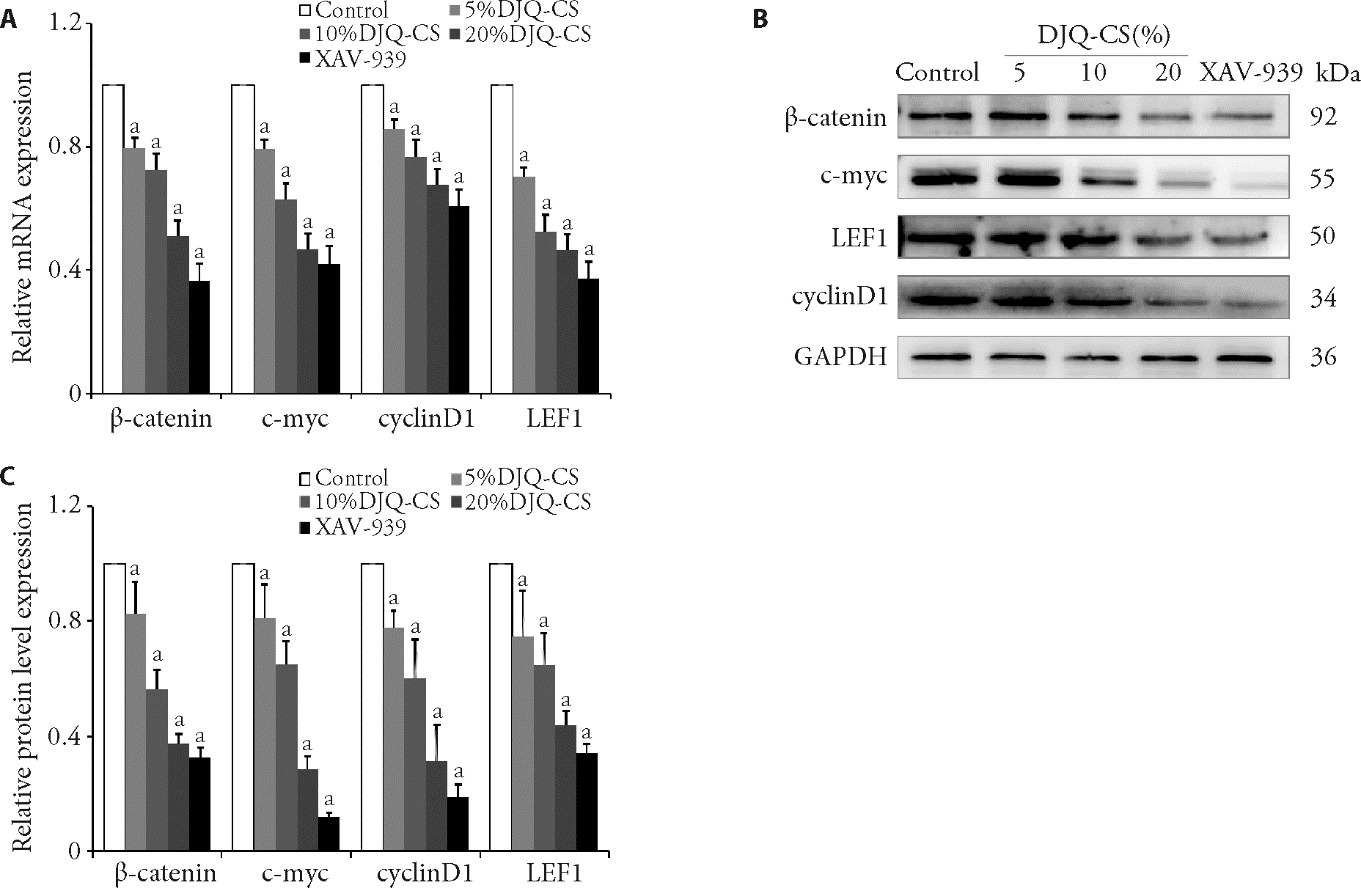
Figure 2 Relative mRNA and protein of Wnt/β-catein of RPMI8226 cells following various treatments assessed by RT-PCR and WB assays A: RT-PCR analysis of β-catenin, c-myc, cyclin D1, and LEF1 mRNA levels after treatment with different doses of DJQ-CS (0%, 5%, 10%, and 20%) and XAV-939 for 24 h; B: WB analysis of β-catenin, c-myc, cyclin D1, and LEF1 protein levels; C: comparison results of β-catenin, c-myc, cyclin D1, and LEF1 protein levels. Control group: treated with 10%FBS for 24 h; 5%DJQ-CS group: treated with 5%DJQ-CS for 24 h; 10%DJQ-CS group: treated with 10%DJQ-CS for 24 h; 20%DJQ-CS group: treated with 20%DJQ-CS for 24 h; XAV-939 group: 2 μmol/mL XAV-939 for 24 h. LEF1: lymphoid enhancer binding factor 1; GAPDH: glyceraldehyde-3-phosphate dehydrogenase; RT-PCR: real-time polymerase chain reaction; WB: western blotting. One-way analysis of variance was used to compare more than two groups, followed by the least significant difference test to detect differences between groups. The data are presented as the mean ± standard deviation (n = 3). Compared with control group, aP < 0.05.
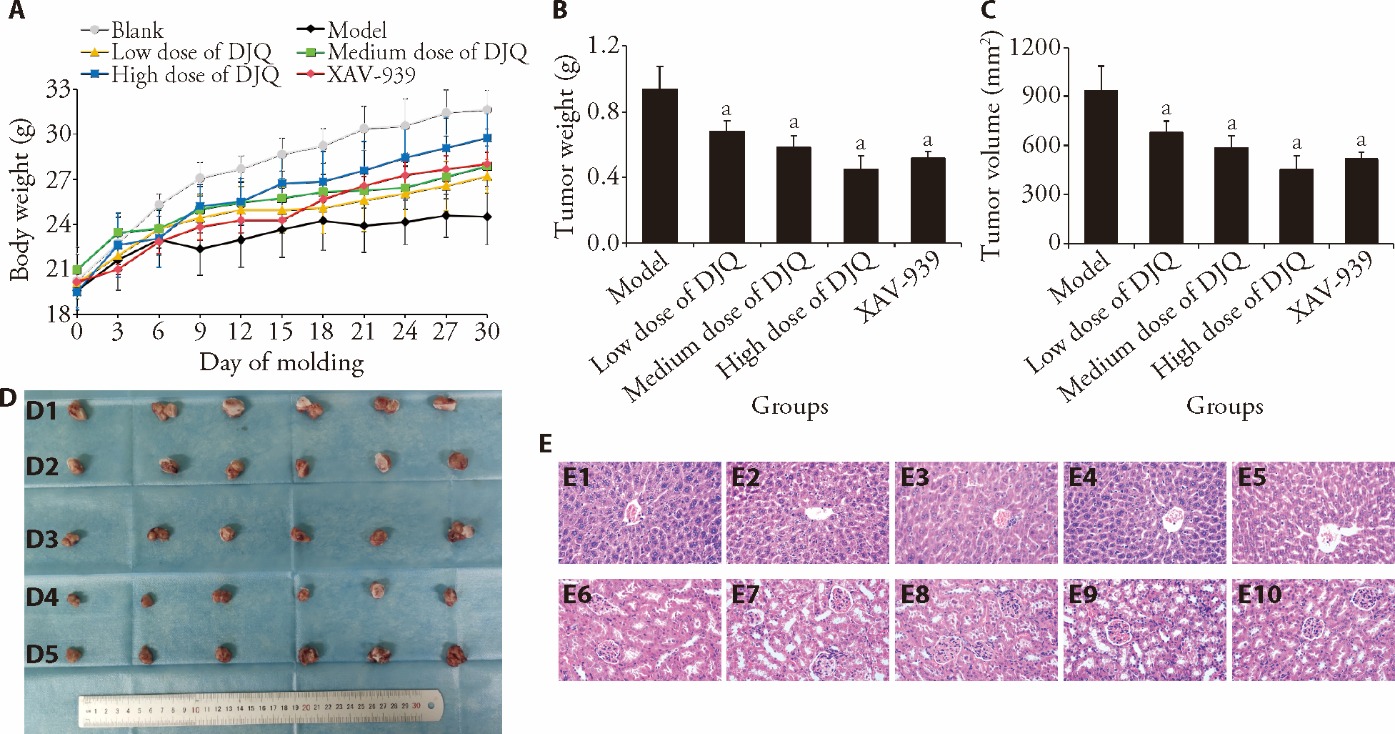
Figure 3 DJQ decoction suppressed xenograft tumor growth in model mice and effects of DJQ decoction on liver and kidney of mouse A: the weight of the mice was determined at 3-day intervals; B: the tumor volume of mice after treated 14-d; C: the tumor weight of mice after treated 14 d; D: the tumor of mice after treated 14 d; D1: the tumor of model mice; D2: the tumor of low dose of DJQ group mice; D3: the tumor of medium dose of DJQ group mice; D4: the tumor of high dose of DJQ group mice; D5: the tumor of XAV-939 group mice; E: the morphological changes in the liver and kidneys were analyzed through HE staining (Scale bar = 100 μm × 200). E1: the liver of model mice; E2: the liver of low dose of DJQ group mice; E3: the liver of medium dose of DJQ group mice; E4: the liver of high dose of DJQ group mice; E5: the liver of XAV-939 group mice. E6: the kidney of model mice; E7: the kidney of low dose of DJQ group mice; E8: the kidney of medium dose of DJQ group mice; E9: the kidney of high dose of DJQ group mice; E10: the kidney of XAV-939 group mice. Blank group: without xenograft model; Model group: without any treatment; Low dose of DJQ group: treated with 18 g/kg DJQ for 14 d; Medium dose of DJQ group: treated with 36 g/kg DJQ for 14 d; High dose of DJQ group: treated with 72 g/kg DJQ for 14 d; XAV-939 group: treated with 2 mg/kg XAV-939 for 14 d. DJQ: Dujieqing decoction; HE: Hematoxylin and eosin staining. One-way analysis of variance was used to compare more than two groups, followed by the least significant difference test to detect differences between groups. The data are presented as the mean ± standard deviation (n = 6). Compared with model group, aP < 0.05.
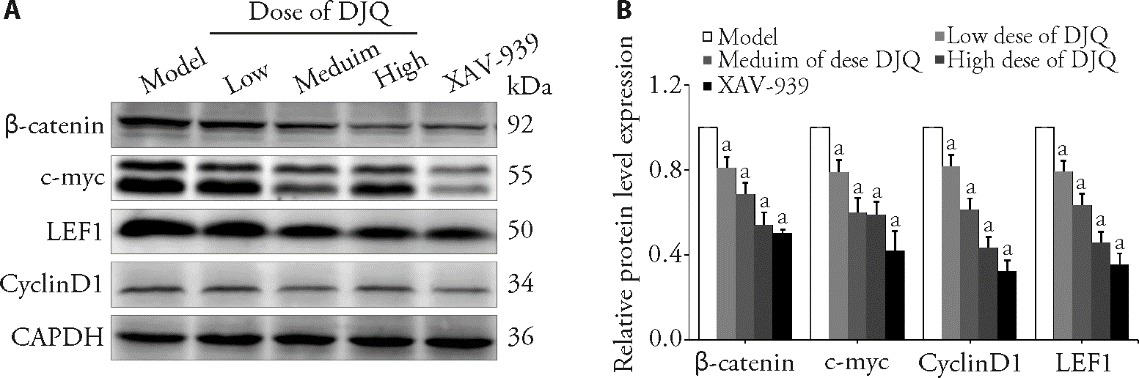
Figure 4 Relative protein of Wnt/β-catenin of model mice following various treatments assessed by WB assays A: Western blot analysis of the protein expression of tumor; B: comparison results of protein. Model group: without any treatment; Low dose of DJQ group: treated with 18 g/kg DJQ for 14 d; Medium dose of DJQ group: treated with 36 g/kg DJQ for 14 d; High dose of DJQ group: treated with 72 g/kg DJQ for 14 d; XAV-939 group: treated with 2 mg/kg XAV-939 for 14 d. DJQ: Dujieqing decoction; WB: Western blotting. One-way analysis of variance was used to compare more than two groups, followed by the least significant difference test to detect differences between groups. The data are presented as the mean ± standard deviation (n = 6). Compared with model group, aP < 0.05.
| 1. | Huang J, Chan SC, Lok V, et al. The epidemiological landscape of multiple myeloma: a global cancer registry estimate of disease burden, risk factors, and temporal trends. Lancet Haematol 2022; 9: e670-7. |
| 2. | Dima D, Jiang D, Singh DJ, et al. Multiple myeloma therapy: emerging trends and challenges. Cancers (Basel) 2022; 14: 4082. |
| 3. | Luo H, Vong CT, Chen H, et al. Naturally occurring anti-cancer compounds: shining from Chinese herbal medicine. Chin Med 2019; 14: 48. |
| 4. |
Fan Y, Ma Z, Zhao L, et al. Anti-tumor activities and mechanisms of Traditional Chinese Medicines formulas: a review. Biomed Pharmacother 2020; 132: 110820.
DOI PMID |
| 5. | Dai H, Ma B, Dai X, et al. Shengma biejia decoction inhibits cell growth in multiple myeloma by inducing autophagy-mediated apoptosis through the ERK/mTOR pathway. Front Pharmacol 2021; 12: 585286. |
| 6. |
Chen P, Wu S, Dong X, Zhou M, Xu P, Chen B. Formosanin C induces autophagy-mediated apoptosis in multiple myeloma cells through the PI3K/AKT/mTOR signaling pathway. Hematology 2022; 27: 977-86.
DOI PMID |
| 7. | Lei Y, Guo YH, Xu JW, et al. To explore the mechanism and experimental verification of Dujieqing decoction (毒结清复方) on multiple myeloma based on network pharmacology. Zhong Yi Yao Dao Bao 2023; 29: 17-23. |
| 8. |
Yu S, Han R, Gan R. The Wnt/β-catenin signalling pathway in haematological neoplasms. Biomark Res 2022; 10: 74.
DOI PMID |
| 9. |
van Andel H, Kocemba KA, Spaargaren M, Pals ST. Aberrant Wnt signaling in multiple myeloma: molecular mechanisms and targeting options. Leukemia 2019; 33: 1063-75.
DOI PMID |
| 10. | Gao Y, Li L, Hou L, Niu B, Ru X, Zhang D. SOX12 promotes the growth of multiple myeloma cells by enhancing Wnt/β-catenin signaling. Exp Cell Res 2020; 388: 111814. |
| 11. |
Chen SY, Zhang GC, Shu QJ. Yangyin Jiedu decoction overcomes gefitinib resistance in non-small cell lung cancer via down-regulation of the PI3K/Akt signalling pathway. Pharm Biol 2021; 59: 1294- 304.
DOI PMID |
| 12. | Lyu M, Liu Q. JMJD2C triggers the growth of multiple myeloma cells via activation of β-catenin. Oncol Rep 2021; 45: 1162-70. |
| 13. |
Yuan Y, Guo M, Gu C, Yang Y. The role of Wnt/β-catenin signaling pathway in the pathogenesis and treatment of multiple myeloma (review). Am J Transl Res 2021; 13: 9932-49.
PMID |
| 14. | Rafae A, van Rhee F, Al Hadidi S. Perspectives on the treatment of multiple myeloma. Oncologist 2024; 29: 200-12. |
| 15. | Bian Y, Wang G, Zhou J, et al. Astragalus Membranaceus (Huangqi) and Rhizoma Curcumae (Ezhu) decoction suppresses colorectal cancer via downregulation of Wnt5/β-Catenin signal. Chin Med. 2022; 17: 11. |
| 16. | Yang Z, Yao Y, Qian C. Study on the effect of Jianpi Yiqi decoction on clinical symptoms, inflammation, oxidative stress, efficacy and adverse reactions in sufferers with colorectal cancer. Biotechnol Genet Eng Rev 2024; 40: 3019-34. |
| 17. | Xin XL, Wang GD, Han R, et al. Mechanism underlying the effect of Liujunzi decoction on advanced-stage non-small cell lung cancer in patients after first-line chemotherapy. J Tradit Chin Med 2022; 42: 108-15. |
| 18. |
Gong H, Chen W, Mi L, et al. Qici Sanling decoction suppresses bladder cancer growth by inhibiting the Wnt/Β-catenin pathway. Pharm Biol 2019; 57: 507-13.
DOI PMID |
| 19. | Cheng Z, Ye F, Xu C, et al. The potential mechanism of Longsheyangquan decoction on the treatment of bladder cancer: Systemic network pharmacology and molecular docking. Front Pharmacol 2022; 13: 932039. |
| 20. | Yu CC, Li Y, Cheng ZJ, Wang X, Mao W, Zhang YW. Active components of traditional Chinese medicinal material for multiple myeloma: current evidence and future directions. Front Pharmacol 2022; 13: 818179. |
| 21. | Zheng J, Chen Y, Zheng Z, et al. In vitro investigation of the cytotoxic activity of emodin 35 derivative on multiple myeloma cell lines. Evid Based Complement Alternat Med 2021; 2021: 6682787. |
| 22. | Gu C, Yin Z, Nie H, et al. Identification of berberine as a novel drug for the treatment of multiple myeloma via targeting UHRF1. BMC Biol 2020; 18: 33. |
| 23. |
Hashemzaei M, Delarami Far A, Yari A, et al. Anticancer and apoptosis-inducing effects of quercetin in vitro and in vivo. Oncol Rep 2017; 38: 819-28.
DOI PMID |
| 24. | Sougiannis AT, VanderVeen B, Chatzistamou I, et al. Emodin reduces tumor burden by diminishing M2-like macrophages in colorectal cancer. Am J Physiol Gastrointest Liver Physiol 2022; 322: G383-95. |
| 25. | Dai G, Wang D, Ma S, et al. ACSL 4 promotes colorectal cancer and is a potential therapeutic target of emodin. Phytomedicine 2022; 102: 154149. |
| 26. | Salama AAA, Allam RM. Promising targets of chrysin and daidzein in colorectal cancer: amphiregulin, CXCL1, and MMP-9. Eur J Pharmacol 2021; 892: 173763. |
| 27. | Liu Y, Luo X, Liu J, et al. Shenlingcao oral liquid for patients with non-small cell lung cancer receiving adjuvant chemotherapy after radical resection: a multicenter randomized controlled trial. Phytomedicine 2023; 113: 154723. |
| 28. | Cichocki F, Zhang B, Wu CY, et al. Nicotinamide enhances natural killer cell function and yields remissions in patients with non-Hodgkin lymphoma. Sci Transl Med 2023; 15: eade3341. |
| 29. |
Tabana YM, Hassan LE, Ahamed MB, et al. Scopoletin, an active principle of tree tobacco (Nicotiana glauca) inhibits human tumor vascularization in xenograft models and modulates ERK1, VEGF-A, and FGF-2 in computer model. Microvasc Res 2016; 107: 17-33.
DOI PMID |
| 30. | Mu Q, Najafi M. Resveratrol for targeting the tumor microenvironment and its interactions with cancer cells. Int Immunopharmacol 2021; 98: 107895. |
| 31. | Wang H, Yu D, Zhang H, et al. Quercetin inhibits the proliferation of multiple myeloma cells by upregulating PTPRR expression. Acta Biochim Biophys Sin (Shanghai) 2021; 53: 1505-15. |
| 32. | Xu YW, Zou LF, Li F. Effect of Quercetin on proliferation and apoptosis of multiple myeloma cells and its related mechanism. Zhong Guo Shi Yan Xue Ye Xue Za Zhi 2020; 28: 1234-9. |
| 33. | Hsu CM, Yen CH, Wang SC, et al. Emodin ameliorates the efficacy of carfilzomib in multiple myeloma cells via apoptosis and autophagy. Biomedicines 2022; 10: 1638. |
| 34. | Ma R, Yu D, Peng Y, et al. Resveratrol induces AMPK and mTOR signaling inhibition-mediated autophagy and apoptosis in multiple myeloma cells. Acta Biochim Biophys Sin (Shanghai) 2021; 53: 775-83. |
| 35. | Geng W, Guo X, Zhang L, et al. Resveratrol inhibits proliferation, migration and invasion of multiple myeloma cells via NEAT1-mediated Wnt/β-catenin signaling pathway. Biomed Pharmacother 2018; 107: 484-94. |
| 36. |
Zhang C, Hao Y, Sun Y, Liu P. Quercetin suppresses the tumorigenesis of oral squamous cell carcinoma by regulating microRNA-22/WNT1/β-catenin axis. J Pharmacol Sci 2019; 140: 128-36.
DOI PMID |
| 37. | Ma W, Liu F, Yuan L, Zhao C, Chen C. Emodin and AZT synergistically inhibit the proliferation and induce the apoptosis of leukemia K562 cells through the EGR1 and the Wnt/β-catenin pathway. Oncol Rep 2020; 43: 260-9. |
| 38. | Razak S, Afsar T, Ullah A, et al. Taxifolin, a natural flavonoid interacts with cell cycle regulators causes cell cycle arrest and causes tumor regression by activating Wnt/β-catenin signaling pathway. BMC Cancer 2018; 18: 1043. |
| 39. | Sun WD, Zhu XJ, Li JJ, Mei YZ, Li WS, Li JH. Nicotinamide N-methyltransferase (NNMT): a key enzyme in cancer metabolism and therapeutic target. Int Immunopharmacol 2024; 142: 113208. |
| 40. | Sakthivel KM, Vishnupriya S, Priya Dharshini LC, Rasmi RR, Ramesh B. Modulation of multiple cellular signalling pathways as targets for anti-inflammatory and anti-tumorigenesis action of Scopoletin. J Pharm Pharmacol 2022; 74: 147-61. |
| 41. | Cilibrasi C, Riva G, Romano G, et al. Resveratrol impairs glioma stem cells proliferation and motility by modulating the Wnt signaling pathway. PLoS One 2017; 12: e0169854. |
| 42. | Zhang Y, Wang X. Targeting the Wnt/β-catenin signaling pathway in cancer. J Hematol Oncol 2020; 13: 165. |
| 43. | Söderholm S, Cantù C. The WNT/β-catenin dependent transcription: a tissue-specific business. WIREs Mech Dis 2021; 13: e1511. |
| 44. | Zheng L, Zheng Q, Yu Z, et al. Liuwei Dihuang pill suppresses metastasis by regulating the wnt pathway and disrupting-catenin/T cell factor interactions in a murine model of triple-negative breast cancer. J Tradit Chin Med 2019; 39: 826-32. |
| 45. | Wu Y, Fang G, Wang X, et al. NUP 153 overexpression suppresses the proliferation of colorectal cancer by negatively regulating Wnt/β-catenin signaling pathway and predicts good prognosis. Cancer Biomark 2019; 24: 61-70. |
| 46. | Tang Y, Song G, Liu H, Yang S, Yu X, Shi L. Silencing of long non-coding RNA HOTAIR alleviates epithelial-mesenchymal transition in pancreatic cancer via the Wnt/β-catenin signaling pathway. Cancer Manag Res 2021; 13: 3247-57. |
| 47. |
Liu X, Zuo X, Sun X, Tian X, Teng Y. Hexokinase 2 promotes cell proliferation and tumor formation through the Wnt/β-catenin pathway-mediated cyclin D1/c-myc upregulation in epithelial ovarian cancer. J Cancer 2022; 13: 2559-69.
DOI PMID |
| 48. | Pandit H, Li Y, Li X, Zhang W, Li S, Martin RCG. Enrichment of cancer stem cells via β-catenin contributing to the tumorigenesis of hepatocellular carcinoma. BMC Cancer 2018; 18: 783. |
| 49. | Liu X, Huang Y, Zhang Y, et al. T-cell factor (TCF/LEF1) binding elements (TBEs) of FasL (Fas ligand or CD95 ligand) bind and cluster Fas (CD95) and form complexes with the TCF-4 and b-catenin transcription factors in vitro and in vivo which result in triggering cell death and/or cell activation. Cell Mol Neurobiol 2016; 36: 1001-13. |
| 50. |
Li C, Zheng X, Han Y, Lyu Y, Lan F, Zhao J. XAV939 inhibits the proliferation and migration of lung adenocarcinoma A549 cells through the WNT pathway. Oncol Lett 2018; 15: 8973-82.
DOI PMID |
| 51. | Pan F, Shen F, Yang L, Zhang L, Guo W, Tian J. Inhibitory effects of XAV939 on the proliferation of small-cell lung cancer H446 cells and Wnt/β-catenin signaling pathway in vitro. Oncol Lett 2018; 16: 1953-8. |
| [1] | ZHAO Ping, HE Xingbo, HAN Xudong, CHEN Xinyue, LI Zhanglong, SONG Jike, XING Wenjia, WU Jiangfeng, GUO Bin, BI Hongsheng. Mechanism of electroacupuncture involve in lens-induced myopia guinea pigs by inhibiting wnt/β-catenin signaling pathway [J]. Journal of Traditional Chinese Medicine, 2025, 45(4): 796-805. |
| [2] | FENG Guiling, ZHOU Xiaolin, SHEN Chengwan, LI Panxiao, ABULIZI ·Abudula. Effects of Huluan decotion (护卵汤) on cyclophosphamide-induced autoimmune premature ovarian failure in murine models [J]. Journal of Traditional Chinese Medicine, 2025, 45(2): 266-271. |
| [3] | ZHU Xuan, LOU An, ZHU Keke, RUAN Mingyu. Effect of Jiedu Huayu decoction (解毒化瘀汤) on oral mucosal Axin and β-catenin expression in oral submucosal fibrosis model rats [J]. Journal of Traditional Chinese Medicine, 2024, 44(4): 688-693. |
| [4] | CHANG Junli, ZHAO Fulai, SUN Xingyuan, MA Xiaoping, ZHAO Peng, ZHOU Chujie, SHI Binhao, GU Wenchao, WANG Yongjun, YANG Yanping. Polyphyllin I enhances tumor necrosis factor-related apoptosis-inducing ligand-induced inhibition of human osteosarcoma cell growth via downregulating the Wnt/β-catenin pathway [J]. Journal of Traditional Chinese Medicine, 2024, 44(2): 251-259. |
| [5] | WU Jieya, HOU Li, ZHANG Xiaoyuan, Elizabeth Gullen, GAO Chong, WANG Jing. Efficacy of Yisui granule (益髓颗粒) on myelodysplastic syndromes in SKM-1 mouse xenograft model through suppressing Wnt/β-catenin signaling pathway [J]. Journal of Traditional Chinese Medicine, 2024, 44(1): 78-87. |
| [6] | YAO Nan, CHEN Guocai, LU Yanyan, XU Xuemeng, ZHAO Chuanxi, HUANG Xuejun, LIU Wengang, PENG Sha, WU Huai. Bushen Qiangjin capsule(补肾强筋胶囊) inhibits the Wnt/β-catenin pathway to ameliorate papain-induced knee osteoarthritis in rats [J]. Journal of Traditional Chinese Medicine, 2021, 41(6): 935-942. |
| [7] | XU Qian, QIN Wei, WU Fangzhen, LIN Yao, HONG Liting, CHEN Dan, HU Xuefeng, CAI Jing. Effect of Roucongrong(Herba Cistanches Deserticolae) decoction on the substantia nigra through Wnt/β-catenin signaling pathway in rats with Parkinson's disease induced by 6-hydroxydopamine hydrochloride [J]. Journal of Traditional Chinese Medicine, 2021, 41(5): 762-770. |
| [8] | DONG Lei, XU Pei. Danzhi Jiangtang capsule (丹蛭降糖胶囊) alleviate hyperglycemiaand periodontitis via Wnt/β-catenin signaling in diabetic rats [J]. Journal of Traditional Chinese Medicine, 2021, 41(4): 608-616. |
| [9] | Zheng Lixiang, Zheng Qing, Yu Zhipeng, Wang Jian, Ren Xiaoying, Gong Yan, Yang Xue, Hunag Ping, Weng Meizhi, Liu Hongning, Liu Haizhou. Liuwei Dihuang pill suppresses metastasis by regulating the wnt pathway and disrupting β-catenin/T cell factor interactions in a murine model of triple-negative breast cancer [J]. Journal of Traditional Chinese Medicine, 2019, 39(06): 826-832. |
| [10] | Guan Huiting, Xie Su, Liu Shangyi, Xie Qing, Hou Shengkai, Liu Huarong, Zhang Yunjie, Hu Yaqing, Zhang Chenyu. Effects of Jiazhu decoction in combination with cyclophosphamide on breast cancer in mice [J]. Journal of Traditional Chinese Medicine, 2019, 39(05): 642-648. |
| [11] | Su Tao, Wang Xinning, Li Chunyu, Bai Jingxuan, Chi-Yan Cheng, Fu Xiuqiong, Li Ting, Yu Zhiling. An ethanolic extract of Bailian(Radix Ampelopsis Japonicae):demonstration of colorectal cancer treatment efficacy via inhibition of β-catenin signaling in vitro [J]. Journal of Traditional Chinese Medicine, 2019, 39(03): 339-345. |
| [12] | Fu Shuping, Yang Li, Hong Hao, Zhang Ronghua. Wnt/β-catenin signaling is involved in the Icariin induced proliferation of bone marrow mesenchymal stem cells [J]. Journal of Traditional Chinese Medicine, 2016, 36(03): 360-368. |
| [13] | Hao Yaning, Zhao Fei, Luo Yuanyuan, Zhang Mei, Li Shasha. Inhibitory effect of oridonin on proliferation of RPMI8226 cells and the possible underlying mechanism [J]. Journal of Traditional Chinese Medicine, 2016, 36(02): 225-230. |
| [14] | Yini Jiang, Daobing Liu, Xiangying Kong, Ying Xu, Weiheng Chen, Na Lin. HuoguⅠformula prevents steroid-induced osteonecrosis in rats by down-regulating PPARγ expression and activating Wnt/LRP5/β-catenin signaling [J]. Journal of Traditional Chinese Medicine, 2014, 34(03): 342-350. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||

