Journal of Traditional Chinese Medicine ›› 2024, Vol. 44 ›› Issue (6): 1194-1203.DOI: 10.19852/j.cnki.jtcm.2024.06.008
• Research Articles • Previous Articles Next Articles
Zuyangping (足疡平) formula promotes skin wound healing in diabetic rats
MENG Junhua1, ZHANG Hong2( ), CAO Yuling3, ZHANG Yu1, WANG Xiong1, SHENG Bi1, AN Jing1, CHEN Yonggang4(
), CAO Yuling3, ZHANG Yu1, WANG Xiong1, SHENG Bi1, AN Jing1, CHEN Yonggang4( )
)
- 1 Department of Pharmacy, Wuhan University Tongren Hospital (the Third Hospital of Wuhan), Wuhan 430060, China
2 Department of Pharmacy, the First People’s Hospital of Jiangxia District, Wuhan 430200, China
3 Department of Pharmacy, Wuhan ASIA General Hospital, Wuhan 430065, China
4 Laboratory Department, Wuhan Center for Clinical Laboratory, Wuhan 430015, China
-
Received:2023-09-11Accepted:2023-12-19Online:2024-12-15Published:2024-11-12 -
Contact:Prof. CHEN Yonggang, Laboratory Department, Wuhan Center for Clinical Laboratory, Wuhan 430015, China. cyg508@163.com Telephone: +86-15172393504
ZHANG Hong, Department of Pharmacy, the First People’s Hospital of Jiangxia District, Wuhan 430200, China. 390479135@qq.com -
Supported by:Hubei Provincial Central Guidance Local Science and Technology Development Project: Wuhan Precision Diagnosis and Treatment Clinical Medical Research Center for Severe Infections(2020ZYYD026);Wuhan Municipal Health Commission Scientific Research Project: Study on the effect of Zuyangping formula on Diabetes Wound Healing through Advanced Glycation End Products Receptor/ Nuclear Factor Kappa-B p65/Nucleotide-binding Oligomerization Domain, Leucine-Rich Repeat and Pyrin Domain-Containing 3 Mediated Inflammatory Reaction(WZ22A01);Wuhan Applied Basic Frontier Project: Development of Scar Eliminating Cream for Burn Scar Clinical Agreement(2020020601012301);Wuhan Clinical Medical Research Project: Preclinical Study of Mitogen-activated Protein Kinase 12-based Pituitary Prolactinoma and Adrenocorticotropin Adenoma in the Treatment of New Drug Barley Maltamine(WX20M02)
Cite this article
MENG Junhua, ZHANG Hong, CAO Yuling, ZHANG Yu, WANG Xiong, SHENG Bi, AN Jing, CHEN Yonggang. Zuyangping (足疡平) formula promotes skin wound healing in diabetic rats[J]. Journal of Traditional Chinese Medicine, 2024, 44(6): 1194-1203.
share this article
| Time point | Normal group (n = 5) | Model group (n = 5) | ZYP group (n = 5) | rhEGF group (n = 5) | SSD group (n = 5) |
|---|---|---|---|---|---|
| Before injection of STZ | 6.59±0.35 | 6.78±0.44 | 6.72±0.43 | 6.70±0.48 | 6.64±0.60 |
| Day 7 after STZ injection | 6.17±0.40 | 29.72±3.30a | 30.44±2.66a | 28.20±1.78a | 29.78±3.34a |
| Day 14 after STZ injection | 6.28±0.23 | 31.94±1.38a | 32.90±0.46a | 30.04±2.86a | 29.30±3.87a |
| Day 7 after treatment | 6.21±0.41 | 30.28±3.44a | 27.32±2.70a | 28.40±3.19a | 28.94±3.47a |
| Day 14 after treatment | 6.26±0.31 | 30.02±2.62a | 24.68±2.06ab | 27.20±3.16a | 28.78±3.82a |
Table 1 Changes in venous blood glucose levels in rats ($\bar{x}±s$, mmol/L)
| Time point | Normal group (n = 5) | Model group (n = 5) | ZYP group (n = 5) | rhEGF group (n = 5) | SSD group (n = 5) |
|---|---|---|---|---|---|
| Before injection of STZ | 6.59±0.35 | 6.78±0.44 | 6.72±0.43 | 6.70±0.48 | 6.64±0.60 |
| Day 7 after STZ injection | 6.17±0.40 | 29.72±3.30a | 30.44±2.66a | 28.20±1.78a | 29.78±3.34a |
| Day 14 after STZ injection | 6.28±0.23 | 31.94±1.38a | 32.90±0.46a | 30.04±2.86a | 29.30±3.87a |
| Day 7 after treatment | 6.21±0.41 | 30.28±3.44a | 27.32±2.70a | 28.40±3.19a | 28.94±3.47a |
| Day 14 after treatment | 6.26±0.31 | 30.02±2.62a | 24.68±2.06ab | 27.20±3.16a | 28.78±3.82a |

Figure 1 Morphological effects on rat wounds A: chromatograms of standard and ZYP extracts; A1: chromatograms of standard; A2: chromatograms of ZYP extracts. B1-B5: representative images of skin wounds at days 0 after surgery from all groups. B6-B10: representative images of skin wounds at days 7 after surgery from all groups. B11-B15: representative images of skin wounds at days 14 after surgery from all groups. B1, B6, B11: Normal group; B2, B7, B12: Model group; B3, B8, B13: ZYP group; B4, B9, B14: rhEGF group; B5, B10, B15: SSD group. C1-C2: wound closure rate at days 7 and 14 post surgery in each group. Normal group: normal rats with skin ulcer; Model group: non-intervention group after the establishment of diabetic ulcer model; ZYP group: after modelling diabetic ulcer, treatment with ZYP formula; rhEGF group: after modelling diabetic ulcer, treatment with epidermal growth factor; SSD group: after modelling diabetic ulcer, treatment with 1% sulfadiazine silver cream. ZYP: Zuyangping; rhEGF: epidermal growth factor; SSD: 1% sulfadiazine silver cream. Values are shown as mean ± standard deviation (n = 5). All the treated groups were compared with the model group using the Dunnett’s test. Student’s t-test was used to compare differences between the normal and model groups. aP < 0.05, compared with the normal group, bP < 0.05, compared with the model group.

Figure 2 Representative immunohistochemical staining of AGEs and CD31 in the granulation tissue of rats (×100) A1-A5: representative images of immunohistochemistry staining of AGEs at days 7 after surgery from all groups; A6-A10: Representative images of immunohistochemistry staining of AGEs at days 14 after surgery from all groups; A11-A15: representative images of immunohistochemistry staining of CD31 at days 7 after surgery from all groups; A16-A20: representative images of immunohistochemistry staining of CD31 at days 14 after surgery from all groups. A1, A6, A11, A16: Normal group; A2, A7, A12, A17: Model group; A3, A8, A13, A18: ZYP group; A4, A9, A14, A19: rhEGF group; A5, A10, A15, A20: SSD group. B1-B2: statistical analysis of AGEs staining positive cells at days 7 and 14 post surgery, cells were calculated by IPP software. C1-C2: statistical analysis of the number of capillary vessels at days 7 and 14 post surgery, capillary vessels per field were calculated by IPP software. Normal group: normal rats with skin ulcer; Model group: Non-intervention group after the establishment of diabetic ulcer model; ZYP group: after modelling diabetic ulcer, treatment with ZYP formula; rhEGF group: after modelling diabetic ulcer, treatment with epidermal growth factor; SSD group: after modelling diabetic ulcer, treatment with 1% sulfadiazine silver cream. AGEs: advanced glycation end products; CD31: platelet endothelial cell adhesion molecule-1; ZYP: Zuyangping; rhEGF: epidermal growth factor; SSD: 1% sulfadiazine silver cream. Values are shown as mean ± standard deviation (n = 3). All the treated groups were compared with the model group using the Dunnett’s test. Student’s t-test was used to compare differences between the normal and model groups. aP < 0.05, compared with the normal group; bP < 0.05, compared with the model group.

Figure 3 Expression of angiogenesis-associated molecules in granulation tissue of diabetic rats at days 7 and 14 post-surgery and the effect of ZYP formula A: Western blotting images at day 7 post surgery; B: after 7 d of surgery, statistical analysis of RAGE expression; C: after 7 d of surgery, statistical analysis of NF-κB p65 expression; D: after 7 d of surgery, statistical analysis of HIF-1α expression; D: after 7 d of surgery, statistical analysis of VEGF expression; F: Western blotting images at day 14 post surgery; G: after 14 d of surgery, statistical analysis of RAGE expression; H: after 14 d of surgery, statistical analysis of NF-κB p65 expression; I: after 14 d of surgery, statistical analysis of HIF-1α expression; J: after 7 d of surgery, statistical analysis of VEGF expression. Normal group: normal rats with skin ulcer; Model group: non-intervention group after the establishment of diabetic ulcer model; ZYP group: after modelling diabetic ulcer, treatment with ZYP formula; rhEGF group: after modelling diabetic ulcer, treatment with epidermal growth factor; SSD group: after modelling diabetic ulcer, treatment with 1% sulfadiazine silver cream. β-actin: beta-actin; RAGE: advanced glycation end products receptor; NF-κB p65: nuclear factor-κB p65; HIF-1α: hypoxia-inducible factor-1α; VEGF: vascular endothelial growth factor; ZYP: Zuyangping; rhEGF: epidermal growth factor; SSD: 1% sulfadiazine silver cream. Values are shown as mean ± standard deviation (n = 3). All the treated groups were compared with the model group using the Dunnett’s test. Student’s t-test was used to compare differences between the normal and model groups. aP < 0.05, compared with the normal group; bP < 0.05, compared with the model group.
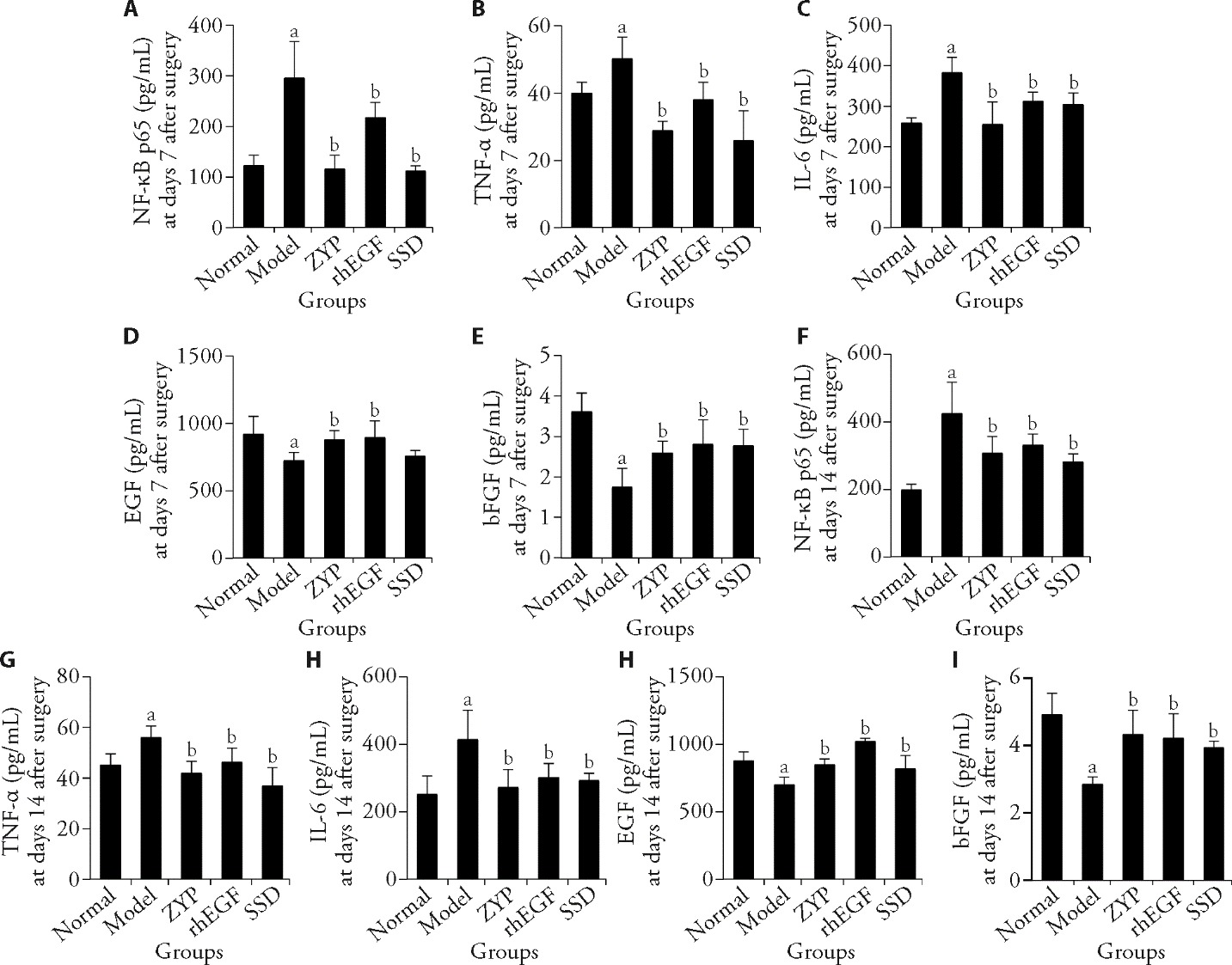
Figure 4 ELISA method was used to detect the expression level of related cytokines in granulation tissue of diabetic rats at days 7 and 14 post-surgery and the effect of ZYP formula A: NF-κB p65 expression level at day 7 post-surgery; B: TNF-α expression level at days 7 post-surgery; C: IL-6 expression level at day 7 post-surgery, D: EGF expression level at day 7 post-surgery; E: bFGF expression level at day 7 post-surgery; F: NF-κB p65 expression level at day 14 post-surgery; G: TNF-α expression level at day 14 post-surgery; H: IL-6 expression level at days 14 post-surgery; I: EGF expression level at days 14 post-surgery; J: bFGF expression level at day 14 post-surgery. Normal group: normal rats with skin ulcer; Model group: non-intervention group after the establishment of diabetic ulcer model; ZYP group: after modelling diabetic ulcer, treatment with ZYP formula; rhEGF group: after modelling diabetic ulcer, treatment with epidermal growth factor; SSD group: after modelling diabetic ulcer, treatment with 1% sulfadiazine silver cream. ELISA: enzyme-linked immunosorbent assay; NF-κB p65: nuclear factor-κB p65; IL-6: interleukin-6; TNF-α: tumor necrosis factor-α; EGF: epidermal growth factor; bFGF: basic fibroblast growth factor; ZYP: Zuyangping; rhEGF: epidermal growth factor; SSD: 1% sulfadiazine silver cream. Values are shown as mean ± standard deviation (n = 5). All the treated groups were compared with the model group using the Dunnett’s test. Student’s t-test was used to compare differences between the normal and model groups. aP < 0.05, compared with the normal group; bP < 0.05, compared with the model group.
| 1. |
Rosenson RS, Fioretto P, Dodson PM. Does microvascular disease predict macrovascular events in type 2 diabetes? Atherosclerosis 2011; 218: 13-8.
DOI PMID |
| 2. |
Jeffcoate WJ, Vileikyte L, Boyko EJ, et al. Current challenges and opportunities in the prevention and management of diabetic foot ulcers. Diabetes Care 2018; 41: 645-52.
DOI PMID |
| 3. |
Daemi A, Lotfi M, Farahpour MR, et al. Topical application of cinnamomum hydroethanolic extract improves wound healing by enhancing re-epithelialization and keratin biosynthesis in streptozotocin-induced diabetic mice. Pharm Biol 2019; 57: 799-806.
DOI PMID |
| 4. | Hong WX, Hu MS, Esquivel M, et al. The role of hypoxia-inducible factor in wound healing. Adv Wound Care (New Rochelle) 2014; 3: 390-9. |
| 5. |
Han Y, Sun T, Tao R, et al. Clinical application prospect of umbilical cord-derived mesenchymal stem cells on clearance of advanced glycation end products through autophagy on diabetic wound. Eur J Med Res 2017; 22: 11.
DOI PMID |
| 6. | Zhou XZ, Luo M, Wu J, et al. Antibacterial effect of foot ulcer cure ointment on ulceration of diabetic rat: an experimental study. Chin J Nosocomiol 2006: 1207-9. |
| 7. | Zhang H, Zhang Y, Cao YL, et al. Effect of Fufang Zuyangping on wound healing of skin ulcer and expression of RAGE/NF-κBP65/VEGF in diabetic rats. Zhong Guo Yi Yuan Yao Xue Za Zhi 2021; 41: 1405-9. |
| 8. | Cai JY, Zhou XZ, Wu J, et al. Clinical study of Zuyangping ointment in the treatment of diabetic foot ulcer. Zhong Guo Yi Shi Za Zhi 2008: 979-80. |
| 9. | Qiu YY, Tang LQ, Wei W. Berberine exerts renoprotective effects by regulating the AGEs-RAGE signaling pathway in mesangial cells during diabetic nephropathy. Mol Cell Endocrinol 2017; 443: 89-105. |
| 10. | Zhang XN, Ma ZJ, Wang Y, et al. Angelica dahurica ethanolic extract improves impaired wound healing by activating angiogenesis in diabetes. PLoS One 2017; 12: e0177862. |
| 11. | Sarandy MM, Novaes RD, Xavier AA, et al. Hydroethanolic extract of strychnos pseudoquina accelerates skin wound healing by modulating the oxidative status and microstructural reorganization of scar tissue in experimental type I diabetes. Biomed Res Int 2017; 2017: 9538351. |
| 12. |
Kant V, Gopal A, Kumar D, et al. Curcumin-induced angiogenesis hastens wound healing in diabetic rats. J Surg Res 2015; 193: 978-88.
DOI PMID |
| 13. |
Yu M, Liu W, Li J, et al. Exosomes derived from atorvastatin-pretreated MSC accelerate diabetic wound repair by enhancing angiogenesis via AKT/eNOS pathway. Stem Cell Res Ther 2020; 11: 350.
DOI PMID |
| 14. | Li G, Ko CN, Li D, et al. A small molecule HIF-1α stabilizer that accelerates diabetic wound healing. Nat Commun 2021; 12: 3363. |
| 15. | Bonab FS, Farahpour MR. Topical co-administration of Pistacia atlantica hull and Quercus infectoria gall hydroethanolic extract improves wound-healing process. Comp Clin Path 2017; 26: 885-92. |
| 16. | Tam JC, Lau KM, Liu CL, et al. The in vivo and in vitro diabetic wound healing effects of a 2-herb formula and its mechanisms of action. J Ethnopharmacol 2011; 134: 831-8. |
| 17. |
Levin ME. Management of the diabetic foot: preventing amputation. South Med J 2002; 95: 10-20.
PMID |
| 18. | Shang XF, Yang CJ, Morris-Natschke SL, et al. Biologically active isoquinoline alkaloids covering 2014-2018. Med Res Rev 2020; 40: 2212-89. |
| 19. |
Chen Q, Mo R, Wu N, et al. Berberine ameliorates diabetes-associated cognitive decline through modulation of aberrant inflammation response and insulin signaling pathway in DM rats. Front Pharmacol 2017; 8: 334.
DOI PMID |
| 20. | Zhang P, He L, Zhang J, et al. Preparation of novel berberine nano-colloids for improving wound healing of diabetic rats by acting Sirt1/NF-kappaB pathway. Colloids Surf B Biointerfaces 2020; 187: 110647. |
| 21. |
Lin CM, Chiu JH, Wu IH, et al. Ferulic acid augments angiogenesis via VEGF, PDGF and HIF-1 alpha. J Nutr Biochem 2010; 21: 627-33.
DOI PMID |
| 22. | Lam HW, Lin HC, Lao SC, et al. The angiogenic effects of Angelica sinensis extract on HUVEC in vitro and zebrafish in vivo. J Cell Biochem 2008; 103: 195-211. |
| 23. |
Yang Y, Chin A, Zhang L, et al. The role of Traditional Chinese Medicines in osteogenesis and angiogenesis. Phytother Res 2014; 28: 1-8.
DOI PMID |
| 24. |
Siddiqui MZ. Boswellia serrata, a potential antiinflammatory agent: an overview. Indian J Pharm Sci 2011; 73: 255-61.
DOI PMID |
| 25. | Dai X, Wang R, Wu Z, et al. Permeation-enhancing effects and mechanisms of borneol and menthol on ligustrazine: a multiscale study using in vitro and coarse-grained molecular dynamics simulation methods. Chem Biol Drug Des 2018; 92: 1830-7. |
| 26. | Liu K, Han S, Gao W, et al. Changes of mineralogical properties and biological activities of gypsum and its calcined products with different phase structures. Evid Based Complement Alternat Med 2021; 2021: 6676797. |
| 27. | Gupta M, Mahajan VK, Mehta KS, et al. Zinc therapy in dermatology: a review. Dermatol Res Pract 2014; 2014: 709152. |
| 28. | Mimura Y, Ihn H, Jinnin M, et al. Epidermal growth factor induces fibronectin expression in human dermal fibroblasts via protein kinase C delta signaling pathway. J Invest Dermatol 2004; 122: 1390-8. |
| 29. | Garcia-Honduvilla N, Cifuentes A, Ortega MA, et al. Immuno-modulatory effect of local rhEGF treatment during tissue repair in diabetic ulcers. Endocr Connect 2018; 7: 584-94. |
| 30. |
Gomez-Villa R, Aguilar-Rebolledo F, Lozano-Platonoff A, et al. Efficacy of intralesional recombinant human epidermal growth factor in diabetic foot ulcers in Mexican patients: a randomized double-blinded controlled trial. Wound Repair Regen 2014; 22: 497-503.
DOI PMID |
| 31. | Muhammad AA, Arulselvan P, Cheah PS, et al. Evaluation of wound healing properties of bioactive aqueous fraction from Moringa oleifera Lam on experimentally induced diabetic animal model. Drug Des Devel Ther 2016; 10: 1715-30. |
| 32. | Kamdi SP, Raval A, Nakhate KT. Effect of apple peel extract on diabetes-induced peripheral neuropathy and wound injury. J Diabetes Metab Disord 2021; 20: 119-30. |
| 33. | Huang JY, Chen L, Zhou ZZ, et al. Effect of Jiedu Shengji ointment on local IL-6 and TNF-α expression in diabetic ulcer rats. Zhong Guo Zhong Yi Ji Chu Yi Xue Za Zhi 2021; 27: 1567-71. |
| 34. | He T, Sun P, Liu B, et al. Puffball spores improve wound healing in a diabetic rat model. Front Endocrinol (Lausanne) 2022; 13: 942549. |
| 35. | Gan D, Su Q, Su H, et al. Burn ointment promotes cutaneous wound healing by modulating the PI3K/AKT/mTOR signaling pathway. Front Pharmacol 2021; 12: 631102. |
| 36. |
Gugliucci A. Formation of fructose-mediated advanced glycation end products and their roles in metabolic and inflammatory diseases. Adv Nutr 2017; 8: 54-62.
DOI PMID |
| 37. |
Richards JE, Hutchinson J, Mukherjee K, et al. Stress hyperglycemia and surgical site infection in stable nondiabetic adults with orthopedic injuries. J Trauma Acute Care Surg 2014; 76: 1070-5.
DOI PMID |
| 38. | Ghosh G, Wang VY, Huang DB, et al. NF-kappa B regulation: lessons from structures. Immunol Rev 2012; 246: 36-58. |
| 39. | Fei J, Ling YM, Zeng MJ, et al. Shixiang plaster, a Traditional Chinese Medicine, promotes healing in a rat model of diabetic ulcer through the receptor for advanced glycation end products (RAGE)/nuclear factor kappa B (NF-kappa B) and vascular endothelial growth factor (VEGF)/vascular cell adhesion molecule-1 (VCAM-1)/endothelial nitric oxide synthase (eNOS) signaling pathways. Med Sci Monit 2019; 25: 9446-57. |
| 40. |
Parmar KM, Shende PR, Katare N, et al. Wound healing potential of Solanum xanthocarpum in streptozotocin-induced diabetic rats. J Pharm Pharmacol 2018; 70: 1389-400.
DOI PMID |
| 41. |
Thangarajah H, Vial IN, Grogan RH, et al. HIF-1alpha dysfunction in diabetes. Cell Cycle 2010; 9: 75-9.
PMID |
| 42. |
Thangarajah H, Yao D, Chang EI, et al. The molecular basis for impaired hypoxia-induced VEGF expression in diabetic tissues. Proc Natl Acad Sci USA 2009; 106: 13505-10.
DOI PMID |
| 43. |
Botusan IR, Sunkari VG, Savu O, et al. Stabilization of HIF-1alpha is critical to improve wound healing in diabetic mice. Proc Natl Acad Sci USA 2008; 105: 19426-31.
DOI PMID |
| 44. |
Sunkari VG, Lind F, Botusan IR, et al. Hyperbaric oxygen therapy activates hypoxia-inducible factor 1 (HIF-1), which contributes to improved wound healing in diabetic mice. Wound Repair Regen 2015; 23: 98-103.
DOI PMID |
| 45. | Chen H, Jia P, Kang H, et al. Upregulating Hif-1alpha by hydrogel nanofibrous scaffolds for rapidly recruiting angiogenesis relative cells in diabetic wound. Adv Healthc Mater 2016; 5: 907-18. |
| [1] | CHENG Kunming, YUAN Jianan, LIU Jun, ZHANG Shengpeng, XU Qixiang, XIE Yong, ZHAO Jingfeng, ZHANG Xiaoxu, TANG Xudong, ZHENG Yongqiu, WANG Zhong. Identifying Qingkailing (清开灵) ingredients-dependent mesenchymal-epithelial transition factor-axiation “π” structuring module with angiogenesis and neurogenesis effects [J]. Journal of Traditional Chinese Medicine, 2024, 44(1): 35-43. |
| [2] | SHI Xiao, WANG Lina, HU Jianpeng, ZHANG Limiao, WANG Jin. Effect of Naoluoxintong formula (脑络欣通方) and its split prescriptions on cerebral vascular regeneration in rats with the cerebral ischemia-reperfusion [J]. Journal of Traditional Chinese Medicine, 2023, 43(6): 1140-1149. |
| [3] | MEI Pingping, FENG Wenzhe, SHI Peng, ZHANG Wenxiu, ZHUANG Yu. Clinical research progress in Traditional Chinese Medicine in treating wound healing after anal fistula surgery [J]. Journal of Traditional Chinese Medicine, 2023, 43(5): 1047-1054. |
| [4] | JIANG Jianzhen, ZHANG Xin, LUO Zhenguo, SU Chengguo, ZHOU Haiyan, JIANG Yuqing, XIAO Xianjun, CHEN Yunfei, ZHU Jun. Efficacy of electroacupuncture stimulating Zusanli (ST36) and Xuanzhong (GB39) on synovial angiogenesis in rats with adjuvant arthritis [J]. Journal of Traditional Chinese Medicine, 2023, 43(5): 955-962. |
| [5] | LI Miao, ZHENG Jialu, WANG Shuangshuang, CHEN Lei, PENG Xiao, CHEN Jinfang, AN Hongmei, HU Bing. Tenglong Buzhong granules (藤龙补中颗粒) inhibits the growth of SW620 human colon cancer in vivo [J]. Journal of Traditional Chinese Medicine, 2022, 42(5): 701-706. |
| [6] | Ivens Takechi Nakagawa, Hamilton Ricardo Alonso, Fabrício Campos Kuroda, José Roberto Passarini Junior, Juan Guzman Quispe Cabanillas, Fernanda Aparecida Sampaio Mendon?a, Gláucia Maria Tech dos Santos, Andrea Aparecida de Aro, Edson Rosa Pimentel, Maria Esméria Corezola do Amaral, Marcelo Augusto Marretto Esquisatto. Comparison of the systemic action of electroacupuncture and laserpuncture in experimental injuries on the back skin of Wistar rats(Rattus novergicus) [J]. Journal of Traditional Chinese Medicine, 2021, 41(5): 779-788. |
| [7] | LI Yuanqi, LI Weili, YU Xinhui, WU Hua, WANG Yuanzhong, JIN Ya, HAO Lele, LIU Mingmin, SONG Xiaoge. Mechanisms of Traditional Chinese Medicine Bushenantai granules (补肾安胎颗粒) in promoting angiogenesis at the maternal-fetal interface of recurrent spontaneous abortion mice [J]. Journal of Traditional Chinese Medicine, 2021, 41(4): 556-563. |
| [8] | Xu Chengyong, Wang Yuguo, Feng Jian, Xu Ran, Dou Yongqi. Extracts from Huangqi(Radix Astragali Mongoliciplus) and Ezhu(Rhizoma Curcumae Phaeocaulis) inhibit Lewis lung carcinoma cell growth in a xenograft mouse model by impairing mitogen-activated protein kinase signaling, vascular endothelial growth factor prod [J]. Journal of Traditional Chinese Medicine, 2019, 39(04): 559-565. |
| [9] | Deng Xin, Liang Xingqiu, Zhou Xiaoxiao, Jiang Manjun, Liang Mingkun, Wang Xinyuan, Zhao Xiaofang, Fu Lei, Liang Jian. Protective effect and mechanisms of Weining granule on N-methyl-N'-nitro-N-nitrosoguanidine-induced gastric cancer in rats [J]. Journal of Traditional Chinese Medicine, 2019, 39(03): 393-401. |
| [10] | Xia Xichao, Ma Yuhong, Wang Fuan, Zheng Xinhua, Liu Yang, Zhang Junfeng, Cui Juan, Shi Bingqin, Li Hongwen, Liu Rongzhi, Zhang Yaping, Cheng Zhaofei, Han Xiaolong. Effects of extracts from Chuanwu(Aconitum Carmichaelii) and Banxia(Rhizoma Pinelliae) on excisional wound healing in a rat's model [J]. Journal of Traditional Chinese Medicine, 2019, 39(01): 65-73. |
| [11] | Sun Zhanxue, Wang Jingjun, Kong Yuhong, Li Nan, Jiang Xiaoyuan, Cao Ting, Jia Yuanning, Zhang Yueyue, Zhang Yisheng, Cheng Jing. Effect of Yupingfeng granules on the skin barrier in atopic dermatitis mice models [J]. Journal of Traditional Chinese Medicine, 2018, 38(06): 872-878. |
| [12] | Li Jing, Bai Zonglu, Du Yuanhao, Li Yongfeng, Zhang Xuezhu, Pang Bo, Zhang Jingjing. Effect of electroacupuncture on expression of Ang/Tie-2 mRNA and protein in rats with acute cerebral infarction [J]. Journal of Traditional Chinese Medicine, 2017, 37(05): 659-666. |
| [13] | Zhao Xinyue, Xi Shengyan, Wang Yanhui, Xu Yangxinzi, Pollock Galia, Su Yu, Cheng Yao, Loy Guanjie, Liu Pei. Efficacy of Ciji Hua'ai Baosheng formula on the expressions of vascular endothelial growth factor, kinase insert domain-containing receptor and basic fibroblast growth factor in mouse models of H_22 hepatocellular carcinoma [J]. Journal of Traditional Chinese Medicine, 2017, 37(01): 88-95. |
| [14] | Gao Junqing, Chen Tao, Jin Huigen, Liu Zongjun, Zhao Deqiang. Effect of calycosin on left ventricular ejection fraction and angiogenesis in rat models with myocardial infarction [J]. Journal of Traditional Chinese Medicine, 2015, 35(02): 160-167. |
| [15] | Yuxin Pang, Dan Wang, Xuan Hu, Hui Wang, Wanjin Fu, Zuowang Fan, Xiaolu Chen, Fulai Yu. Effect of volatile oil from Blumea Balsamifera(L.) DC. leaves on wound healing in mice [J]. Journal of Traditional Chinese Medicine, 2014, 34(06): 716-724. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||

