Journal of Traditional Chinese Medicine ›› 2025, Vol. 45 ›› Issue (1): 100-106.DOI: 10.19852/j.cnki.jtcm.2025.01.008
Previous Articles Next Articles
Promising effects of Persian shallot extract on the serum markers and blood pressure of patients with metabolic syndrome: a double-blinded randomized controlled trial
Neda Naimipoor1, Zahra Bagheri-Hosseinabadi1, Mohammad Reza Hajizadeh1, Mitra Abbasifard2, Mohammad Reza Mirzaei3, Akram Ghadiri Anari4, Maryam Mohammad-Sadeghipour5,6, Mehdi Mahmoodi5,6( )
)
- 1 Zahra Bagheri-Hosseinabadi, Mohammad Reza Hajizadeh, Department of Clinical Biochemistry, School of Medicine, Rafsanjan University of Medical Sciences, Rafsanjan 77181759, Iran
2 Department of Internal Medicine, School of Medicine, Ali-Ibn Abi-Talib Hospital, Rafsanjan University of Medical Sciences, Rafsanjan 7717937555, Iran
3 Molecular Medicine Research Center, Research Institute of Basic Medical Sciences, Rafsanjan University of Medical Sciences, Rafsanjan 7718175911, Iran
4 Diabetes Research Center, Shahid Sadoughi University of Medical Sciences, Yazd 8917693571, Iran
5 Department of Clinical Biochemistry, Afzalipour School of Medicine, Kerman University of Medical Sciences, Kerman 7616914115, Iran
6 Physiology Research Center, Institute of Neuropharmacology, Kerman University of Medical Sciences, Kerman 7619813159, Iran
-
Received:2023-09-22Accepted:2024-04-15Online:2025-02-15Published:2025-01-10 -
Contact:Prof. Mehdi Mahmoodi, Department of Clinical Biochemistry, Afzalipour School of Medicine, Kerman University of Medical Sciences, Kerman, 7616914115, Iran.me.mahmoodi@kmu.ac.ir Telephone: +98-9131914855
Cite this article
Neda Naimipoor, Zahra Bagheri-Hosseinabadi, Mohammad Reza Hajizadeh, Mitra Abbasifard, Mohammad Reza Mirzaei, Akram Ghadiri Anari, Maryam Mohammad-Sadeghipour, Mehdi Mahmoodi. Promising effects of Persian shallot extract on the serum markers and blood pressure of patients with metabolic syndrome: a double-blinded randomized controlled trial[J]. Journal of Traditional Chinese Medicine, 2025, 45(1): 100-106.
share this article
| Variable | Placebo group (n = 25) | Intervention group (n = 25) | aP value | |
|---|---|---|---|---|
| Age (years) | 43.8±7.3 | 45.1±6.9 | 0.51 | |
| Sex [n (%)] | Male | 11 (44) | 12 (48) | 0.50 |
| Female | 14 (56) | 13 (52) | ||
| Height (cm) | 167.2±8.9 | 170.9±9.5 | 0.26 | |
| Weight (Kg) | 78.5±10.3 | 81.5±10.4 | 0.11 | |
| BMI (kg/m2) | 26.9±0.9 | 27.4±1.5 | 0.16 | |
| Waist circumference (cm) | 97.4±5.2 | 98.9±6.1 | 0.23 | |
| Systolic blood pressure (mm Hg) | 132.5±4.8 | 132.5±5.0 | 0.91 | |
| Diastolic blood pressure (mm Hg) | 82.3±1.5 | 82.7±1.9 | 0.38 | |
Table 1 Comparison of demographic characteristics between the intervention and placebo group
| Variable | Placebo group (n = 25) | Intervention group (n = 25) | aP value | |
|---|---|---|---|---|
| Age (years) | 43.8±7.3 | 45.1±6.9 | 0.51 | |
| Sex [n (%)] | Male | 11 (44) | 12 (48) | 0.50 |
| Female | 14 (56) | 13 (52) | ||
| Height (cm) | 167.2±8.9 | 170.9±9.5 | 0.26 | |
| Weight (Kg) | 78.5±10.3 | 81.5±10.4 | 0.11 | |
| BMI (kg/m2) | 26.9±0.9 | 27.4±1.5 | 0.16 | |
| Waist circumference (cm) | 97.4±5.2 | 98.9±6.1 | 0.23 | |
| Systolic blood pressure (mm Hg) | 132.5±4.8 | 132.5±5.0 | 0.91 | |
| Diastolic blood pressure (mm Hg) | 82.3±1.5 | 82.7±1.9 | 0.38 | |
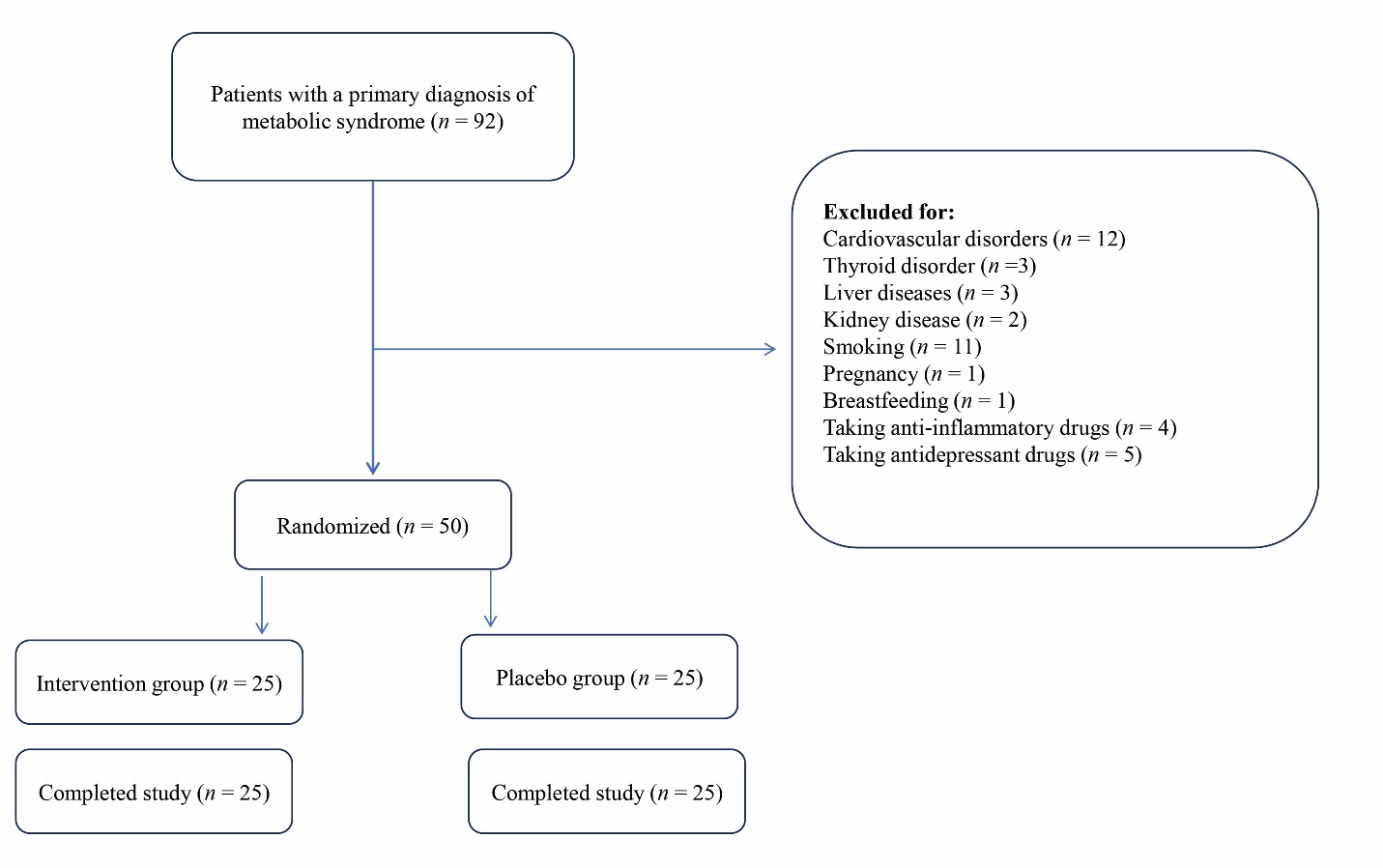
Figure 1 Flow chart of the study Placebo group received gelatin capsules filled with cellulose (600 mg) twice a day for 12 weeks. Intervention group received gelatin capsules filled with Persian shallot extract (600 mg) twice a day for 3 months.
| Variable | Intervention group (n = 25)? | Placebo group (n = 25)? | Between two groups | |||||
|---|---|---|---|---|---|---|---|---|
| Before | After | P valuea | Before | After | P valuea | P value (before)b | P value (after)b | |
| Serum levels of MDA (μmol/L) | 5.17±0.78 | 3.81±0.75 | <0.001 | 4.87±0.66 | 4.45±0.67 | <0.001 | 0.146 | 0.003 |
| Total antioxidant capacity (μmol/L) | 0.62±0.11 | 1.03±0.19 | <0.001 | 0.61±0.12 | 0.71±0.11 | <0.001 | 0.823 | <0.001 |
| SOD activity level (U/mL) | 43.69±5.27 | 45.58±6.03 | 0.159 | 41.45±6.39 | 42.14±6.80 | 0.571 | 0.154 | 0.065 |
| Serum ox-LDL levels (ng/L) | 278.39±32.22 | 241.32±37.71 | <0.001 | 277.77±41.19 | 264.70±33.95 | 0.002 | 0.953 | 0.026 |
| Serum level of Apo-H (ng/mL) | 19.12±2.86 | 17.15±2.56 | 0.001 | 18.08±2.55 | 17.15±2.17 | 0.080 | 0.180 | 0.995 |
| FBS (mg/dL) | 116.96±8.88 | 99.12±8.77 | <0.001 | 110.44±10.67 | 105.36±10.54 | <0.001 | 0.023 | 0.027 |
| Serum cholesterol level (mg/dL) | 213.36±19.49 | 191.52±16.56 | <0.001 | 210.24±17.57 | 201.56±17.83 | <0.001 | 0.627 | 0.026 |
| Serum HDL level (mg/dL) | 40.56±2.61 | 45±3.34 | <0.001 | 42.32±2.77 | 42.56±2.61 | 0.109 | 0.026 | 0.006 |
| Serum LDL level (mg/dL) | 120.32±12.36 | 108.64±10.90 | <0.001 | 121.40±11.19 | 117.16±10.62 | <0.001 | 0.748 | 0.003 |
| Serum TG level (mg/dL) | 193.20±14.24 | 171.52±13.58 | <0.001 | 191.12±17.51 | 189.00±17.81 | 0.236 | 0.647 | <0.001 |
| Systolic blood pressure (mm Hg) | 132.52±5.00 | 125.88±4.20 | <0.001 | 132.52±4.80 | 132.12±5.04 | 0.412 | 1.00 | <0.001 |
| Diastolic blood pressure (mm Hg)? | 82.72±1.90 | 81.64±1.35 | 0.001 | 82.28±1.45 | 82.48±1.47 | 0.197 | 0.380 | 0.048 |
Table 2 The comparison of the outcome measures before and after the intervention between and within intervention and Placebo groups
| Variable | Intervention group (n = 25)? | Placebo group (n = 25)? | Between two groups | |||||
|---|---|---|---|---|---|---|---|---|
| Before | After | P valuea | Before | After | P valuea | P value (before)b | P value (after)b | |
| Serum levels of MDA (μmol/L) | 5.17±0.78 | 3.81±0.75 | <0.001 | 4.87±0.66 | 4.45±0.67 | <0.001 | 0.146 | 0.003 |
| Total antioxidant capacity (μmol/L) | 0.62±0.11 | 1.03±0.19 | <0.001 | 0.61±0.12 | 0.71±0.11 | <0.001 | 0.823 | <0.001 |
| SOD activity level (U/mL) | 43.69±5.27 | 45.58±6.03 | 0.159 | 41.45±6.39 | 42.14±6.80 | 0.571 | 0.154 | 0.065 |
| Serum ox-LDL levels (ng/L) | 278.39±32.22 | 241.32±37.71 | <0.001 | 277.77±41.19 | 264.70±33.95 | 0.002 | 0.953 | 0.026 |
| Serum level of Apo-H (ng/mL) | 19.12±2.86 | 17.15±2.56 | 0.001 | 18.08±2.55 | 17.15±2.17 | 0.080 | 0.180 | 0.995 |
| FBS (mg/dL) | 116.96±8.88 | 99.12±8.77 | <0.001 | 110.44±10.67 | 105.36±10.54 | <0.001 | 0.023 | 0.027 |
| Serum cholesterol level (mg/dL) | 213.36±19.49 | 191.52±16.56 | <0.001 | 210.24±17.57 | 201.56±17.83 | <0.001 | 0.627 | 0.026 |
| Serum HDL level (mg/dL) | 40.56±2.61 | 45±3.34 | <0.001 | 42.32±2.77 | 42.56±2.61 | 0.109 | 0.026 | 0.006 |
| Serum LDL level (mg/dL) | 120.32±12.36 | 108.64±10.90 | <0.001 | 121.40±11.19 | 117.16±10.62 | <0.001 | 0.748 | 0.003 |
| Serum TG level (mg/dL) | 193.20±14.24 | 171.52±13.58 | <0.001 | 191.12±17.51 | 189.00±17.81 | 0.236 | 0.647 | <0.001 |
| Systolic blood pressure (mm Hg) | 132.52±5.00 | 125.88±4.20 | <0.001 | 132.52±4.80 | 132.12±5.04 | 0.412 | 1.00 | <0.001 |
| Diastolic blood pressure (mm Hg)? | 82.72±1.90 | 81.64±1.35 | 0.001 | 82.28±1.45 | 82.48±1.47 | 0.197 | 0.380 | 0.048 |
| Variable | Type Ⅲ sum of squares | Df (degree of freedom) | Mean square | F test | P value | Partial eta squared |
|---|---|---|---|---|---|---|
| FBS before the intervention | 3679.44 | 1 | 3679.437 | 207.61 | <0.001 | 0.82 |
| 1629.17 | 1 | 1629.17 | 91.93 | <0.001 | 0.66 | |
| HDL before the intervention | 390.58 | 1 | 390.58 | 441.43 | <0.001 | 0.90 |
| 208.11 | 1 | 208.11 | 235.21 | <0.001 | 0.83 |
Table 3 Results of the covariance test to compare the mean total score of FBS and HDL-C after the intervention in the placebo and intervention groups
| Variable | Type Ⅲ sum of squares | Df (degree of freedom) | Mean square | F test | P value | Partial eta squared |
|---|---|---|---|---|---|---|
| FBS before the intervention | 3679.44 | 1 | 3679.437 | 207.61 | <0.001 | 0.82 |
| 1629.17 | 1 | 1629.17 | 91.93 | <0.001 | 0.66 | |
| HDL before the intervention | 390.58 | 1 | 390.58 | 441.43 | <0.001 | 0.90 |
| 208.11 | 1 | 208.11 | 235.21 | <0.001 | 0.83 |
| 1. |
Saklayen MG. The global epidemic of the metabolic syndrome. Curr Hypertens Rep 2018; 20: 12-20.
DOI PMID |
| 2. | Alemany M. The metabolic syndrome, a human disease. Int J Mol Med Sci 2024; 25: 2251-347. |
| 3. |
Kim D, Touros A, Kim WR. Nonalcoholic fatty liver disease and metabolic syndrome. Clin Liver Dis 2018; 22: 133-40.
DOI PMID |
| 4. |
Pasquali R. Metabolic syndrome in polycystic ovary syndrome. Front Horm Res 2018; 49: 114-30.
DOI PMID |
| 5. |
Dwyer AA, Quinton R. The metabolic syndrome in central hypogonadotrophic hypogonadism. Front Horm Res 2018; 49: 156-69.
DOI PMID |
| 6. | Hess PL, Al‐Khalidi HR, Friedman DJ, et al. The metabolic syndrome and risk of sudden cardiac death: the atherosclerosis risk in communities study. J Am Heart Assoc 2017; 6: e006103-11. |
| 7. | Hao Y, Zhu YJ, Zou S, et al. Metabolic syndrome and psoriasis: Mechanisms and future directions. Front Immunol 2021; 12: 711060-70. |
| 8. | Libowitz MR, Nurmi EL. The burden of antipsychotic-induced weight gain and metabolic syndrome in children. Front Psychiatry 2021; 12: 623681-705. |
| 9. |
Bagherifard A, Kadijani AA, Yahyazadeh H, et al. The value of serum total oxidant to the antioxidant ratio as a biomarker of knee osteoarthritis. Clin Nutr ESPEN 2020; 38: 118-23.
DOI PMID |
| 10. |
Dziegielewska-Gesiak S. Metabolic syndrome in an aging society-role of oxidant-antioxidant imbalance and inflammation markers in disentangling atherosclerosis. Clin Interv Aging 2021; 16: 1057-70.
DOI PMID |
| 11. | Rubio-Ruiz ME, Guarner-Lans V, Cano-Martínez A, et al. Resveratrol and quercetin administration improves antioxidant DEFENSES and reduces fatty liver in metabolic syndrome rats. Molecules 2019; 24: 1297-315. |
| 12. | Monserrat-Mesquida M, Quetglas-Llabrés M, Capó X, et al. Metabolic syndrome is associated with oxidative stress and proinflammatory state. Antioxidants 2020; 9: 236-50. |
| 13. | Francisqueti FV, Chiaverini LCT, Santos KCd, et al. The role of oxidative stress on the pathophysiology of metabolic syndrome. Rev Assoc Med Bras 2017; 63: 85-91. |
| 14. |
Patti AM, Al-Rasadi K, Giglio RV, et al. Natural approaches in metabolic syndrome management. Arch Med Sci 2018; 14: 422-41.
DOI PMID |
| 15. | Wang J, Liao B, Wang C, Zhong O, Lei X, Yang Y. Effects of antioxidant supplementation on metabolic disorders in obese patients from randomized clinical controls: a Meta-analysis and systematic review. Oxid Med Cell Longev 2022; 2022: 7255413-33. |
| 16. | Zhang S, Xu M, Zhang W, Liu C, Chen S. Natural polyphenols in metabolic syndrome: protective mechanisms and clinical applications. Int J Mol Sci 2021; 22: 6110. |
| 17. | Abdelrahman M, Ariyanti NA, Sawada Y, et al. Metabolome-Based Discrimination analysis of shallot landraces and bulb onion cultivars associated with differences in the amino acid and flavonoid profiles. Molecules 2020; 25: 5300-13. |
| 18. | Kothari D, Lee WD, Niu KM, Kim SK. The genus Allium as poultry feed additive: a review. Animals 2019; 9: 1032-53. |
| 19. |
De Greef D, Barton EM, Sandberg EN, et al., editors. Anticancer potential of garlic and its bioactive constituents: a systematic and comprehensive review. Semin Cancer Biol 2021; 73: 219-64.
DOI PMID |
| 20. | Ghafarifarsani H, Hoseinifar SH, Talebi M, et al. Combined and singular effects of ethanolic extract of persian shallot (Allium hirtifolium Boiss) and synbiotic Biomin® IMBO on growth performance, serum-and mucus-immune parameters and antioxidant defense in Zebrafish (Danio rerio). Animals 2021; 11: 2995-3010. |
| 21. | Ghafarifarsani H, Yousefi M, Hoseinifar SH, et al. Beneficial effects of Persian shallot (Allium hirtifolium) extract on growth performance, biochemical, immunological and antioxidant responses of rainbow trout oncorhynchus mykiss fingerlings. Aquaculture 2022; 555: 738162-78. |
| 22. | Omidifar N, Nili-Ahmadabadi A, Gholami A, Dastan D, Ahmadimoghaddam D, Nili-Ahmadabadi H. Biochemical and histological evidence on the protective effects of Allium hirtifolium boiss (Persian Shallot) as an herbal supplement in cadmium-induced hepatotoxicity. Evid Based Complement Alternat Med 2020; 2020: 7457504-12. |
| 23. | Sun W, Shahrajabian MH, Cheng Q. The insight and survey on medicinal properties and nutritive components of shallot. J Med Plant Res 2019; 13: 452-7. |
| 24. | Grewal AS, Sharma N, Singh S. In silico docking studies of compounds from Persian shallot as allosteric glucokinase. Plant Arch 2022; 22: 547-70. |
| 25. | Khaleghi S, Bahrami G, Mahmoodi M, Asgari V, Mostafaie A. Hypoglycemic effect of hydroalcoholic extract and hexane fraction of persian shallot (Allium hirtifolium boiss) extract in streptozotocin-induced diabetic rats. JRPS 2016; 5: 33-40. |
| 26. |
Hosseini FS, Falahati-Pour SK, Hajizadeh MR, et al. Persian shallot, Allium hirtifolium Boiss, induced apoptosis in human hepatocellular carcinoma cells. Cytotechnology 2017; 69: 551-63.
DOI PMID |
| 27. | Satvati SAR, Shooriabi M, Amin M, Shiezadeh F. Evaluation of the antimicrobial activity of Tribulus terrestris, Allium sativum, Salvia officinalis, and Allium hirtifolium Boiss against Enterococcus faecalis. Int J Enteric Pathog 2017; 5: 63-7. |
| 28. |
Mehriardestani M, Aliahmadi A, Toliat T, Rahimi R. Medicinal plants and their isolated compounds showing anti-trichomonas vaginalis-activity. Biomed Pharmacother 2017; 88: 885-93.
DOI PMID |
| 29. |
Eftekhari MH, Akbarzadeh M, MH Dabbaghmanesh, et al. The effect of calcitriol on lipid profile and oxidative stress in hyperlipidemic patients with type 2 diabetes mellitus. ARYA Atheroscler 2014; 10: 82-8.
PMID |
| 30. |
Altun HK, Ermumcu MSK, Kurklu NS. Evaluation of dietary supplement, functional food and herbal medicine use by dietitians during the COVID-19 pandemic. Public Health Nutr 2021; 24: 861-9.
DOI PMID |
| 31. |
Barkat MA, Goyal A, Barkat HA, Salauddin M, Pottoo FH, Anwer ET. Herbal medicine: clinical perspective and regulatory status. Comb Chem High Throughput Screen 2021; 24: 1573-82.
DOI PMID |
| 32. | Xu YXZ, Xi S, Qian X. Evaluating Traditional Chinese Medicine and herbal products for the treatment of gestational diabetes mellitus. J Diabetes Res 2019; 2019: 9182595. |
| 33. | Heshmat-Ghahdarijani K, Soltani R, Ghanadian M, Soleymani H. The effect of Allium hirtifolium bulb on serum lipid profile in adult patients with hyperlipidemia: a randomized double-blind placebo-controlled clinical trial. Complement Ther Clin Pract 2022; 49: 101654-61. |
| 34. | Karthikkumar V, Anbu S, Rajasekar P. Beneficial biological role of Allium hirtifolium on various diseases. RJPT 2020; 13: 1009-14. |
| 35. |
Mehdi M, Javad H, Seyed-Mostafa HZ, Mohammadreza M, Ebrahim M. The effect of Persian shallot (Allium hirtifolium Boiss.) extract on blood sugar and serum levels of some hormones in diabetic rats. Pak J Pharm Sci 2013; 26: 397-402.
PMID |
| 36. | Panahandeh J, Farhadi N, Motallebi Azar A, Alizadeh Salteh S. Evaluation of Persian shallot (Allium hirtifolium) ecotypes for phytochemical components and antioxidant activity. JMPB 2016; 5: 217-26. |
| 37. |
Rahimi-Madiseh M, Heidarian E, Kheiri S, Rafieian-Kopaei M. Effect of hydroalcoholic Allium ampeloprasum extract on oxidative stress, diabetes mellitus and dyslipidemia in alloxan-induced diabetic rats. Biomed Pharmacother 2017; 86: 363-7.
DOI PMID |
| 38. | Liu DS, Wang SL, Li JM, Liang ES, Yan MZ, Gao W. Allicin improves carotid artery intima‑media thickness in coronary artery disease patients with hyperhomocysteinemia. Exp Ther Med 2017; 14: 1722-6. |
| 39. | Marón FJM, Camargo AB, Manucha W. Allicin pharmacology: common molecular mechanisms against neuroinflammation and cardiovascular diseases. Life Sci 2020; 249: 117513-20. |
| 40. | Salehi B, Zucca P, Orhan IE, et al. Allicin and health: a comprehensive review. Trends Food Sci Technol 2019; 86: 502-16. |
| 41. | Sánchez-Gloria JL, Arellano-Buendía AS, Juárez-Rojas JG, et al. Cellular mechanisms underlying the cardioprotective role of allicin on cardiovascular diseases. Int J Mol Sci 2022; 23: 9082-103. |
| 42. | Lu J, Fang B, Meng Z, Zheng Y, Tian X, Guan S. Protective effects of allicin on 1, 3-DCP-induced lipid metabolism disorder in HepG2 cells. Biomed Pharmacother 2017; 96: 1411-17. |
| 43. | Shi Xe, Zhou X, Chu X, et al. Allicin improves metabolism in high-fat diet-induced obese mice by modulating the gut microbiota. Nutrients 2019; 11: 2909-23. |
| 44. | Ma L, Chen S, Li S, Deng L, Li Y, Li H. Effect of allicin against ischemia/hypoxia-induced H9c2 myoblast apoptosis via eNOS/NO pathway-mediated antioxidant activity. Evid Based Complement Alternat Med 2018; 2018: 3207973-83. |
| 45. | García Trejo EMÁ, Arellano Buendía AS, Sánchez Reyes O, et al. The beneficial effects of allicin in chronic kidney disease are comparable to losartan. Int J Mol Sci 2017; 18: 1980-99. |
| 46. |
Nazeri Z, Azizidoost S, Cheraghzadeh M, Mohammadi A, Kheirollah A. Increased protein expression of ABCA1, HMG-CoA reductase, and CYP46A1 induced by garlic and allicin in the brain mouse and astrocytes-isolated from C57BL/6J. Avicenna J Phytomed 2021; 11: 473-83.
DOI PMID |
| 47. | Cheng B, Li T, Li F. Use of Network pharmacology to investigate the mechanism by which Allicin ameliorates lipid metabolism disorder in HepG2 cells. Evid Based Complement Alternat Med 2021; 2021: 3956504-15. |
| 48. | Cui T, Liu W, Chen S, Yu C, Li Y, Zhang JY. Antihypertensive effects of allicin on spontaneously hypertensive rats via vasorelaxation and hydrogen sulfide mechanisms. Biomed Pharmacother 2020; 128: 110240-52. |
| 49. | Russo B, Picconi F, Malandrucco I, Frontoni S. Flavonoids and insulin-resistance: from molecular evidences to clinical trials. Int J Mol Sci 2019; 20: 2061-79. |
| 50. | He C, Liu X, Jiang Z, Geng S, Ma H, Liu B. Interaction mechanism of flavonoids and α-glucosidase: experimental and molecular modelling studies. Foods 2019; 8: 355-64. |
| 51. | Nishimura M, Muro T, Kobori M, Nishihira J. Effect of daily ingestion of quercetin-rich onion powder for 12 weeks on visceral fat: a randomised, double-blind, placebo-controlled, parallel-group study. Nutrients 2020; 12: 91-103. |
| 52. | Sok Yen F, Shu Qin C, Tan Shi Xuan S, et al. Hypoglycemic effects of plant flavonoids: a review. Evid Based Complement Alternat Med 2021; 2021: 2057333-45. |
| [1] | XIA Xichao, XUE Shipeng, SONG Guoying, LI Bin, WANG Huiping, QIU Ju, Wang Jihong, LIU Qingchun, MA Yuhong, OUYANG Jingfeng. Anti-oxidative and immunological role of Cyclocarya paliurus polysaccharide on the liver injury of diabetic rats [J]. Journal of Traditional Chinese Medicine, 2024, 44(6): 1146-1152. |
| [2] | PENG Wan, NI Hengfan, GUO Dale, DENG Yun, DAI Manyun. Farnesoid X receptor regulators from natural products and their biological function [J]. Journal of Traditional Chinese Medicine, 2023, 43(3): 618-626. |
| [3] | Esma Anissa Trad Khodja, Abd El Hamid Khabtane, Rabah Arhab, Djamila Benouchenne, Mohamed Sabri Bensaad, Chawki Bensouici, Ramazan Erenler. In vitro assessment of antioxidant, neuroprotective, anti-urease and anti-tyrosinase capacities of Tamarix africana leaves extracts [J]. Journal of Traditional Chinese Medicine, 2023, 43(2): 252-264. |
| [4] | Naser Mirazi, Sheida Hesami, Alireza Nourian, Abdolkarim Hosseini. Protective efficacy of dark chocolate in letrozole-induced ovary toxicity model rats: hormonal, biochemical, and histopathological investigation [J]. Journal of Traditional Chinese Medicine, 2022, 42(5): 741-748. |
| [5] | Kamal Dawood, Roohullah, Rabbi Fazle, Naz Attiqa, Bilal Muhammad. In-vitro and in-vivo pharmacological screening of Iris albicans [J]. Journal of Traditional Chinese Medicine, 2022, 42(1): 9-16. |
| [6] | Elham A.Abd-Allah, Nouf S.Al-Abbas, Mona M.Atia, Fawzia Alzahrani, El-Mokhtar M.Ahmed, Soad S.Ali, Soad K.Al Jaouni. Can Fig and Olive Ameliorate the toxicity Induced by 2-nitropropane in some organs of mice? role of inflammatory versus anti-inflammatory genes [J]. Journal of Traditional Chinese Medicine, 2021, 41(6): 891-899. |
| [7] | XIA Xichao, LI Bin, QIU Ju, TIAN Gang, CHEN Changdong, LA Ming, ZHANG Ke, QI Jinxu, LI Yanyan, GAO Huashan, SHAO Xiangyang, SU Congying, WANG Mengqi, OUYANG Jingfeng. Antioxidative and immunological effects of Cyclocarya paliurus polysaccharides on the spleen injury of diabetic rats [J]. Journal of Traditional Chinese Medicine, 2021, 41(5): 739-746. |
| [8] | TANG Chengfang, GAO Yang, Gulibairemu Yusuyin, MAO Yan, LI Yujun, WANG Yandong, GU Zhengyi. Anti-cataract effects of Dajizhi(Euphorbium) eye drops on selenite-induced cataracts in rats [J]. Journal of Traditional Chinese Medicine, 2021, 41(5): 747-752. |
| [9] | Mohsen Akbaribazm, Fatemeh Khazaei, Leila Naseri, Mona Pazhouhi, Mohammad Zamanian, Mozafar Khazaei. Pharmacological and therapeutic properties of the Red Clover(Trifolium pratense L.): an overview of the new findings [J]. Journal of Traditional Chinese Medicine, 2021, 41(4): 642-649. |
| [10] | ?nder Aybast?er;. Efficacy of methanol-water extract of Inula helenium root against oxidative DNA damage [J]. Journal of Traditional Chinese Medicine, 2021, 41(2): 293-300. |
| [11] | CHEN Xiaoqing, ZHANG Yong, HUANG Chunlai, FU Tingting, TAO Qinghua, MA Liqiang, WANG Liping. Efficacy of Huanglian root decoction(黄连煎剂) on kidney injury in rat's model of metabolic syndrome [J]. Journal of Traditional Chinese Medicine, 2021, 41(1): 117-124. |
| [12] | KONG Deyan, LUO Jiefeng, SHI Shengliang, HUANG Zhenhua. Efficacy of tanshinone ⅡA and mesenchymal stem cell treatment of learning and memory impairment in a rat model of vascular dementia [J]. Journal of Traditional Chinese Medicine, 2021, 41(1): 133-139. |
| [13] | Hou Jiguang, Fang Fang, Kang Shunai, Wang Zhicheng, Yang Yanming. Curcumin from Jianghuang (Rhizoma Curcumae Longae) protects against exposure to ultraviolet B by antioxidation and attenuating mitochondrion-dependent apoptosis [J]. Journal of Traditional Chinese Medicine, 2020, 40(5): 782-791. |
| [14] | Emel Akta?, Hilal Yildiran. Antioxidant and ntiinflammatory efficacy of curcumin on lung tissue in rats with sepsis [J]. Journal of Traditional Chinese Medicine, 2020, 40(5): 820-826. |
| [15] | Seval Yilmaz, Emre Kaya, Erhan Yilmaz, Ahmet Kavakli, Suleyman Gurbuz, Mustafa Ozkaraca. Effect of acupuncture therapy on fracture healing in rats with femur fractures [J]. Journal of Traditional Chinese Medicine, 2020, 40(2): 275-283. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||

