Journal of Traditional Chinese Medicine ›› 2024, Vol. 44 ›› Issue (6): 1067-1081.DOI: 10.19852/j.cnki.jtcm.20240927.002
• Meta-Analyses • Next Articles
Network Meta-analysis of the clinical efficacy and safety of kidney-tonifying and bone-strengthening therapies for the treatment of rheumatoid arthritis with kidney deficiency type
GAN Chang1,2,3, TAO Qingwen2,3, YI Haoying1,2,3, BIAN Yuting1,2,3, WANG Jianming2,3( )
)
- 1 School of Traditional Chinese Medicine, Beijing University of Chinese Medicine, Beijing 100029, China
2 Department of Traditional Chinese Medicine Rheumatology, China-Japan Friendship Hospital, Beijing 100029, China
3 Beijing Key Lab for Immune-Mediated Inflammatory Diseases, China-Japan Friendship Hospital, Beijing 100029, China
-
Received:2023-09-10Accepted:2023-12-22Online:2024-12-15Published:2024-09-27 -
Contact:Prof. WANG Jianming, Department of Traditional Chinese Medicine Rheumatology, China-Japan Friendship Hospital, Beijing 100029, China. wangjianming@zryhyy.com.cn Telephone: +86-13910890713 -
Supported by:National Natural Science Foundation of China: Therapy which Invigorating Kidney and Strengthening Bone Regulates the Balance between Osteoblast and Osteoclast in Treating Rheumatoid Arthritis Bone Destruction: a Mechanism Study(82074223);National Natural Science Foundation of China: Therapy which Invigorating Kidney and Strengthening Bone Regulates the Balance between Osteoblast and Osteoclast in Treating Rheumatoid Arthritis Bone Destruction: a Mechanism Study(82274435);Fifth Batch of National Training Program for Clinical Excellence in Chinese Medicine(2022178)
Cite this article
GAN Chang, TAO Qingwen, YI Haoying, BIAN Yuting, WANG Jianming. Network Meta-analysis of the clinical efficacy and safety of kidney-tonifying and bone-strengthening therapies for the treatment of rheumatoid arthritis with kidney deficiency type[J]. Journal of Traditional Chinese Medicine, 2024, 44(6): 1067-1081.
share this article
| Included study | Cases (male/female, n) | Age (years) | Duration of illness (years) | Intervention | Treatment duration (weeks) | Outcome indicator | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T | C | T | C | T | C | T | C | |||||||||||||||||||||
| Chen D et al 2018 | 9/16 | 8/17 | 34.4± 15.54 | 34.45±15.85 | 6.75±3.97 | 6.67±3.62 | Bushen Quhan Zhiwang decoction + Methotrexate | Methotrexate | 24 | ①② | ||||||||||||||||||
| Chen F et al 2016 | 11/24 | 13/22 | 37.52± 6.61 | 37.34±6.48 | 2.2±0.51 | 2.1±0.37 | YJC+Methotrexate+Leflunomide+Celecoxib | Methotrexate+Leflunomide+Celecoxib | 8 | ①②③④ | ||||||||||||||||||
| Chen L et al 2021 | 31/144 | 33/142 | 47.28± 12.19 | 49.11±11.62 | ≤1 | WT+Methotrexate+Acetaminophen | Methotrexate+WT analog+Acetaminophen | 12 | ② | |||||||||||||||||||
| Chen XL et al 2020 | 7/23 | 8/22 | 41.6±5.2 | 42.8± 4.7 | 3.2 | 3.3 | DJD+Leflunomide | Leflunomide | 8 | ④ | ||||||||||||||||||
| Chen YG et al 2019 | 2/23 | 4/21 | 51.4±5.8 | 53.6± 8.6 | 8.0±3.9 | 7.9±4.1 | DJD+Methotrexate+Leflunomide+Meloxicam | Methotrexate+Leflunomide+Meloxicam | 12 | ①②③④ | ||||||||||||||||||
| Du DY et al 2019 | 16/32 | 18/30 | 44.75± 6.73 | 44.80±6.69 | 4.24±1.61 | 4.24±1.61 | DJD+Methotrexate+Meloxicam | Methotrexate+meloxicam | 4 | ①②③④ | ||||||||||||||||||
| Gao Y et al 2015 | 14/46 | 15/45 | 64.0±3.2 | <1 | YJC+Methotrexate+Etoricoxib | Methotrexate+Etoricoxib | 12 | ②③④ | ||||||||||||||||||||
| He CX et al 2018 | - | - | 49.77± 11.43 | 49.74±11.59 | 7.13±6.74 | 6.8±6.04 | Bushen Quhan Zhiwang decoction+Methotrexate | Methotrexate | 12 | ①②③④ | ||||||||||||||||||
| He YK et al 2015 | 3/26 | 4/23 | 42.86± 9.54 | 47.26±13.56 | 11.34±7.35 | 12.04±7.88 | BJTF+Methotrexate | Methotrexate | 12 | ② | ||||||||||||||||||
| He YK et al 2018 | 9/29 | 12/28 | 48.14± 10.24 | 49.59±11.78 | 10.51±6.98 | 11.01±6.58 | BJTF+Methotrexate | Methotrexate | 24 | ①③④ | ||||||||||||||||||
| Hou HL et al 2013 | 6/24 | 7/23 | 52.63± 11.57 | 51.86±11.09 | 3.90±3.14 | 5.01±4.54 | WP+Methotrexate+Meloxicam | Methotrexate+Meloxicam | 4 | ①③④ | ||||||||||||||||||
| Ji HW et al 2012 | - | - | 26-64 | 0.25~20 | WT+Methotrexate | Methotrexate | 8 | ①②③④ | ||||||||||||||||||||
| Li N et al 2014 | 14/36 | 12/38 | 46.5±5.1 | 46.1± 4.8 | 5.2±2.3 | 5.4±2.2 | Bushen Quhan Zhiwang decoction+Methotrexate | Methotrexate | 20 | ①② | ||||||||||||||||||
| Li SQ et al 2017 | 15/28 | 13/30 | 43.2±6.3 | 41.7± 6.3 | 4.1±2.8 | 4.9±2.7 | Bushen Quhan Zhiwang decoction+Methotrexate | Methotrexate | 20 | ①② | ||||||||||||||||||
| Li XL et al 2015 | 10/24 | 11/23 | 43.5±7.2 | 44.7± 7.2 | 7.2±2.1 | 7.14±2.2 | QTOL+Methotrexate | Methotrexate | 8 | ①③④ | ||||||||||||||||||
| Liang J et al 2015 | 6/28 | 9/19 | 35.2± 13.6 | 36.1± 12.2 | 1.4±0.6 | 1.3±0.5 | Bushen Quhan Zhiwang decoction+Methotrexate | Methotrexate | 3 | ①②③④ | ||||||||||||||||||
| Lin GY et al 2016 | 42/33 | 40/35 | 47.4±4.2 | 46.4± 4.8 | 5.6±2.2 | 5.5±2.0 | Bushen Quhan Zhiwang decoction+Methotrexate | Methotrexate | 20 | ①② | ||||||||||||||||||
| Luo W et al 2013 | - | - | 20-55 | - | - | Bushen Quhan Zhiwang decoction+Methotrexate+Total Glucosides of Peony | Methotrexate+Total Glucosides of Peony | 24 | ①②③④ | |||||||||||||||||||
| Miao XY et al 2012 | 9/21 | 11/19 | 48.8 | 48.9 | 6.1 | 5.95 | WD+Methotrexate+Diclofenac Sodium | Methotrexate+Diclofenac Sodium | 12 | ②③ | ||||||||||||||||||
| Qiu SB et al 2018 | 3/27 | 2/28 | 48.47± 6.54 | 49.82±5.84 | 7.42±5.37 | 6.84±6.34 | DJD+Methotrexate+Hydroxychloroquine+Meloxicam | Methotrexate+Hydroxychloroquine+Meloxicam | 24 | ①③④ | ||||||||||||||||||
| Shen JY et al 2019 | 11/24 | 10/25 | 58.49± 11.976 | 60.63±11.324 | - | - | BJTF+Methotrexate | Methotrexate | 24 | ①③④ | ||||||||||||||||||
| Included study | Cases (male/female, n) | Age (years) | Duration of illness (years) | Intervention | Treatment duration (weeks) | Outcome indicator | ||||||||||||||||||||||
| T | C | T | C | T | C | T | C | |||||||||||||||||||||
| Shi Y et al 2019 | 15/25 | 15/25 | 36.5±1.2 | 35.5± 2.7 | 6.5±1.2 | 4.5±0.7 | DJD+Methotrexate+Meloxicam | Methotrexate+Meloxicam | 12 | ① | ||||||||||||||||||
| Sun GM et al 2021 | 5/10 | 7/8 | 43.28± 7.45 | 43.84± 6.73 | 4.34± 2.58 | 4.27± 2.93 | WT+Methotrexate | Methotrexate | 8 | ①③④ | ||||||||||||||||||
| Wan LY et al 2016 | - | - | - | - | - | - | DJD+Leflunomide | Leflunomide | 24 | ①④ | ||||||||||||||||||
| Wang G et al 2021 | 12/18 | 10/20 | 42.5±9.8 | 45.3± 10.4 | 5.8±2.7 | 6.0±2.5 | Bushen Quhan Zhiwang decoction+Methotrexate | Methotrexate | 24 | ①③④ | ||||||||||||||||||
| Wang JM et al 2013 | 31/89 | 33/87 | 31.62± 14.28 | 33.93± 12.46 | 6.56± 4.63 | 7.17± 5.82 | Bushen Quhan Zhiwang decoction+Methotrexate | Methotrexate | 24 | ①②③④ | ||||||||||||||||||
| Wang JM et al et al 2018 | 27/73 | 29/71 | 38.37± 12.28 | 40.13± 12.00 | 5.33± 2.57 | 5.16± 2.64 | Bushen Qinghua Zhiwang decoction+Methotrexate | Methotrexate | 24 | ①②③④ | ||||||||||||||||||
| Wang X et al 2019 | 7/41 | 9/39 | 46.75± 9.84 | 45.60± 7.51 | 0.65± 0.276 | 0.62± 0.24 | WP+Methotrexate+Celecoxib | Methotrexate+Celecoxib | 12 | ①②③④ | ||||||||||||||||||
| Xu L et al 2012 | 5/25 | 5/25 | 54.20± 8.25 | 54.47± 8.68 | 8.93± 8.12 | 8.16± 8.06 | QTOL+Methotrexate+Hydroxychloroquine Sulphate+Celecoxib | Methotrexate+Hydro-xychloroquine Sulphate+Celecoxib | 8 | ①③④ | ||||||||||||||||||
| Xu ZY et al 2020 | 15/25 | 17/23 | 42.71± 3.09 | 42.36± 3.57 | 4.90± 2.21 | 4.85± 2.63 | Bushen Qinghua Zhiwang decoction+Methotrexate | Methotrexate | 24 | ①②③④ | ||||||||||||||||||
| Xue QL et al 2016 | 33/57 | 32/58 | 43.2±1.8 | 42.9± 2.3 | 7.3±1.5 | 7.2±0.8 | Bushen Quhan Zhiwang decoction +Methotrexate | Methotrexate | 24 | ①②③④ | ||||||||||||||||||
| Yan WC et al 2015 | 21/9 | 19/11 | 48.93 | 48.9 | 6.1 | 5.91 | WD+Methotrexate | Methotrexate | 12 | ①② | ||||||||||||||||||
| Yang F et al 2020 | 18/22 | 21/19 | 55.23± 3.35 | 54.44± 3.31 | 3.23± 1.12 | 3.21± 1.11 | DJD+Methotrexate | Methotrexate | 8 | ①②③④ | ||||||||||||||||||
| Yao MS et al 2013 | 3/27 | 5/25 | 45.17± 10.79 | 47.42± 11.47 | 3.98± 2.35 | 4.12± 2.56 | DJD+Methotrexate+Celebrex | Methotrexate+Celebrex | 8 | ①②③④ | ||||||||||||||||||
| Zhang BC et al 2017 | 27/23 | 26/24 | 44.53± 4.47 | 45.32± 3.68 | 8.21± 5.04 | 7.12± 5.2 | Bushen Quhan Zhiwang decoction+Methotrexate | Methotrexate | 20 | ① | ||||||||||||||||||
| Zhang L et al 2017 | 17/33 | 15/35 | 53.2± 10.1 | 53.1± 11.2 | 6.58± 2.41 | 6.56± 2.53 | Bushen Quhan Zhiwang decoction+Methotrexate | Methotrexate | 24 | ①② | ||||||||||||||||||
| Zhang WJ et al 2013 | 9/21 | 8/22 | 43.6±5.3 | 42.5± 4.7 | 0.29~8.6 | 0.36- 6.9 | DJD+Leflunomide | Leflunomide | 16 | ①③④ | ||||||||||||||||||
| Zhao HY et al 2021 | 3/18 | 5/19 | 53.17± 10.11 | 52.87± 10.07 | 2.48± 0.96 | 2.51± 0.93 | DJD+Antirheumatic drugs of Western Medicine | Western Medicine Antirheumatic Drugs | 24 | ③④ | ||||||||||||||||||
| Su DC et al 2022 | 20/22 | 19/23 | 48.23± 5.16 | 48.15± 5.07 | 6.31± 1.92 | 6.34± 1.89 | DJD+Methotrexate+Meloxicam | Methotrexate+Meloxicam | 12 | ① | ||||||||||||||||||
| Lan TY et al 2023 | 18/58 | 21/56 | 61.24± 10.23 | 61.01± 8.97 | 8.61± 6.99 | 8.16± 4.52 | BZD+cDMARDs | cDMARDs | 48 | ③④ | ||||||||||||||||||
| Liu X 2022 | 11/21 | 10/22 | 58.27± 6.74 | 57.86± 6.05 | 1.73± 0.42 | 1.65± 0.38 | DJD+Leflunomide+Celebrex | Leflunomide+Celebrex | 8 | ①②③ | ||||||||||||||||||
| Yao CH et al 2023 | 2/20 | 3/17 | 55.36± 9.85 | 53.5± 12.48 | 6.54 (2.88, 9.25) | 8.33 (4.88, 13.25) | BZD+cDMARDs | cDMARDs | 48 | ①②③④ | ||||||||||||||||||
Table 1 Basic characteristics of the included literature
| Included study | Cases (male/female, n) | Age (years) | Duration of illness (years) | Intervention | Treatment duration (weeks) | Outcome indicator | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T | C | T | C | T | C | T | C | |||||||||||||||||||||
| Chen D et al 2018 | 9/16 | 8/17 | 34.4± 15.54 | 34.45±15.85 | 6.75±3.97 | 6.67±3.62 | Bushen Quhan Zhiwang decoction + Methotrexate | Methotrexate | 24 | ①② | ||||||||||||||||||
| Chen F et al 2016 | 11/24 | 13/22 | 37.52± 6.61 | 37.34±6.48 | 2.2±0.51 | 2.1±0.37 | YJC+Methotrexate+Leflunomide+Celecoxib | Methotrexate+Leflunomide+Celecoxib | 8 | ①②③④ | ||||||||||||||||||
| Chen L et al 2021 | 31/144 | 33/142 | 47.28± 12.19 | 49.11±11.62 | ≤1 | WT+Methotrexate+Acetaminophen | Methotrexate+WT analog+Acetaminophen | 12 | ② | |||||||||||||||||||
| Chen XL et al 2020 | 7/23 | 8/22 | 41.6±5.2 | 42.8± 4.7 | 3.2 | 3.3 | DJD+Leflunomide | Leflunomide | 8 | ④ | ||||||||||||||||||
| Chen YG et al 2019 | 2/23 | 4/21 | 51.4±5.8 | 53.6± 8.6 | 8.0±3.9 | 7.9±4.1 | DJD+Methotrexate+Leflunomide+Meloxicam | Methotrexate+Leflunomide+Meloxicam | 12 | ①②③④ | ||||||||||||||||||
| Du DY et al 2019 | 16/32 | 18/30 | 44.75± 6.73 | 44.80±6.69 | 4.24±1.61 | 4.24±1.61 | DJD+Methotrexate+Meloxicam | Methotrexate+meloxicam | 4 | ①②③④ | ||||||||||||||||||
| Gao Y et al 2015 | 14/46 | 15/45 | 64.0±3.2 | <1 | YJC+Methotrexate+Etoricoxib | Methotrexate+Etoricoxib | 12 | ②③④ | ||||||||||||||||||||
| He CX et al 2018 | - | - | 49.77± 11.43 | 49.74±11.59 | 7.13±6.74 | 6.8±6.04 | Bushen Quhan Zhiwang decoction+Methotrexate | Methotrexate | 12 | ①②③④ | ||||||||||||||||||
| He YK et al 2015 | 3/26 | 4/23 | 42.86± 9.54 | 47.26±13.56 | 11.34±7.35 | 12.04±7.88 | BJTF+Methotrexate | Methotrexate | 12 | ② | ||||||||||||||||||
| He YK et al 2018 | 9/29 | 12/28 | 48.14± 10.24 | 49.59±11.78 | 10.51±6.98 | 11.01±6.58 | BJTF+Methotrexate | Methotrexate | 24 | ①③④ | ||||||||||||||||||
| Hou HL et al 2013 | 6/24 | 7/23 | 52.63± 11.57 | 51.86±11.09 | 3.90±3.14 | 5.01±4.54 | WP+Methotrexate+Meloxicam | Methotrexate+Meloxicam | 4 | ①③④ | ||||||||||||||||||
| Ji HW et al 2012 | - | - | 26-64 | 0.25~20 | WT+Methotrexate | Methotrexate | 8 | ①②③④ | ||||||||||||||||||||
| Li N et al 2014 | 14/36 | 12/38 | 46.5±5.1 | 46.1± 4.8 | 5.2±2.3 | 5.4±2.2 | Bushen Quhan Zhiwang decoction+Methotrexate | Methotrexate | 20 | ①② | ||||||||||||||||||
| Li SQ et al 2017 | 15/28 | 13/30 | 43.2±6.3 | 41.7± 6.3 | 4.1±2.8 | 4.9±2.7 | Bushen Quhan Zhiwang decoction+Methotrexate | Methotrexate | 20 | ①② | ||||||||||||||||||
| Li XL et al 2015 | 10/24 | 11/23 | 43.5±7.2 | 44.7± 7.2 | 7.2±2.1 | 7.14±2.2 | QTOL+Methotrexate | Methotrexate | 8 | ①③④ | ||||||||||||||||||
| Liang J et al 2015 | 6/28 | 9/19 | 35.2± 13.6 | 36.1± 12.2 | 1.4±0.6 | 1.3±0.5 | Bushen Quhan Zhiwang decoction+Methotrexate | Methotrexate | 3 | ①②③④ | ||||||||||||||||||
| Lin GY et al 2016 | 42/33 | 40/35 | 47.4±4.2 | 46.4± 4.8 | 5.6±2.2 | 5.5±2.0 | Bushen Quhan Zhiwang decoction+Methotrexate | Methotrexate | 20 | ①② | ||||||||||||||||||
| Luo W et al 2013 | - | - | 20-55 | - | - | Bushen Quhan Zhiwang decoction+Methotrexate+Total Glucosides of Peony | Methotrexate+Total Glucosides of Peony | 24 | ①②③④ | |||||||||||||||||||
| Miao XY et al 2012 | 9/21 | 11/19 | 48.8 | 48.9 | 6.1 | 5.95 | WD+Methotrexate+Diclofenac Sodium | Methotrexate+Diclofenac Sodium | 12 | ②③ | ||||||||||||||||||
| Qiu SB et al 2018 | 3/27 | 2/28 | 48.47± 6.54 | 49.82±5.84 | 7.42±5.37 | 6.84±6.34 | DJD+Methotrexate+Hydroxychloroquine+Meloxicam | Methotrexate+Hydroxychloroquine+Meloxicam | 24 | ①③④ | ||||||||||||||||||
| Shen JY et al 2019 | 11/24 | 10/25 | 58.49± 11.976 | 60.63±11.324 | - | - | BJTF+Methotrexate | Methotrexate | 24 | ①③④ | ||||||||||||||||||
| Included study | Cases (male/female, n) | Age (years) | Duration of illness (years) | Intervention | Treatment duration (weeks) | Outcome indicator | ||||||||||||||||||||||
| T | C | T | C | T | C | T | C | |||||||||||||||||||||
| Shi Y et al 2019 | 15/25 | 15/25 | 36.5±1.2 | 35.5± 2.7 | 6.5±1.2 | 4.5±0.7 | DJD+Methotrexate+Meloxicam | Methotrexate+Meloxicam | 12 | ① | ||||||||||||||||||
| Sun GM et al 2021 | 5/10 | 7/8 | 43.28± 7.45 | 43.84± 6.73 | 4.34± 2.58 | 4.27± 2.93 | WT+Methotrexate | Methotrexate | 8 | ①③④ | ||||||||||||||||||
| Wan LY et al 2016 | - | - | - | - | - | - | DJD+Leflunomide | Leflunomide | 24 | ①④ | ||||||||||||||||||
| Wang G et al 2021 | 12/18 | 10/20 | 42.5±9.8 | 45.3± 10.4 | 5.8±2.7 | 6.0±2.5 | Bushen Quhan Zhiwang decoction+Methotrexate | Methotrexate | 24 | ①③④ | ||||||||||||||||||
| Wang JM et al 2013 | 31/89 | 33/87 | 31.62± 14.28 | 33.93± 12.46 | 6.56± 4.63 | 7.17± 5.82 | Bushen Quhan Zhiwang decoction+Methotrexate | Methotrexate | 24 | ①②③④ | ||||||||||||||||||
| Wang JM et al et al 2018 | 27/73 | 29/71 | 38.37± 12.28 | 40.13± 12.00 | 5.33± 2.57 | 5.16± 2.64 | Bushen Qinghua Zhiwang decoction+Methotrexate | Methotrexate | 24 | ①②③④ | ||||||||||||||||||
| Wang X et al 2019 | 7/41 | 9/39 | 46.75± 9.84 | 45.60± 7.51 | 0.65± 0.276 | 0.62± 0.24 | WP+Methotrexate+Celecoxib | Methotrexate+Celecoxib | 12 | ①②③④ | ||||||||||||||||||
| Xu L et al 2012 | 5/25 | 5/25 | 54.20± 8.25 | 54.47± 8.68 | 8.93± 8.12 | 8.16± 8.06 | QTOL+Methotrexate+Hydroxychloroquine Sulphate+Celecoxib | Methotrexate+Hydro-xychloroquine Sulphate+Celecoxib | 8 | ①③④ | ||||||||||||||||||
| Xu ZY et al 2020 | 15/25 | 17/23 | 42.71± 3.09 | 42.36± 3.57 | 4.90± 2.21 | 4.85± 2.63 | Bushen Qinghua Zhiwang decoction+Methotrexate | Methotrexate | 24 | ①②③④ | ||||||||||||||||||
| Xue QL et al 2016 | 33/57 | 32/58 | 43.2±1.8 | 42.9± 2.3 | 7.3±1.5 | 7.2±0.8 | Bushen Quhan Zhiwang decoction +Methotrexate | Methotrexate | 24 | ①②③④ | ||||||||||||||||||
| Yan WC et al 2015 | 21/9 | 19/11 | 48.93 | 48.9 | 6.1 | 5.91 | WD+Methotrexate | Methotrexate | 12 | ①② | ||||||||||||||||||
| Yang F et al 2020 | 18/22 | 21/19 | 55.23± 3.35 | 54.44± 3.31 | 3.23± 1.12 | 3.21± 1.11 | DJD+Methotrexate | Methotrexate | 8 | ①②③④ | ||||||||||||||||||
| Yao MS et al 2013 | 3/27 | 5/25 | 45.17± 10.79 | 47.42± 11.47 | 3.98± 2.35 | 4.12± 2.56 | DJD+Methotrexate+Celebrex | Methotrexate+Celebrex | 8 | ①②③④ | ||||||||||||||||||
| Zhang BC et al 2017 | 27/23 | 26/24 | 44.53± 4.47 | 45.32± 3.68 | 8.21± 5.04 | 7.12± 5.2 | Bushen Quhan Zhiwang decoction+Methotrexate | Methotrexate | 20 | ① | ||||||||||||||||||
| Zhang L et al 2017 | 17/33 | 15/35 | 53.2± 10.1 | 53.1± 11.2 | 6.58± 2.41 | 6.56± 2.53 | Bushen Quhan Zhiwang decoction+Methotrexate | Methotrexate | 24 | ①② | ||||||||||||||||||
| Zhang WJ et al 2013 | 9/21 | 8/22 | 43.6±5.3 | 42.5± 4.7 | 0.29~8.6 | 0.36- 6.9 | DJD+Leflunomide | Leflunomide | 16 | ①③④ | ||||||||||||||||||
| Zhao HY et al 2021 | 3/18 | 5/19 | 53.17± 10.11 | 52.87± 10.07 | 2.48± 0.96 | 2.51± 0.93 | DJD+Antirheumatic drugs of Western Medicine | Western Medicine Antirheumatic Drugs | 24 | ③④ | ||||||||||||||||||
| Su DC et al 2022 | 20/22 | 19/23 | 48.23± 5.16 | 48.15± 5.07 | 6.31± 1.92 | 6.34± 1.89 | DJD+Methotrexate+Meloxicam | Methotrexate+Meloxicam | 12 | ① | ||||||||||||||||||
| Lan TY et al 2023 | 18/58 | 21/56 | 61.24± 10.23 | 61.01± 8.97 | 8.61± 6.99 | 8.16± 4.52 | BZD+cDMARDs | cDMARDs | 48 | ③④ | ||||||||||||||||||
| Liu X 2022 | 11/21 | 10/22 | 58.27± 6.74 | 57.86± 6.05 | 1.73± 0.42 | 1.65± 0.38 | DJD+Leflunomide+Celebrex | Leflunomide+Celebrex | 8 | ①②③ | ||||||||||||||||||
| Yao CH et al 2023 | 2/20 | 3/17 | 55.36± 9.85 | 53.5± 12.48 | 6.54 (2.88, 9.25) | 8.33 (4.88, 13.25) | BZD+cDMARDs | cDMARDs | 48 | ①②③④ | ||||||||||||||||||
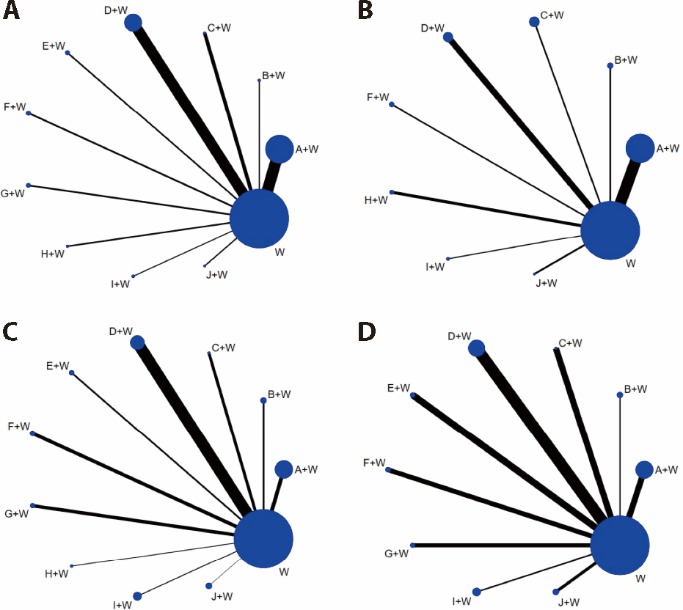
Figure 2 Evidence network diagrams for four outcome indicators A: evidence network diagram for total effective rate; B: evidence network diagram for incidence of adverse reactions; C: evidence network diagram for CRP; D: evidence network diagram for ESR. W: conventional Western Medicine; A + W: Bushen Quhan Zhiwang decoction + conventional Western Medicine; B + W: Yishen Juanbi capsules + conventional Western Medicine; C + W: Wangbi tablets + conventional Western Medicine; D + W: Duhuo Jisheng decoction + conventional Western Medicine; E + W: Bushen Jiedu Tongluo formula + conventional Western Medicine; F + W: Wanbikang pills + conventional Western Medicine; G + W: Qiwei Tongbi oral liquid + conventional Western Medicine; H + W: Wanbi decoction + conventional Western Medicine; I + W: Bushen Qinghua Zhiwang decoction + conventional Western Medicine; J + W: Bushen Zhiwang decoction + conventional Western Medicine.
| Item | Number of studies (n) | Test group (events/total, n) | Control group (events/total, n) | Effect measure RR (95% CI) | Heterogeneity (I 2, %) |
|---|---|---|---|---|---|
| Bushen Quhan Zhiwang decoction | 12 | 554/675 | 431/652 | 1.24 (1.17, 1.32)a | 0.0 |
| YJC | 1 | 31/33 | 24/32 | 1.25 (1.01, 1.56)a | - |
| WT | 2 | 23/25 | 12/25 | 1.92 (1.25, 2.72)a | 0.0 |
| DJD | 10 | 297/377 | 219/377 | 1.28 (1.19, 1.37)a | 0.0 |
| BJTF | 2 | 51/70 | 30/70 | 1.70 (1.26, 2.30)a | 0.0 |
| WP | 2 | 65/74 | 47/75 | 1.41 (1.03, 1.93)a | 53.6 |
| QTOL | 2 | 51/64 | 40/64 | 1.27 (1.02, 1.59)a | 0.0 |
| WD | 1 | 29/30 | 21/30 | 1.38 (1.08, 1.76)a | - |
| Bushen Qinghua Zhiwang decoction | 1 | 37/40 | 30/40 | 1.23 (1.01, 1.51)a | - |
| BZD | 1 | 20/22 | 14/20 | 1.20 (0.95, 1.78) | - |
Table 2 Direct pairwise Meta-analysis of the total effective rate
| Item | Number of studies (n) | Test group (events/total, n) | Control group (events/total, n) | Effect measure RR (95% CI) | Heterogeneity (I 2, %) |
|---|---|---|---|---|---|
| Bushen Quhan Zhiwang decoction | 12 | 554/675 | 431/652 | 1.24 (1.17, 1.32)a | 0.0 |
| YJC | 1 | 31/33 | 24/32 | 1.25 (1.01, 1.56)a | - |
| WT | 2 | 23/25 | 12/25 | 1.92 (1.25, 2.72)a | 0.0 |
| DJD | 10 | 297/377 | 219/377 | 1.28 (1.19, 1.37)a | 0.0 |
| BJTF | 2 | 51/70 | 30/70 | 1.70 (1.26, 2.30)a | 0.0 |
| WP | 2 | 65/74 | 47/75 | 1.41 (1.03, 1.93)a | 53.6 |
| QTOL | 2 | 51/64 | 40/64 | 1.27 (1.02, 1.59)a | 0.0 |
| WD | 1 | 29/30 | 21/30 | 1.38 (1.08, 1.76)a | - |
| Bushen Qinghua Zhiwang decoction | 1 | 37/40 | 30/40 | 1.23 (1.01, 1.51)a | - |
| BZD | 1 | 20/22 | 14/20 | 1.20 (0.95, 1.78) | - |

Figure 3 Comparative-corrected funnel plots for four outcome indicators A: comparative-corrected funnel plot for total effective rate; B: comparative-corrected funnel plot for incidence of adverse reactions; C: comparative-corrected funnel plot for CRP; D: comparative-corrected funnel plot for ESR. W: conventional Western Medicine; A + W: Bushen Quhan Zhiwang decoction + conventional Western Medicine; B + W: Yishen Juanbi capsules + conventional Western Medicine; C + W: Wangbi tablets + conventional Western Medicine; D + W: Duhuo Jisheng decoction + conventional Western Medicine; E + W: Bushen Jiedu Tongluo formula + conventional Western Medicine; F + W: Wanbikang pills + conventional Western Medicine; G + W: Qiwei Tongbi oral liquid + conventional Western Medicine; H + W: Wanbi decoction + conventional Western Medicine; I + W: Bushen Qinghua Zhiwang decoction + conventional Western Medicine; J + W: Bushen Zhiwang decoction + conventional Western Medicine. CRP: C-reactive protein; ESR: Erythrocyte sedimentation rate.
| Item | Number of studies (n) | Test group (events/total, n) | Control group (events/total, n) | Effect measure RR (95% CI) | Heterogeneity (I 2, %) |
|---|---|---|---|---|---|
| Bushen Quhan Zhiwang decoction | 11 | 31/591 | 124/568 | 0.24 (0.17, 0.36)a | 0.0 |
| YJC | 2 | 12/93 | 12/92 | 0.98 (0.47, 2.03) | 0.0 |
| WT | 2 | 32/183 | 30/185 | 1.08 (0.69, 1.68) | 15.9 |
| DJD | 5 | 15/175 | 32/175 | 0.47 (0.27, 0.83)a | 49.9 |
| WP | 2 | 14/74 | 16/75 | 0.89 (0.47, 1.69) | 0.0 |
| WD | 2 | 2/60 | 7/60 | 0.29 (0.06, 1.32) | 0.0 |
| Bushen Qinghua Zhiwang decoction | 1 | 2/40 | 8/40 | 0.25 (0.06, 1.11) | - |
| BZD | 1 | 1/22 | 1/20 | 0.91 (0.06, 13.59) | - |
Table 3 Direct pairwise Meta-analysis of the incidence of adverse reactions
| Item | Number of studies (n) | Test group (events/total, n) | Control group (events/total, n) | Effect measure RR (95% CI) | Heterogeneity (I 2, %) |
|---|---|---|---|---|---|
| Bushen Quhan Zhiwang decoction | 11 | 31/591 | 124/568 | 0.24 (0.17, 0.36)a | 0.0 |
| YJC | 2 | 12/93 | 12/92 | 0.98 (0.47, 2.03) | 0.0 |
| WT | 2 | 32/183 | 30/185 | 1.08 (0.69, 1.68) | 15.9 |
| DJD | 5 | 15/175 | 32/175 | 0.47 (0.27, 0.83)a | 49.9 |
| WP | 2 | 14/74 | 16/75 | 0.89 (0.47, 1.69) | 0.0 |
| WD | 2 | 2/60 | 7/60 | 0.29 (0.06, 1.32) | 0.0 |
| Bushen Qinghua Zhiwang decoction | 1 | 2/40 | 8/40 | 0.25 (0.06, 1.11) | - |
| BZD | 1 | 1/22 | 1/20 | 0.91 (0.06, 13.59) | - |
| Item | Number of studies (n) | Test group (n) | Control group (n) | Effect measure MD (95% CI) | Heterogeneity (I 2, %) | |
|---|---|---|---|---|---|---|
| Bushen Quhan Zhiwang decoction | 6 | 348 | 348 | -1.96 (-3.79, -0.12)a | 85.4 | |
| YJC | 2 | 93 | 92 | -4.28 (-5.33, -3.23)a | 0.0 | |
| WT | 2 | 25 | 25 | -2.63 (-5.00, -0.26)a | 0.0 | |
| DJD | 8 | 259 | 259 | -4.48 (-5.26, -3.70)a | 80.5 | |
| BJTF | 2 | 70 | 70 | -5.75 (-17.89, 6.38) | 84.8 | |
| WP | 2 | 74 | 75 | -8.79 (-22.79, 5.20) | 86.4 | |
| QTOL | 2 | 64 | 64 | -10.53 (-17.28, -3.78)a | 54.2 | |
| WD | 1 | 30 | 30 | -3.12 (-5.85, -0.39)a | - | |
| Bushen Qinghua Zhiwang decoction | 2 | 140 | 140 | -4.78 (-10.79, 1.24) | 96.4 | |
| BZD | 2 | 98 | 97 | -0.46 (-0.91, -0.01)a | 78.5 | |
Table 4 Direct pairwise Meta-analysis of CRP
| Item | Number of studies (n) | Test group (n) | Control group (n) | Effect measure MD (95% CI) | Heterogeneity (I 2, %) | |
|---|---|---|---|---|---|---|
| Bushen Quhan Zhiwang decoction | 6 | 348 | 348 | -1.96 (-3.79, -0.12)a | 85.4 | |
| YJC | 2 | 93 | 92 | -4.28 (-5.33, -3.23)a | 0.0 | |
| WT | 2 | 25 | 25 | -2.63 (-5.00, -0.26)a | 0.0 | |
| DJD | 8 | 259 | 259 | -4.48 (-5.26, -3.70)a | 80.5 | |
| BJTF | 2 | 70 | 70 | -5.75 (-17.89, 6.38) | 84.8 | |
| WP | 2 | 74 | 75 | -8.79 (-22.79, 5.20) | 86.4 | |
| QTOL | 2 | 64 | 64 | -10.53 (-17.28, -3.78)a | 54.2 | |
| WD | 1 | 30 | 30 | -3.12 (-5.85, -0.39)a | - | |
| Bushen Qinghua Zhiwang decoction | 2 | 140 | 140 | -4.78 (-10.79, 1.24) | 96.4 | |
| BZD | 2 | 98 | 97 | -0.46 (-0.91, -0.01)a | 78.5 | |
| Item | Number of studies (n) | Test group (n) | Control group (n) | Effect measure MD (95% CI) | Heterogeneity (I 2, %) |
|---|---|---|---|---|---|
| Bushen Quhan Zhiwang decoction | 6 | 348 | 325 | -2.63 (-3.49, -1.77)a | 39.8 |
| YJC | 2 | 93 | 92 | -7.88 (-8.78, -6.99)a | 41.1 |
| WT | 2 | 25 | 25 | -8.51 (-14.95, -2.07)a | 19.4 |
| DJD | 9 | 317 | 317 | -10.13 (-14.04, -6.22)a | 92.6 |
| BJTF | 2 | 70 | 70 | -2.99 (-12.20, 6.23) | 0.0 |
| WP | 2 | 74 | 75 | -6.73 (-12.59, -0.86)a | 0.0 |
| QTOL | 2 | 64 | 64 | -13.31 (-17.81, -8.82)a | 0.0 |
| Bushen Qinghua Zhiwang decoction | 2 | 140 | 140 | -10.66 (-23.16, 1.83) | 96.4 |
| BZD | 2 | 98 | 97 | -12.38 (-16.36, -8.40)a | 78.5 |
Table 5 Direct pairwise Meta-analysis of ESR
| Item | Number of studies (n) | Test group (n) | Control group (n) | Effect measure MD (95% CI) | Heterogeneity (I 2, %) |
|---|---|---|---|---|---|
| Bushen Quhan Zhiwang decoction | 6 | 348 | 325 | -2.63 (-3.49, -1.77)a | 39.8 |
| YJC | 2 | 93 | 92 | -7.88 (-8.78, -6.99)a | 41.1 |
| WT | 2 | 25 | 25 | -8.51 (-14.95, -2.07)a | 19.4 |
| DJD | 9 | 317 | 317 | -10.13 (-14.04, -6.22)a | 92.6 |
| BJTF | 2 | 70 | 70 | -2.99 (-12.20, 6.23) | 0.0 |
| WP | 2 | 74 | 75 | -6.73 (-12.59, -0.86)a | 0.0 |
| QTOL | 2 | 64 | 64 | -13.31 (-17.81, -8.82)a | 0.0 |
| Bushen Qinghua Zhiwang decoction | 2 | 140 | 140 | -10.66 (-23.16, 1.83) | 96.4 |
| BZD | 2 | 98 | 97 | -12.38 (-16.36, -8.40)a | 78.5 |
| A+W | 0.21 (0.08, 0.56)a | 0.19 (0.09, 0.38)a | 0.55 (0.23, 1.28) | - | 0.24 (0.10, 0.59)a | - | 0.78 (0.15, 4.17) | 0.98 (0.18, 5.21) | 0.23 (0.01, 4.02) | 0.21 (0.14, 0.31)a |
|---|---|---|---|---|---|---|---|---|---|---|
| 0.53 (0.10, 2.79) | B+W | 0.89 (0.31, 2.53) | 2.59 (0.82, 8.20) | - | 1.14 (0.34, 3.76) | - | 3.72 (0.59, 23.52) | 4.64 (0.73, 29.36) | 1.08 (0.06, 21.15) | 0.98 (0.40, 2.36) |
| 0.31 (0.06, 1.52) | 0.59 (0.06, 5.68) | C+W | 2.92 (1.16, 7.38) | - | 1.28 (0.48, 3.42) | - | 4.19 (0.76, 23.24) | 5.23 (0.94, 29.01) | 1.22 (0.07, 22.00) | 1.10 (0.63, 1.93) |
| 0.70 (0.41, 1.17) | 1.32 (0.24, 7.17) | 2.22 (0.44, 11.15) | D+W | - | 0.44 (0.15, 1.31) | - | 1.44 (0.24, 8.50) | 1.79 (0.30, 10.61) | 0.42 (0.02, 7.83) | 0.38 (0.18, 0.79)a |
| 0.74 (0.33, 1.63) | 1.40 (0.23, 8.44) | 2.36 (0.42, 13.19) | 1.06 (0.45, 2.50) | E+W | - | - | - | - | - | - |
| 0.63 (0.26, 1.53) | 1.19 (0.19, 7.51) | 2.00 (0.34, 11.76) | 0.90 (0.35, 2.33) | 0.85 (0.28, 2.61) | F+W | - | 3.28 (0.54, 20.00) | 4.09 (0.67, 24.97) | 0.95 (0.05, 18.23) | 0.86 (0.38, 1.93) |
| 1.12 (0.48, 2.65) | 2.13 (0.34, 13.21) | 3.59 (0.62, 20.68) | 1.61 (0.65, 4.03) | 1.52 (0.51, 4.55) | 1.79 (0.56, 5.77) | G+W | - | - | - | - |
| 0.22 (0.03, 1.90) | 0.42 (0.03, 6.16) | 0.70 (0.05, 9.88) | 0.32 (0.04, 2.80) | 0.30 (0.03, 2.87) | 0.35 (0.04, 3.50) | 0.20 (0.02, 1.93) | H+W | 1.25 (0.13, 12.30) | 0.29 (0.01, 7.62) | 0.26 (0.05, 1.32) |
| 0.66 (0.16, 2.71) | 1.26 (0.15, 10.69) | 2.12 (0.27, 16.92) | 0.95 (0.23, 4.04) | 0.90 (0.19, 4.29) | 1.06 (0.21, 5.33) | 0.59 (0.12, 2.92) | 3.02 (0.24, 38.55) | I+W | 0.23 (0.01, 6.12) | 0.21 (0.04, 1.06) |
| 0.64 (0.11, 3.71) | 1.21 (0.11, 13.16) | 2.03 (0.20, 20.97) | 0.92 (0.15, 5.49) | 0.86 (0.13, 5.71) | 1.02 (0.15, 7.03) | 0.57 (0.08, 3.86) | 2.90 (0.18, 45.76) | 0.96 (0.10, 8.82) | J+W | 0.90 (0.05, 15.49) |
| 2.73 (2.03, 3.65)a | 5.17 (1.00, 26.60)a | 8.71 (1.84, 41.26)a | 3.92 (2.55, 6.03)a | 3.70 (1.76, 7.75)a | 4.35 (1.87, 10.14)a | 2.43 (1.08, 5.45)a | 12.43 (1.46, 105.74)a | 4.11 (1.04, 16.29)a | 4.29 (0.75, 24.42) | W |
Table 6 Network Meta-analysis of the total effective rate (lower left) and network Meta-analysis of the incidence of adverse reactions (upper right)
| A+W | 0.21 (0.08, 0.56)a | 0.19 (0.09, 0.38)a | 0.55 (0.23, 1.28) | - | 0.24 (0.10, 0.59)a | - | 0.78 (0.15, 4.17) | 0.98 (0.18, 5.21) | 0.23 (0.01, 4.02) | 0.21 (0.14, 0.31)a |
|---|---|---|---|---|---|---|---|---|---|---|
| 0.53 (0.10, 2.79) | B+W | 0.89 (0.31, 2.53) | 2.59 (0.82, 8.20) | - | 1.14 (0.34, 3.76) | - | 3.72 (0.59, 23.52) | 4.64 (0.73, 29.36) | 1.08 (0.06, 21.15) | 0.98 (0.40, 2.36) |
| 0.31 (0.06, 1.52) | 0.59 (0.06, 5.68) | C+W | 2.92 (1.16, 7.38) | - | 1.28 (0.48, 3.42) | - | 4.19 (0.76, 23.24) | 5.23 (0.94, 29.01) | 1.22 (0.07, 22.00) | 1.10 (0.63, 1.93) |
| 0.70 (0.41, 1.17) | 1.32 (0.24, 7.17) | 2.22 (0.44, 11.15) | D+W | - | 0.44 (0.15, 1.31) | - | 1.44 (0.24, 8.50) | 1.79 (0.30, 10.61) | 0.42 (0.02, 7.83) | 0.38 (0.18, 0.79)a |
| 0.74 (0.33, 1.63) | 1.40 (0.23, 8.44) | 2.36 (0.42, 13.19) | 1.06 (0.45, 2.50) | E+W | - | - | - | - | - | - |
| 0.63 (0.26, 1.53) | 1.19 (0.19, 7.51) | 2.00 (0.34, 11.76) | 0.90 (0.35, 2.33) | 0.85 (0.28, 2.61) | F+W | - | 3.28 (0.54, 20.00) | 4.09 (0.67, 24.97) | 0.95 (0.05, 18.23) | 0.86 (0.38, 1.93) |
| 1.12 (0.48, 2.65) | 2.13 (0.34, 13.21) | 3.59 (0.62, 20.68) | 1.61 (0.65, 4.03) | 1.52 (0.51, 4.55) | 1.79 (0.56, 5.77) | G+W | - | - | - | - |
| 0.22 (0.03, 1.90) | 0.42 (0.03, 6.16) | 0.70 (0.05, 9.88) | 0.32 (0.04, 2.80) | 0.30 (0.03, 2.87) | 0.35 (0.04, 3.50) | 0.20 (0.02, 1.93) | H+W | 1.25 (0.13, 12.30) | 0.29 (0.01, 7.62) | 0.26 (0.05, 1.32) |
| 0.66 (0.16, 2.71) | 1.26 (0.15, 10.69) | 2.12 (0.27, 16.92) | 0.95 (0.23, 4.04) | 0.90 (0.19, 4.29) | 1.06 (0.21, 5.33) | 0.59 (0.12, 2.92) | 3.02 (0.24, 38.55) | I+W | 0.23 (0.01, 6.12) | 0.21 (0.04, 1.06) |
| 0.64 (0.11, 3.71) | 1.21 (0.11, 13.16) | 2.03 (0.20, 20.97) | 0.92 (0.15, 5.49) | 0.86 (0.13, 5.71) | 1.02 (0.15, 7.03) | 0.57 (0.08, 3.86) | 2.90 (0.18, 45.76) | 0.96 (0.10, 8.82) | J+W | 0.90 (0.05, 15.49) |
| 2.73 (2.03, 3.65)a | 5.17 (1.00, 26.60)a | 8.71 (1.84, 41.26)a | 3.92 (2.55, 6.03)a | 3.70 (1.76, 7.75)a | 4.35 (1.87, 10.14)a | 2.43 (1.08, 5.45)a | 12.43 (1.46, 105.74)a | 4.11 (1.04, 16.29)a | 4.29 (0.75, 24.42) | W |
| A+W | -2.67 (-11.62, 6.28) | -6.18 (-18.05, 5.69) | -6.26 (-12.35, -0.17)a | 0.86 (-12.05, 13.76) | -2.34 (-13.49, 8.81) | -8.47 (-18.93, 1.99) | - | -6.68 (-15.65, 2.30) | -8.02 (-17.56, 1.53) | 3.88 (-0.76, 8.52) |
|---|---|---|---|---|---|---|---|---|---|---|
| -2.56 (-9.24, 4.11) | B+W | -3.51 (-16.88, 9.85) | -3.59 (-12.22, 5.04) | 3.53 (-10.74, 17.80) | 0.33 (-12.37, 13.03) | -5.80 (-17.89, 6.29) | - | -4.01 (-14.86, 6.84) | -5.35 (-16.66, 5.97) | 6.55 (-1.11, 14.21) |
| 0.82 (-6.37, 8.01) | 3.38 (-5.30, 12.07) | C+W | -0.08 (-11.70, 11.54) | 7.04 (-9.23, 23.31) | 3.84 (-11.09, 18.77) | -2.29 (-16.72, 12.14) | - | -0.50 (-13.86, 12.87) | -1.84 (-15.60, 11.93) | 10.06 (-0.88, 21.01) |
| -4.11 (-8.65, 0.43) | -1.54 (-8.22, 5.13) | -4.93 (-12.14, 2.28) | D+W | 7.12 (-5.56, 19.80) | 3.92 (-6.97, 14.81) | -2.21 (-12.39, 7.98) | - | -0.42 (-9.06, 8.22) | -1.76 (-10.99, 7.48) | 10.14 (6.17, 14.11)a |
| -1.16 (-7.80, 5.49) | 1.41 (-6.84, 9.66) | -1.98 (-10.66, 6.71) | 2.95 (-3.67, 9.57) | E+W | -3.20 (-18.94, 12.54) | -9.33 (-24.58, 5.93) | - | -7.54 (-21.82, 6.75) | -8.87 (-23.52, 5.77) | 3.02 (-9.02, 15.06) |
| -4.07 (-11.60, 3.46) | -1.50 (-10.49, 7.49) | -4.89 (-14.33, 4.56) | 0.04 (-7.41, 7.50) | -2.91 (-11.83, 6.00) | F+W | -6.13 (-19.92, 7.66) | - | -4.34 (-17.06, 8.38) | -5.68(-18.79, 7.44) | 6.22 (-3.91, 16.35) |
| -8.05 (-15.42, -0.68)a | -5.48 (-14.33, 3.36) | -8.87 (-18.14, 0.40) | -3.94 (-11.27, 3.39) | -6.89 (-15.69, 1.90) | -3.98 (-13.40, 5.44) | G+W | - | 1.79 (-10.33, 13.91) | 0.45 (-12.08, 12.99) | 12.35 (2.98, 21.72)a |
| -0.69 (-9.33, 7.95) | 1.88 (-8.04, 11.80) | -1.51 (-11.77, 8.76) | 3.42 (-5.22, 12.06) | 0.47 (-9.44, 10.38) | 3.38 (-7.16, 13.92) | 7.36 (-3.05, 17.78) | H+W | - | - | - |
| -2.33 (-8.67, 4.00) | 0.23 (-7.77, 8.22) | -3.16 (-11.57, 5.26) | 1.77 (-4.56, 8.10) | -1.18 (-9.15, 6.80) | 1.73 (-7.02, 10.48) | 5.71 (-2.88, 14.31) | -1.65 (-11.34, 8.04) | I+W | -1.34 (-12.67, 10.00) | 10.56 (2.88, 18.24)a |
| 1.60 (-4.64, 7.83) | 4.16 (-3.76, 12.09) | 0.78 (-7.57, 9.13) | 5.71 (-0.54, 11.95) | 2.75 (-5.16, 10.66) | 5.66 (-3.03, 14.35) | 9.65 (1.11, 18.18)a | 2.28 (-7.35, 11.92) | 3.93 (-3.70, 11.57) | J+W | 11.90 (3.56, 20.23)a |
| 2.43 (-0.80, 5.67) | 5.00 (-0.85, 10.85) | 1.61 (-4.80, 8.03) | 6.54 (3.31, 9.77)a | 3.59 (-2.24, 9.42) | 6.50 (-0.35, 13.36) | 10.48 (3.83, 17.14)a | 3.12 (-4.89, 11.13) | 4.77 (-0.68, 10.22) | 0.84 (-4.51, 6.18) | W |
Table 7 Network Meta-analysis of CRP (lower left) and network Meta-analysis of ESR (upper right)
| A+W | -2.67 (-11.62, 6.28) | -6.18 (-18.05, 5.69) | -6.26 (-12.35, -0.17)a | 0.86 (-12.05, 13.76) | -2.34 (-13.49, 8.81) | -8.47 (-18.93, 1.99) | - | -6.68 (-15.65, 2.30) | -8.02 (-17.56, 1.53) | 3.88 (-0.76, 8.52) |
|---|---|---|---|---|---|---|---|---|---|---|
| -2.56 (-9.24, 4.11) | B+W | -3.51 (-16.88, 9.85) | -3.59 (-12.22, 5.04) | 3.53 (-10.74, 17.80) | 0.33 (-12.37, 13.03) | -5.80 (-17.89, 6.29) | - | -4.01 (-14.86, 6.84) | -5.35 (-16.66, 5.97) | 6.55 (-1.11, 14.21) |
| 0.82 (-6.37, 8.01) | 3.38 (-5.30, 12.07) | C+W | -0.08 (-11.70, 11.54) | 7.04 (-9.23, 23.31) | 3.84 (-11.09, 18.77) | -2.29 (-16.72, 12.14) | - | -0.50 (-13.86, 12.87) | -1.84 (-15.60, 11.93) | 10.06 (-0.88, 21.01) |
| -4.11 (-8.65, 0.43) | -1.54 (-8.22, 5.13) | -4.93 (-12.14, 2.28) | D+W | 7.12 (-5.56, 19.80) | 3.92 (-6.97, 14.81) | -2.21 (-12.39, 7.98) | - | -0.42 (-9.06, 8.22) | -1.76 (-10.99, 7.48) | 10.14 (6.17, 14.11)a |
| -1.16 (-7.80, 5.49) | 1.41 (-6.84, 9.66) | -1.98 (-10.66, 6.71) | 2.95 (-3.67, 9.57) | E+W | -3.20 (-18.94, 12.54) | -9.33 (-24.58, 5.93) | - | -7.54 (-21.82, 6.75) | -8.87 (-23.52, 5.77) | 3.02 (-9.02, 15.06) |
| -4.07 (-11.60, 3.46) | -1.50 (-10.49, 7.49) | -4.89 (-14.33, 4.56) | 0.04 (-7.41, 7.50) | -2.91 (-11.83, 6.00) | F+W | -6.13 (-19.92, 7.66) | - | -4.34 (-17.06, 8.38) | -5.68(-18.79, 7.44) | 6.22 (-3.91, 16.35) |
| -8.05 (-15.42, -0.68)a | -5.48 (-14.33, 3.36) | -8.87 (-18.14, 0.40) | -3.94 (-11.27, 3.39) | -6.89 (-15.69, 1.90) | -3.98 (-13.40, 5.44) | G+W | - | 1.79 (-10.33, 13.91) | 0.45 (-12.08, 12.99) | 12.35 (2.98, 21.72)a |
| -0.69 (-9.33, 7.95) | 1.88 (-8.04, 11.80) | -1.51 (-11.77, 8.76) | 3.42 (-5.22, 12.06) | 0.47 (-9.44, 10.38) | 3.38 (-7.16, 13.92) | 7.36 (-3.05, 17.78) | H+W | - | - | - |
| -2.33 (-8.67, 4.00) | 0.23 (-7.77, 8.22) | -3.16 (-11.57, 5.26) | 1.77 (-4.56, 8.10) | -1.18 (-9.15, 6.80) | 1.73 (-7.02, 10.48) | 5.71 (-2.88, 14.31) | -1.65 (-11.34, 8.04) | I+W | -1.34 (-12.67, 10.00) | 10.56 (2.88, 18.24)a |
| 1.60 (-4.64, 7.83) | 4.16 (-3.76, 12.09) | 0.78 (-7.57, 9.13) | 5.71 (-0.54, 11.95) | 2.75 (-5.16, 10.66) | 5.66 (-3.03, 14.35) | 9.65 (1.11, 18.18)a | 2.28 (-7.35, 11.92) | 3.93 (-3.70, 11.57) | J+W | 11.90 (3.56, 20.23)a |
| 2.43 (-0.80, 5.67) | 5.00 (-0.85, 10.85) | 1.61 (-4.80, 8.03) | 6.54 (3.31, 9.77)a | 3.59 (-2.24, 9.42) | 6.50 (-0.35, 13.36) | 10.48 (3.83, 17.14)a | 3.12 (-4.89, 11.13) | 4.77 (-0.68, 10.22) | 0.84 (-4.51, 6.18) | W |
| Treatment | Total effective rate | Incidence of adverse reactions | CRP | ESR |
|---|---|---|---|---|
| A+W | 30.6 | 85.7a | 36.7 | 27.0 |
| B+W | 62.2 | 27.2 | 59.8 | 43.5 |
| C+W | 78.2 | 20.8 | 31.6 | 63.2 |
| D+W | 52.8 | 66.4 | 74.7 | 67.4 |
| E+W | 49.7 | - | 47.8 | 28.4 |
| F+W | 57.8 | 33.4 | 71.1 | 43.3 |
| G+W | 27.8 | - | 92.8a | 76.8a |
| H+W | 82.8b | 73.9 | 42.8 | - |
| I+W | 54.2 | 81.2 | 57.8 | 68.4 |
| J+W | 52.5 | 36.1 | 22.4 | 75.4 |
| W | 1.4 | 25.6 | 12.5 | 6.6 |
Table 8 SUCRA values for each intervention
| Treatment | Total effective rate | Incidence of adverse reactions | CRP | ESR |
|---|---|---|---|---|
| A+W | 30.6 | 85.7a | 36.7 | 27.0 |
| B+W | 62.2 | 27.2 | 59.8 | 43.5 |
| C+W | 78.2 | 20.8 | 31.6 | 63.2 |
| D+W | 52.8 | 66.4 | 74.7 | 67.4 |
| E+W | 49.7 | - | 47.8 | 28.4 |
| F+W | 57.8 | 33.4 | 71.1 | 43.3 |
| G+W | 27.8 | - | 92.8a | 76.8a |
| H+W | 82.8b | 73.9 | 42.8 | - |
| I+W | 54.2 | 81.2 | 57.8 | 68.4 |
| J+W | 52.5 | 36.1 | 22.4 | 75.4 |
| W | 1.4 | 25.6 | 12.5 | 6.6 |
| 1. | Aletaha D, Neogi T, Silman AJ, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 2010; 62: 2569-81. |
| 2. | Chinese Rheumatology Association. 2018 Chinese guideline for the diagnosis and treatment of rheumatoid arthritis. Zhong Hua Nei Ke Za Zhi 2018; 57: 242-51. |
| 3. | Tian XP, Li MT, Zeng XF. The challenges and opportunities for the management of rheumatoid arthritis in China:an annual report of 2019. Chin J Intern Med 2021; 60: 593-8. |
| 4. | Tanaka E, Hoshi D, Igarashi A, et al. Analysis of direct medical and nonmedical costs for care of rheumatoid arthritis patients using the large cohort database, IORRA. Mod Rheumatol 2013; 23: 742-51. |
| 5. |
Aletaha D, Smolen JS. Diagnosis and management of rheumatoid arthritis: a review. JAMA 2018; 320: 1360-72.
DOI PMID |
| 6. |
Yamanaka H, Tanaka E, Nakajima A, et al. A large observational cohort study of rheumatoid arthritis, IORRA: providing context for today’s treatment options. Mod Rheumatol 2020; 30: 1-6.
DOI PMID |
| 7. | Wang Y, Chen S, Du K, et al. Traditional herbal medicine: therapeutic potential in rheumatoid arthritis. J Ethnopharmacol 2021; 279: 114368. |
| 8. | Jiao S. A further discussion on the identification and treatment of Wangbi based on syndrome differentiation. Zhong Yi Za Zhi 1997; 8: 504-5. |
| 9. | Yao C, Dou D, Xu C, et al. Systematic review of kidney tonifying therapy in treatment of rheumatoid arthritis. Zhong Guo Zhong Yi Ji Chu Yi Xue Za Zhi 2021; 27: 1414-19. |
| 10. | Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021; 372: n71. |
| 11. | Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988; 31: 315-24. |
| 12. | Diagnostic basis, classification of syndromes, and assessment of efficacy of Wangbi—Chinese medicine industry standard of the People’s Republic of China,Diagnostic and efficacy criteria for internal medicine diseases (ZY/T001.1-94). Liaoning Zhong Yi Yao Da Xue Xue Bao 2016; 18: 217. |
| 13. | McGrath S, Zhao X, Steele R, Thombs BD, Benedetti A. Estimating the sample mean and standard deviation from commonly reported quantiles in Meta-analysis. Stat Methods Med Res 2020; 29: 2520-37. |
| 14. | Cumpston M, Li T, Page MJ, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev 2019; 10: ED000142. |
| 15. |
Debray TPA, Moons KGM, Riley RD. Detecting small-study effects and funnel plot asymmetry in Meta-analysis of survival data: a comparison of new and existing tests. Res Synth Methods 2018; 9: 41-50.
DOI PMID |
| 16. |
Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol 2011; 64: 383-94.
DOI PMID |
| 17. | Puhan MA, Schünemann HJ, Murad MH, et al. A GRADE Working Group approach for rating the quality of treatment effect estimates from network Meta-analysis. BMJ 2014; 349: g5630. |
| 18. | Shim S, Yoon BH, Shin IS, Bae JM. Network Meta-analysis: application and practice using Stata. Epidemiol Health. 2017; 39: e2017047. |
| 19. | Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in Meta-analyses. BMJ 2003; 327: 557-60. |
| 20. | Chen D. Analysis of the efficacy and safety of the combination of Bushen Quhan Zhiwang decoction and Methotrexate in the treatment of rheumatoid arthritis with syndrome of kidney deficiency and cold. Shuang Zu Yu Bao Jian 2018; 27: 157-9. |
| 21. | Chen F, Min CH, Zhou Y, Zhang HM. Clinical observation on 35 cases of rheumatoid arthritis treated with Yishen Juanbi capsule combined with Western Medicine. Zhong Yi Za Zhi 2016; 57: 1045-8. |
| 22. | Chen L, Yan XP, Shi GY, Zhao CQ. Clinical observation on Wangbi tablet treating on early rheumatoid arthritis with syndrome of liver and kidney deficiency and syndrome of wind-dampness blocking collaterals. Zhong Hua Zhong Yi Yao Za Zhi 2021; 36: 2400-3. |
| 23. | Chen XL, Yin ZJ, Wang HJ. Clinical efficacy of Duhuo Jisheng decoction in the treatment of rheumatoid arthritis. Neimenggu Zhong Yi Yao 2020; 39: 22-3. |
| 24. | Chen YG. Clinical study on Jiawei Duhuo Jisheng decoction in treating rheumatoid arthritis of liver and kidney deficiency pattern. Guangxi: Guangxi University of Traditional Chinese Medicine, 2019: 1-43. |
| 25. | Du DY. Clinical study on the combination of Chinese and Western Medicines in the treatment of rheumatoid arthritis with liver and kidney deficiency syndrome. Shi Yong Zhong Yi Yao Za Zhi 2019; 35: 1137-8. |
| 26. | Gao Y. Clinical observation on Yishen Juanbi Capsule combined with Western Medicine for treatment of elder-onset rheumatoid arthritis. Zhong Cao Yao 2015; 46: 1653-5. |
| 27. | He CX. Clinical study on the treatment of rheumatoid arthritis by Bushen Quhan Zhiwang decoction and the effect on TNF-α and MMP-13 in the joints of CIA rats. Beijing: Beijing University of Traditional Chinese Medicine, 2018: 1-29. |
| 28. | He YK, Yang G, Lyu M, Zheng Y, Xu X, Pan X. Effects of kidney-nourishing, toxin-relieving and collateral-unblocking decoction on IL-1, IL-6 and TNF-α in patients with rheumatoid arthritis. Liaoning Zhong Yi Yao Za Zhi 2015; 42: 1451-3. |
| 29. | He YK, Yang GH, Zheng YQ, et al. Effect of Bushen Tongluo Jiedu decoction on the angiogenesis relative factors in the serum of patients with rheumatoid arthritis. Tianjin Zhong Yi Yao 2018; 35: 732-5. |
| 30. | Hou HL. Wenbikang pill treatment the Yin deficiency of liver and kidney of the rheumatoid arthritis to observe the clinical efficacy. Hunan: Hunan University of Traditional Chinese Medicine, 2013: 1-25. |
| 31. | Ji HW, Luo Q, Cao XJ, et al. Clinical efficacy of Wangbi Tablet in the treatment of rheumatoid arthritis of liver and kidney deficiency with blood stasis syndrome. In: Lou YQ, Wang CD, Wu DH, Zhang FC, Fan YS, editors. Medical and health science. CACM 2012: Proceedings of the 16th National Rheumatism Conference of the China Association of Chinese Medicine; 2012 Aug 18; Huangshan, China. Beijing: China Association of Chinese Medicine, 2012: 197-9. |
| 32. | Li N. Evaluation on efficacy and safety of Zhiwang decoction combined with Methotrexate for rheumatoid arthritis with kidney deficiency and cold excession. Zhong Yi Lin Chuang Yan Jiu 2014; 6: 8-9. |
| 33. | Li SQ, Li N, Qin JJ, Yang XY. Clinical study on the treatment of rheumatoid arthritis with kidney deficiency and coldness syndrome in combination with methotrexate by Bushen Quhan Zhiwang decoction. Neimenggu Zhong Yi Yao 2017; 36: 54-5. |
| 34. | Li XL. Clinical observation of 34 cases of rheumatoid arthritis treated with Qiwei Tongbi oral liquid with conventional Western Medicine. Zhong Yi Yao Dao Bao 2015; 21: 69-71. |
| 35. | Liang J, Pan MC, Wu XG, Yang LL, Su G. Clinical observation of 62 cases of rheumatoid arthritis treated with Bushen Quhan Zhiwang decoction. Gansu Ke Ji 2015; 31: 149-51. |
| 36. | Lin GY, Liang D, Liu LH, Wang JP. Efficacy of Bushen Quhan Zhiwang decoction combined with Methotrexate in the treatment of rheumatoid arthritis with kidney deficiency and cold syndrome. Shi Jie Zui Xin Yi Xue Xin Xi Wen Zhai 2016; 16: 95. |
| 37. | Luo W, Fu Q, Yan XP. Clinical observation of rheumatoid arthritis treated with Strong Kidney-bone formula. Zhong Hua Zhong Yi Yao Za Zhi 2013; 28: 2813-16. |
| 38. | Miao XY. The research of influence about Wan Bi decoction on inflammatory indicators of rheumatoid arthritis (kidney-deficiency and blood-stasis syndrome). Henan: Henan University of Chinese Medicine, 2015: 1-26. |
| 39. | Qiu SB, Wu JY, Jiang JY, Qiu F, Tang Y, Chen YQ. Clinical efficacy of Duhuo Jisheng decoction as adjuvant therapy for rheumatoid arthritis with liver-kidney deficiency pattern. Guangxi Yi Xue 2018; 40: 2675-77, 81. |
| 40. | Shen JN. Effect of Bushen Jiedu Tongluo recipe combined with methotrexate on bone corrosion of rheumatoid arthritis. Shanghai: Shanghai University of Traditional Chinese Medicine, 2019: 1-47. |
| 41. | Shi Y, Yang L, Jiang Y, Mou FX, Xie L, Zou QH. Clinical observation of Duhuo Jisheng soup in treatment of rheumatoid arthritis of liver and kidney deficiency type. Lin Chuang He Li Yong Yao Za Zhi 2019; 12: 24-6. |
| 42. | Sun GM, Gao B, Zhang D. Observation on clinical efficacy of Wangbi tablets combined with methotrexate in the treatment of rheumatoid arthritis with syndrome of liver and kidney deficiency. Zhong Guo Shi Yong Yi Yao 2021; 16: 147-9. |
| 43. | Wan LY, Yang K, Mei XP. Clinical research on the combination of Duhuo Jisheng decoction and Leflunomide in the treatment of rheumatoid arthritis. Da Zhong Ke Ji 2016; 18: 71-3, 8. |
| 44. | Wang G, Wang T, Dang P. Clinical observation on 30 cases of rheumatoid arthritis treated with Bushen Quhan Zhiwang decoction combined with Methotrexate. Zhong Guo Zhong Yi Ji Chu Yi Xue Za Zhi 2021; 27: 1298-300. |
| 45. | Wang JM, Tao QW, Zhang YZ, Xu Y, Yan XP. Treating rheumatoid arthritis patients of shen deficiency and cold invading syndrome by Bushen Quhan Zhiwang decoction combined Methotrexate: an evaluation of clinical efficacy and safety. Zhong Guo Zhong Xi Yi Jie He Za Zhi 2013; 33: 614-8. |
| 46. | Wang JM, Zhang YZ, Ma X, Zhang YZ, Yan XP, Tao QW. The evaluation of clinical efficacy and safety of Bushenqinghuazhiwang decoction combined with methotrexate in the treatment of rheumatoid arthritis belonging to dampness-heat injury kidney syndrome. Zhong Ri You Hao Yi Yuan Xue Bao 2018; 32: 67-70. |
| 47. | Wang X, Li WY. Clinical observation of Wanbikang pill in treating rheumatoid arthritis of liver and kidney Yin deficiency syndrome. Hubei Zhong Yi Za Zhi 2019; 41: 12-5. |
| 48. | Xu L, Chen YY, Xu CS, et al. 30 cases of rheumatoid arthritis treated by Qiwei Tongbi oral liquid. Nanjing Zhong Yi Yao Da Xue Xue Bao 2012; 28: 586-8. |
| 49. | Xu ZY, Ma YJ. Efficacy of Chinese medicine in treating rheumatoid arthritis with damp-heat-injured kidney syndrome by combining Methotrexate with Bushen Qinghua Zhiwang decoction. Zhong Hua Yang Sheng Bao Jian 2020; 38: 10-1. |
| 50. | Xue QL, Pang HM, Zhang YJ. Clinical observation of Bushen-quhan-zhiwang decoction combined with methotrexate on the treatment of rheumatoid arthritis. Hebei Zhong Yi 2016; 38: 1333-7. |
| 51. | Yan WC. Methotrexate combined obstinate arthralgia soup treatment of rheumatoid arthritis (deficiency and blood stasis resistance type) of clinical observation. Henan: Henan University of Chinese Medicine, 2015: 1-34. |
| 52. | Yang F, Shen JY, Cao H. Evaluation of clinical effect of modified Duhuo Jisheng decoction combined with Methotrexate in the treatment of rheumatoid arthritis with liver and kidney deficiency. Shi Jie Zhong Xi Yi Jie He Za Zhi 2020; 15: 1494-7. |
| 53. | Yao MS. The effectness of Duhuo Jisheng decoction cure the rheumatoid arthritis of liver and kidney deficiency. Guangzhou: Guangzhou University of Chinese Medicine, 2013: 1-46. |
| 54. | Zhang BC. Efficacy of Bushen Quhan Zhiwang decoction combined with Methotrexate in the treatment of rheumatoid arthritis with kidney deficiency and cold syndrome. Shi Yong Zhong Yi Yao Za Zhi 2017; 33: 1402-3. |
| 55. | Zhang L. Efficacy of Bushen Quhan Zhiwang decoction combined with Methotrexate in the treatment of rheumatoid arthritis. Heilongjiang Zhong Yi Yao 2017; 46: 25-6. |
| 56. | Zhang WJ, Yang H, Huang JC, Ma FQ. On the treatment of 30 cases with rheumatoid arthritis due to liver and kidney deficiency by Duhuo Jisheng decoction combined with Leflunomide. Feng Shi Bing Yu Guan Jie Yan 2013; 2: 24-6. |
| 57. | Zhao HY, Zhang HT, Guo DD, Zhang XR. Effects of Duhuo Jisheng decoction on Th17/Treg balance in rheumatoid arthritis of liver-kidney insufficiency, cold-dampness obstruction pattern. Xi Bu Zhong Yi Yao 2021; 34: 8-12. |
| 58. | Su DC, Li C, Zhou L. Effect of Duhuo Jizhi decoction on rheumatoid arthritis with deficiency of liver and kidney. Dang Dai Yi Yao Lun Cong 2022; 20: 168-71. |
| 59. | Lan TY, Wang ZH, Yan ZR, et al. The delaying effect of Bushen Zhiwang decoction on bone destruction in rheumatoid arthritis patients with a pattern of deficiency of both liver and kidney based on modified total Sharp score. Beijing Zhong Yi Yao Da Xue Xue Bao 2023; 46: 557-63. |
| 60. | Liu X, Xia P, Zhou Q, et al. Effect of Duhuo Jisheng decoction on early cartilage destruction markers in patients with rheumatoid arthritis with liver-kidney Yin eficiency syndrome. Guo Ji Zhong Yi Zhong Yao Za Zhi 2022; 44: 1370-4. |
| 61. | Yao CH, Xu C, Zhang YZ. Clinical observation on delaying progression of bone erosion in rheumatoid arthritis with Bushen Zhiwang decoction. Zhong Guo Zhong Yi Ji Chu Yi Xue Za Zhi 2023; 29: 976-81. |
| 62. | Zeng XF, Zhu S, Tan A, Xie X. Disease burden and quality of life of rheumatoid arthritis in China: a systematic review. Zhong Guo Xun Zheng Yi Xue Za Zhi 2013; 13: 300-7. |
| 63. | Ma X, Guo Z, Tao QW, Yan ZR, Liu Z, Xu Y. Mechanism of Zhiwang decoction combined with Methotrexate against bone destruction through regulating RANKL/OPG pathway in rats with collagen-induced arthritis. Zhong Guo Shi Yan Fang Ji Xue Za Zhi 2022; 28: 46-54. |
| 64. | Wang JM, Tao QW, Zhang YZ, et al. Treating rheumatoid arthritis patients of Shen deficiency and cold invading syndrome by Bushen Quhan Zhiwang decoction combined methotrexate: an evaluation of clinical efficacy and safety. Zhong Guo Zhong Xi Yi Jie He Za Zhi 2013; 33: 614-8. |
| 65. | Ou-Yang SH, Jiang T, Zhu L, Yi T. Dioscorea nipponica Makino: a systematic review on its ethnobotany, phytochemical and pharmacological profiles. Chem Cent J 2018; 12: 57. |
| 66. | Ke Z, Zhang X, Geng T, et al. Research on molecular mechanism of Qiwei Tongbi oral liquid in treatment of rheumatoid arthritis via network pharmacology. Zhong Cao Yao 2020; 51: 4489-97. |
| [1] | WANG Chao, WU Qiong, LI Ping, WANG Zhigang, LOU Xusheng, LI Yuanyuan, ZHANG Lin. Effect of Traditional Chinese Medicine combined with Western Medicine on blood lipid levels and inflammatory factors in patients with angina pectoris in coronary heart disease identified as intermingled phlegm and blood stasis syndrome: a network Meta-analysis [J]. Journal of Traditional Chinese Medicine, 2023, 43(4): 640-649. |
| [2] | HUANG Zhaohan, FANG Yuan, WANG Xiaolu, HAN Yue, YU Qi, WANG Tong. Effectiveness of acupuncture-related therapies on schizophrenia: a Bayesian network Meta-analysis [J]. Journal of Traditional Chinese Medicine, 2023, 43(2): 239-251. |
| [3] | Zhang Dan, Wang Kaihuan, Zheng Jiewen, Wu Jiarui, Duan Xiaojiao, Ni Mengwei, Liu Shuyu, Zhang Bing, Zhao Yi. Comparative efficacy and safety of Chinese herbal injections combined with transcatheter hepatic arterial chemoembolization in treatment of liver cancer: a bayesian network Meta-analysis [J]. Journal of Traditional Chinese Medicine, 2020, 40(2): 167-187. |
| [4] | Wang Haixia, Mo Shu, Yang Li, Wang Panpan, Sun Kehuan, Xiong Yingquan, Liu Hengrui, Liu Xiaoguang, Wu Zhidi, Ou Ling, Li Xiaoyun, Peng Xunqian, Peng Bojia, He Haibin, Tian Ya, Zhang Ronghua, Zhu Xiaofeng. Effectiveness associated with different therapies for senile osteoporosis: a network Meta-analysis [J]. Journal of Traditional Chinese Medicine, 2020, 40(1): 17-27. |
| [5] | Tan Di, Wu Jiarui, Jing Zhiwei, Duan Xiaojiao, Cui Yingying, Liu Shi. Efficacy and safety of ginkgo injections in the treatment of angina pectoris caused by coronary heart disease in China: a network Meta-analysis and systematic review [J]. Journal of Traditional Chinese Medicine, 2019, 39(03): 285-296. |
| [6] | Liu Shi, Wu Jiarui, Zhang Dan, Tan Di. What are the best Salvia miltiorrhiza injection classes for treatment of unstable angina pectoris? A systematic review and network Meta-analysis [J]. Journal of Traditional Chinese Medicine, 2018, 38(03): 321-338. |
| [7] | Tan Di, Wu Jiarui, Liu Shi, Zhang Dan, Cui Yingying, Zhang Xiaomeng, Zhang Bing. Injections of ginkgo in the treatment of cerebral infarction: a systematic review and network Meta-analysis [J]. Journal of Traditional Chinese Medicine, 2018, 38(01): 1-11. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||


