Journal of Traditional Chinese Medicine ›› 2025, Vol. 45 ›› Issue (5): 1028-1039.DOI: 10.19852/j.cnki.jtcm.2025.05.009
• Original Articles • Previous Articles Next Articles
Dampness syndrome aggravates T helper 17/regulatory T imbalance to promote renal injury in rats with experimental membranous nephropathy
SHAN Wenjun1, GU Haowen1, GUAN Haiyu1, LI Ping2,3,4,5, WANG Yi1, HAN Miaoru1, WANG Houchun1, HUANG Xiaoyan2,3,4( ), BAO Kun2,3,4,5(
), BAO Kun2,3,4,5( )
)
- 1 Guangzhou University of Chinese Medicine, Guangzhou 510145, China
2 State Key Laboratory of Dampness Syndrome of Chinese Medicine, the Second Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangzhou 510006, China
3 Guangdong-Hong Kong-Macau Joint Lab on Chinese Medicine and Immune Disease Research, Guangzhou 510006, China
4 Nephrology Department, Guangdong Provincial Hospital of Chinese Medicine, Guangzhou 510006, China
5 Guangdong Provincial Key Laboratory of Chinese Medicine for Prevention and Treatment of Refractory Chronic Disease, the Second Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangzhou 510006, China
-
Received:2024-09-12Accepted:2025-01-14Online:2025-10-15Published:2025-09-15 -
Contact:Prof. BAO Kun, State Key Laboratory of Dampness Syndrome of Chinese Medicine, the Second Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangzhou 510006, China; Guangdong-Hong Kong-Macau Joint Lab on Chinese Medicine and Immune Disease Research, Guangzhou 510006, China; Nephrology Department, Guangdong Provincial Hospital of Chinese Medicine, Guangzhou 510006, China; Guangdong Provincial Key Laboratory of Chinese Medicine for Prevention and Treatment of Refractory Chronic Disease, the Second Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangzhou 510006, China. baokun@aliyun.com;
Prof. HUANG Xiaoyan, NState Key Laboratory of Dampness Syndrome of Chinese Medicine, the Second Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangzhou 510006, China; Guangdong-Hong Kong-Macau Joint Lab on Chinese Medicine and Immune Disease Research, Guangzhou 510006, China; Nephrology Department, Guangdong Provincial Hospital of Chinese Medicine, Guangzhou 510006, China. hxyan2013@126.com,Telephone: + 86-20-39318891 -
About author:SHAN Wenjun and GU Haowen are co-first authors and contributed equally to this work -
Supported by:Special Project of State Key Laboratory of Dampness Syndrome of Chinese Medicine: a Randomized Controlled Clinical Study of Sanqi Qushi Granules in the Treatment of Membranous Nephropathy(SZ2021ZZ36);a Cohort Study on the Pathogenesis and Evolution of Dampness Syndrome in Idiopathic Membranous Nephropathy and Its Material Basis(SZ2021ZZ09);the National Natural Science Foundation of China: Research on the Role of Damp Nephropathy based on the Metabolic Disorders-T helper 17/Regulatory T Imbalance Evil in the Progression of Membranous(81974565);the Guangzhou Science and Technology Plan Project: Exploring the Mechanism of Treating Membranous Nephropathy based on Dampness from the Perspective of Regulating Amino Acid Metabolism Disorders(2023A03J0746);Multi-omics Study to Explore the Material Basis of Dampness Syndrome in Membranous Nephropathy and the Intervention Mechanism of Sanqi Qushi Decoction(2024A03J0117);a Multimodal Machine Learning Prediction Model based on Pathological Images, Transcriptomics and Traditional Chinese Medicine Syndromes to Explore the Prognosis-related lncRNA Molecules of Membranous Nephropathy and the Intervention Mechanism of Sanqi Qushi Decoction(2025A03J4062);Research Project of Guangdong Provincial Hospital of Traditional Chinese Medicine: Construction of a Risk Management Prediction Model for Membranous Nephropathy Based on Artificial Intelligence Technology(YN2023HL03);Study on the Pathogenesis Evolution and Microbiological Mechanism of Membranous Nephropathy with Dampness Syndrome Based on the Changes of Tongue Coating Microecology(YN2023MB10);Post-doctoral Research Project of Guangdong Provincial Hospital of Chinese Medicine: Study on the Mechanism of Yinyang Shengmai Method in Regulating Myocardial Infarction in Diabetic Rats based on Ang/Vascular Endothelial Growth Factor-Mediated Angiogenesis(10814);Funding Project of Guangdong Provincial Administration of Traditional Chinese Medicine: Study on the Mechanism of "Dampness Stagnation in Blood Collaterals" inducing Vascular Aging Based on Oxidative Stress-inflammation Crosstalk and Intervention of Traditional Chinese Medicine(20233021);China Postdoctoral Science Foundation Funded Project: Study on the Mechanism of Shenmai Injection in Promoting Angiogenesis after Myocardial Infarction in Rats with Qi and Yin Deficiency Type Diabetic Nephropathy(2023M730810)
Cite this article
SHAN Wenjun, GU Haowen, GUAN Haiyu, LI Ping, WANG Yi, HAN Miaoru, WANG Houchun, HUANG Xiaoyan, BAO Kun. Dampness syndrome aggravates T helper 17/regulatory T imbalance to promote renal injury in rats with experimental membranous nephropathy[J]. Journal of Traditional Chinese Medicine, 2025, 45(5): 1028-1039.
share this article
Figure 1 Comparison of the appearance of rats in each group A: appearance of rats in the NC group; B: appearance of rats in the PHN group; C: appearance of rats in the PHN + DS group; D: appearance of rats in the DS group. NC group: injected with saline (0.5 mL per 100 g body weight) into the tail vein once and gavaged with distilled water (1 mL per 100 g body weight) daily (n = 6); PHN group: injected with anti-Fx1A serum (0.5 mL per 100 g body weight) into the tail vein once and gavaged with lard (1 mL per 100 g body weight) every other day, a 60% cold sucrose solution (0.5 mL per 100 g body weight), and Chinese Baijiu with 56% purity (0.5 mL per 100 g body weight) every other day (n = 6); PHN+DS group: injected with anti-Fx1A serum (0.5 mL per 100 g body weight) into the tail vein once and gavaged with distilled water (1 mL per 100 g body weight) daily (n = 6); DS group: injected with saline (0.5 mL per 100 g body weight) into the tail vein once and gavaged with lard (1 mL per 100 g body weight) every other day, a 60% cold sucrose solution (0.5 mL per 100 g body weight), and Chinese Baijiu with 56% purity (0.5 mL per 100 g body weight) every other day (n = 6). NC: normal control; PHN: passive Heymann nephritis; DS: dampness syndrome.
| Group | Day 0 | Day 6 | Day 12 | Day 18 | Day 24 | Day 30 |
|---|---|---|---|---|---|---|
| NC | 23.00 | 23.50 | 23.67 | 24.67 | 26.17 | 27.00 |
| PHN | 23.25 | 23.00 | 23.17 | 23.20 | 21.00 | 19.50 |
| PHN+DS | 22.80 | 19.50 | 21.00 | 20.17 | 18.83 | 18.17 |
| DS | 23.10 | 20.50 | 20.50 | 21.33 | 21.67 | 22.17 |
Table 1 The average daily feed intake of rats in each group at different time points (g)
| Group | Day 0 | Day 6 | Day 12 | Day 18 | Day 24 | Day 30 |
|---|---|---|---|---|---|---|
| NC | 23.00 | 23.50 | 23.67 | 24.67 | 26.17 | 27.00 |
| PHN | 23.25 | 23.00 | 23.17 | 23.20 | 21.00 | 19.50 |
| PHN+DS | 22.80 | 19.50 | 21.00 | 20.17 | 18.83 | 18.17 |
| DS | 23.10 | 20.50 | 20.50 | 21.33 | 21.67 | 22.17 |
| Group | n | Day 0 | Day 6 | Day 12 | Day 18 | Day 24 | Day 30 |
|---|---|---|---|---|---|---|---|
| NC | 6 | 43.00 | 42.83 | 42.83 | 43.67 | 44.17 | 45.17 |
| PHN | 6 | 42.00 | 43.00 | 43.00 | 43.58 | 41.25 | 41.30 |
| PHN+DS | 6 | 42.50 | 33.67 | 32.83 | 34.17 | 32.67 | 28.17 |
| DS | 6 | 40.56 | 33.50 | 33.42 | 33.50 | 33.25 | 31.17 |
Table 2 The average daily water intake of rats in each group at different time points (mL)
| Group | n | Day 0 | Day 6 | Day 12 | Day 18 | Day 24 | Day 30 |
|---|---|---|---|---|---|---|---|
| NC | 6 | 43.00 | 42.83 | 42.83 | 43.67 | 44.17 | 45.17 |
| PHN | 6 | 42.00 | 43.00 | 43.00 | 43.58 | 41.25 | 41.30 |
| PHN+DS | 6 | 42.50 | 33.67 | 32.83 | 34.17 | 32.67 | 28.17 |
| DS | 6 | 40.56 | 33.50 | 33.42 | 33.50 | 33.25 | 31.17 |
| Group | n | Day 0 | Day 6 | Day 12 | Day 18 | Day 24 | Day 30 |
|---|---|---|---|---|---|---|---|
| NC | 6 | 217±13 | 244±17 | 279±32 | 323±30 | 367±20 | 395±25 |
| PHN | 6 | 219±7 | 255±9 | 280±16 | 320±18 | 351±29 | 322±19b |
| PHN+DS | 6 | 221±6 | 247±14 | 263±19 | 309±26 | 338±29 | 300±29b |
| DS | 6 | 220±10 | 240±16 | 260±18 | 302±16 | 331±19a | 355±23bcd |
Table 3 Comparison of the body weight of rats in each group at different time points (g, x - ± s)
| Group | n | Day 0 | Day 6 | Day 12 | Day 18 | Day 24 | Day 30 |
|---|---|---|---|---|---|---|---|
| NC | 6 | 217±13 | 244±17 | 279±32 | 323±30 | 367±20 | 395±25 |
| PHN | 6 | 219±7 | 255±9 | 280±16 | 320±18 | 351±29 | 322±19b |
| PHN+DS | 6 | 221±6 | 247±14 | 263±19 | 309±26 | 338±29 | 300±29b |
| DS | 6 | 220±10 | 240±16 | 260±18 | 302±16 | 331±19a | 355±23bcd |
| Group | n | Week 1 | Week 2 | Week 3 |
|---|---|---|---|---|
| NC | 6 | 6.46±1.76 | 9.01±1.46 | 6.66±1.15 |
| PHN | 6 | 12.73±9.62 | 42.36±9.89c | 55.70±13.95c |
| PHN+DS | 6 | 15.97±5.11a | 57.26±16.08c | 93.94±19.10ce |
| DS | 6 | 5.40±1.04b | 8.26±2.23df | 5.70±2.06df |
Table 4 Comparison of the proteinuria levels of rats in each group at different time points (mg, x - ± s)
| Group | n | Week 1 | Week 2 | Week 3 |
|---|---|---|---|---|
| NC | 6 | 6.46±1.76 | 9.01±1.46 | 6.66±1.15 |
| PHN | 6 | 12.73±9.62 | 42.36±9.89c | 55.70±13.95c |
| PHN+DS | 6 | 15.97±5.11a | 57.26±16.08c | 93.94±19.10ce |
| DS | 6 | 5.40±1.04b | 8.26±2.23df | 5.70±2.06df |
| Group | n | ALB (g/L) | TC (mmol/L) | TG (mmol/L) | SCr (μmol/L) |
|---|---|---|---|---|---|
| NC | 6 | 22.50±1.01 | 1.05±0.26 | 0.42±0.08 | 20.61±1.49 |
| PHN | 6 | 19.72±1.51a | 5.59±1.11a | 3.08±0.35a | 34.03±5.07a |
| PHN+DS | 6 | 18.82±2.07a | 7.71±0.65ac | 3.88±0.69a | 39.72±6.64a |
| DS | 6 | 21.01±1.73b | 1.42±0.34de | 0.61±0.13de | 21.20±1.88de |
Table 5 Comparison of the ALB, TC, TG, SCr levels of rats in each group at different time points ( x - ± s)
| Group | n | ALB (g/L) | TC (mmol/L) | TG (mmol/L) | SCr (μmol/L) |
|---|---|---|---|---|---|
| NC | 6 | 22.50±1.01 | 1.05±0.26 | 0.42±0.08 | 20.61±1.49 |
| PHN | 6 | 19.72±1.51a | 5.59±1.11a | 3.08±0.35a | 34.03±5.07a |
| PHN+DS | 6 | 18.82±2.07a | 7.71±0.65ac | 3.88±0.69a | 39.72±6.64a |
| DS | 6 | 21.01±1.73b | 1.42±0.34de | 0.61±0.13de | 21.20±1.88de |

Figure 2 Comparison of the renal pathology of rats in each group (×400) A-D: HE staining of renal tissue from rats; E-H: PAS staining of renal tissue from rats; J-L: Masson staining of renal tissue from rats; M-P: PASM staining of renal tissue from rats; A, E, J, M: NC group; B, F, J, N: PHN group; C, G, K, O: PHN+DS group; D, H, L, P: DS group. NC group: injected with saline (0.5 mL per 100 g body weight) into the tail vein once and gavaged with distilled water (1 mL per 100 g body weight) daily (n = 6); PHN group: injected with anti-Fx1A serum (0.5 mL per 100 g body weight) into the tail vein once and gavaged with lard (1 mL per 100 g body weight) every other day, a 60% cold sucrose solution (0.5 mL per 100 g body weight), and Chinese Baijiu with 56% purity (0.5 mL per 100 g body weight) every other day (n = 6); PHN + DS group: injected with anti-Fx1A serum (0.5 mL per 100 g body weight) into the tail vein once and gavaged with distilled water (1 mL per 100 g body weight) daily (n = 6); DS group: injected with saline (0.5 mL per 100 g body weight) into the tail vein once and gavaged with lard (1 mL per 100 g body weight) every other day, a 60% cold sucrose solution (0.5 mL per 100 g body weight), and Chinese Baijiu with 56% purity (0.5 mL per 100 g body weight) every other day (n = 6). NC: normal control; PHN: passive Heymann nephritis; DS: dampness syndrome; HE: hematoxylin and eosin; PAS: periodic acid-schiff; PASM: periodic acid-silver methenamine.

Figure 3 Comparison of the podocyte injury of rats in each group (×400) A-D: nephrin expression in the renal tissue of rats; E-H: podocin expression in the renal tissue of rats; I-L: synaptopodin expression in the renal tissue of rats; A, E, I: NC group; B, F, J: PHN group; C, G, K: PHN+DS group; D, H, L: DS group. All pictures were stained by immunohistochemical method, with PBS used as the negative control. NC group: injected with saline (0.5 mL per 100 g body weight) into the tail vein once and gavaged with distilled water (1 mL per 100 g body weight) daily (n = 6); PHN group: injected with anti-Fx1A serum (0.5 mL per 100 g body weight) into the tail vein once and gavaged with lard (1 mL per 100 g body weight) every other day, a 60% cold sucrose solution (0.5 mL per 100 g body weight), and Chinese Baijiu with 56% purity (0.5 mL per 100 g body weight) every other day (n = 6); PHN + DS group: injected with anti-Fx1A serum (0.5 mL per 100 g body weight) into the tail vein once and gavaged with distilled water (1 mL per 100 g body weight) daily (n = 6); DS group: injected with saline (0.5 mL per 100 g body weight) into the tail vein once and gavaged with lard (1 mL per 100 g body weight) every other day, a 60% cold sucrose solution (0.5 mL per 100 g body weight), and Chinese Baijiu with 56% purity (0.5 mL per 100 g body weight) every other day (n = 6). NC: normal control; PHN: passive Heymann nephritis; DS: dampness syndrome; PBS: phosphate-buffered saline.
| Group | n | Nephrin | Podocin | Synaptopodin |
|---|---|---|---|---|
| NC | 6 | 0.233±0.017 | 0.221±0.014 | 0.243±0.010 |
| PHN | 6 | 0.202±0.013a | 0.189±0.010a | 0.202±0.025a |
| PHN+DS | 6 | 0.171±0.013ab | 0.165±0.018ab | 0.201±0.015a |
| DS | 6 | 0.226±0.013bc | 0.213±0.014bc | 0.234±0.014bc |
Table 6 Comparison of the average optical density of nephrin, podocin, and synaptopodin in kidneys of rats in each group ( x - ± s)
| Group | n | Nephrin | Podocin | Synaptopodin |
|---|---|---|---|---|
| NC | 6 | 0.233±0.017 | 0.221±0.014 | 0.243±0.010 |
| PHN | 6 | 0.202±0.013a | 0.189±0.010a | 0.202±0.025a |
| PHN+DS | 6 | 0.171±0.013ab | 0.165±0.018ab | 0.201±0.015a |
| DS | 6 | 0.226±0.013bc | 0.213±0.014bc | 0.234±0.014bc |
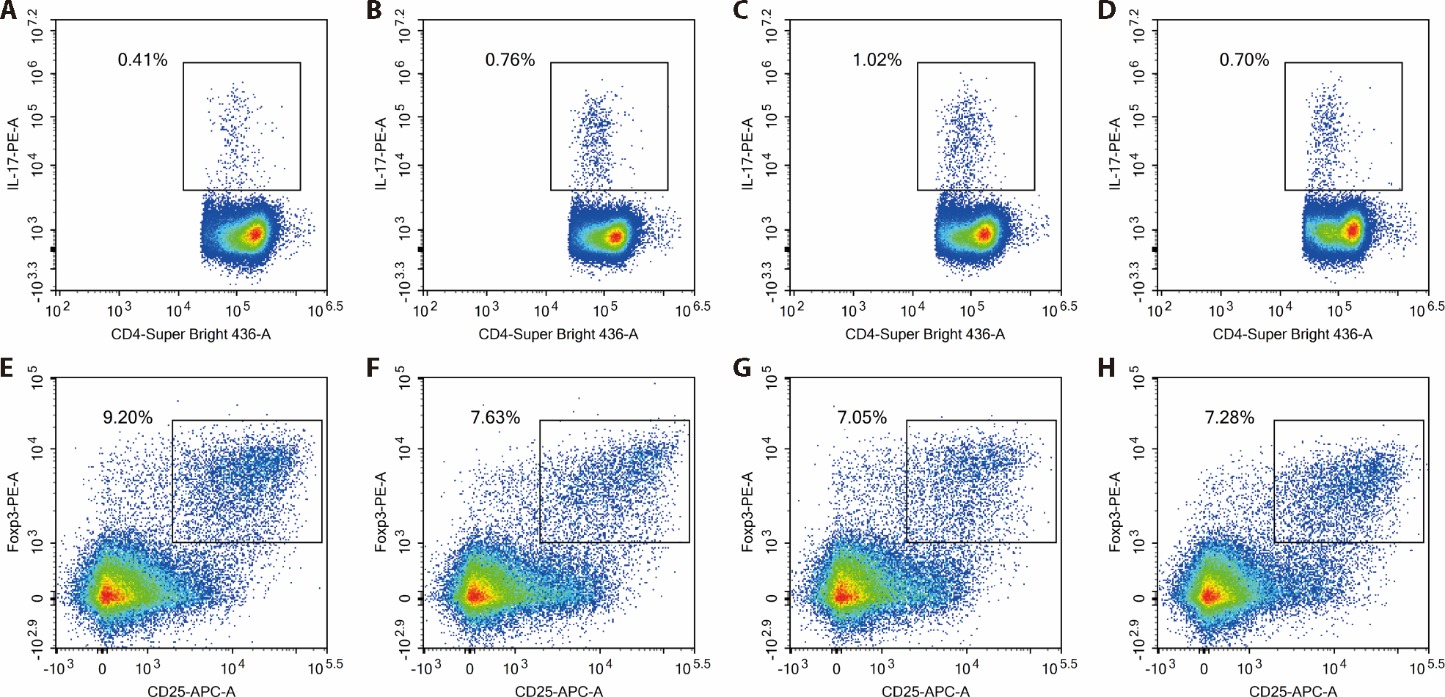
Figure 4 Comparison of the percentages of Th17, Treg in PBMCs of rats o in each group A-D: percentages of Th17 cells in PBMCs of rats; E-H: percentages of Treg cells in PBMCs of rats; A, E: NC group; B, F: PHN group; C, G: PHN + DS group; D, H: DS group. NC group: injected with saline (0.5 mL per 100 g body weight) into the tail vein once and gavaged with distilled water (1 mL per 100 g body weight) daily (n = 6); PHN group: injected with anti-Fx1A serum (0.5 mL per 100 g body weight) into the tail vein once and gavaged with lard (1 mL per 100 g body weight) every other day, a 60% cold sucrose solution (0.5 mL per 100 g body weight), and Chinese Baijiu with 56% purity (0.5 mL per 100 g body weight) every other day (n = 6); PHN + DS group: injected with anti-Fx1A serum (0.5 mL per 100 g body weight) into the tail vein once and gavaged with distilled water (1 mL per 100 g body weight) daily (n = 6); DS group: injected with saline (0.5 mL per 100 g body weight) into the tail vein once and gavaged with lard (1 mL per 100 g body weight) every other day, a 60% cold sucrose solution (0.5 mL per 100 g body weight), and Chinese Baijiu with 56% purity (0.5 mL per 100 g body weight) every other day (n = 6). NC: normal control; PHN: passive Heymann nephritis; DS: dampness syndrome; Th17: T helper 17; Treg: regulatory T; PBMCs: peripheral blood mononuclear cells.
| Group | n | Th17 | Treg | Th17/Treg |
|---|---|---|---|---|
| NC | 6 | 0.508±0.093 | 8.867±1.746 | 0.060±0.155 |
| PHN | 6 | 0.752±0.227a | 8.553±1.144 | 0.090±0.029 |
| PHN+DS | 6 | 0.935±0.134b | 6.647±1.429ac | 0.152±0.042bd |
| DS | 6 | 0.800±0.183b | 6.808±1.332ac | 0.115±0.026be |
Table 7 Comparison of the percentages of Th17, Treg and the ratio of Th17/Treg in PBMCs of rats in each group ( x - ± s)
| Group | n | Th17 | Treg | Th17/Treg |
|---|---|---|---|---|
| NC | 6 | 0.508±0.093 | 8.867±1.746 | 0.060±0.155 |
| PHN | 6 | 0.752±0.227a | 8.553±1.144 | 0.090±0.029 |
| PHN+DS | 6 | 0.935±0.134b | 6.647±1.429ac | 0.152±0.042bd |
| DS | 6 | 0.800±0.183b | 6.808±1.332ac | 0.115±0.026be |
| Group | n | IL-17 | IL-6 | TGF-β1 |
|---|---|---|---|---|
| NC | 6 | 144±43 | 4±0 | 314±83 |
| PHN | 6 | 452±141a | 5±1 | 182±64b |
| PHN+DS | 6 | 787±189bc | 6±1a | 136±66b |
| DS | 6 | 146±39cd | 5±1 | 132±55b |
Table 8 Comparison of the IL-17, IL-6, TGF-β1 levels in serum of rats in each group (pg/mL, x - ± s)
| Group | n | IL-17 | IL-6 | TGF-β1 |
|---|---|---|---|---|
| NC | 6 | 144±43 | 4±0 | 314±83 |
| PHN | 6 | 452±141a | 5±1 | 182±64b |
| PHN+DS | 6 | 787±189bc | 6±1a | 136±66b |
| DS | 6 | 146±39cd | 5±1 | 132±55b |
| 1. |
Ronco P, Beck L, Debiec H, et al. Membranous nephropathy. Nat Rev Dis Primers 2021; 7: 69.
DOI PMID |
| 2. | Claudio P. Primary membranous nephropathy: an endless story. J Nephrol 2023; 36: 563-74. |
| 3. | Li LS, Liu ZH. Epidemiologic data of renal diseases from a single unit in China: analysis based on 13 519 renal biopsies. Kidney Int 2004; 66: 920-3. |
| 4. | Couser WG. Primary membranous nephropathy. Clin J Am Soc Nephrol 2017; 12: 983-97. |
| 5. | Lai WL, Yeh TH, Chen PM, et al. Membranous nephropathy: a review on the pathogenesis, diagnosis, and treatment. J Formos Med Assoc 2015; 114: 102-11. |
| 6. | Zhu P, Zhou FD, Wang SX, Zhao MH, Wang HY. Increasing frequency of idiopathic membranous nephropathy in primary glomerular disease: a 10-year renal biopsy study from a single Chinese nephrology centre. Nephrology (Carlton) 2015; 20: 560-6. |
| 7. |
Xie J, Chen N. Primary glomerulonephritis in mainland China: an overview. Contrib Nephrol. 2013; 181: 1-11.
DOI PMID |
| 8. | Motavalli R, Etemadi J, Kahroba H, Mehdizadeh A, Yousefi M. Immune system-mediated cellular and molecular mechanisms in idiopathic membranous nephropathy pathogenesis and possible therapeutic targets. Life Sci 2019; 238: 116923. |
| 9. | Paquissi FC, Abensur H. The Th17/IL-17 axis and kdney diseases, with focus on lupus nephritis. Front Med (Lausanne) 2021; 8: 654912. |
| 10. | Zhu X, Li S, Zhang Q, et al. Correlation of increased Th17/Treg cell ratio with endoplasmic reticulum stress in chronic kidney disease. Medicine (Baltimore) 2018; 97: e10748. |
| 11. | Motavalli R, Etemadi J, Soltani-Zangbar MS, et al. Altered Th17/Treg ratio as a possible mechanism in pathogenesis of idiopathic membranous nephropathy. Cytokine 2021; 141: 155452. |
| 12. |
Rosenzwajg M, Languille E, Debiec H, et al. B- and T-cell subpopulations in patients with severe idiopathic membranous nephropathy may predict an early response to rituximab. Kidney Int 2017; 92: 227-37.
DOI PMID |
| 13. | Chen Y, Sun BG, Zhang SJ, Chen ZX, Hardi CF, Xiang T. Observations of TCRVβ gene expression in rats with dampness syndrome. Evid Based Complement Alternat Med 2014; 2014: 373608. |
| 14. | Hong X, Li C, Cai F, et al. Construction of questionnaire for damp type of membranous nephropathy and correlation between urine protein and damp syndrome. Guangzhou Zhong Yi Yao Da Xue Xue Bao 2021; 38: 2260-7. |
| 15. | Zhang S, Chen Z, Lin Y. Effect of Chinese medicine for resolving dampness on activated and functional T lymphocyte subsets in chronic hepatitis B patients with dampness syndrome. Zhong Guo Zhong Xi Yi Jie He Za Zhi 2006; 26: 1078-81. |
| 16. | Gong Y, Liu L, He X, et al. The th17/treg immune balance in ulcerative colitis patients with two different chinese syndromes: dampness-heat in large intestine and spleen and kidney Yang deficiency syndrome. Evid Based Complement Alternat Med 2015; 2015: 264317. |
| 17. |
Glassock RJ. The pathogenesis of idiopathic membranous nephropathy: a 50-year odyssey. Am J Kidney Dis 2010; 56: 157-67.
DOI PMID |
| 18. | Wang CS, Greenbaum LA. Nephrotic syndrome. Pediatr Clin North Am 2019; 66: 73-85. |
| 19. | Tu DQ, Jin J, Hu X, Ren Y, Zhao L, He Q. Curcumin improves the renal autophagy in rat experimental membranous nephropathy via regulating the PI3K/AKT/mTOR and Nrf2/HO-1 signaling pathways. Biomed Res Int 2020; 2020: 7069052. |
| 20. |
Glassock RJ. Diagnosis and natural course of membranous nephropathy. Semin Nephrol 2003; 23: 324-32.
DOI PMID |
| 21. |
Frazier KS, Obert LA. Drug-induced glomerulonephritis: the spectre of biotherapeutic and antisense oligonucleotide immune activation in the kidney. Toxicol Pathol 2018; 46: 904-17.
DOI PMID |
| 22. | Jefferson JA, Pippin JW, Shankland SJ. Experimental models of membranous nephropathy. Drug Discov Today Dis Models 2010; 7: 27-33. |
| 23. | Kaysen GA, Paukert TT, Menke DJ, Couser WG, MH H. Plasma volume expansion is necessary for edema formation in the rate with Heymann nephritis. Am J Physiol 1985; 248: F247-253. |
| 24. | Fau ZM, Druet P. Passive Heymann's nephritis as a model of immune glomerulonephritis mediated by antibodies to immunoglobulins. Clin Exp Immunol 1980; 41: 189-95. |
| 25. | Wang XH, Yang YN, Liang Y, et al. Structural modulation of gut microbiota during alleviation of experimental passive Heymann nephritis in rats by a traditional Chinese herbal formula. Biomed Pharmacother. 2022; 145: 112475. |
| 26. |
Lu A, Jiang M, Zhang C, Chan K. An integrative approach of linking Traditional Chinese Medicine pattern classification and biomedicine diagnosis. J Ethnopharmacol 2012; 141: 549-56.
DOI PMID |
| 27. | Hao Y, Yuan X, Qian P, Bai G, Wang Y. The serum analysis of dampness syndrome in patients with coronary heart disease and chronic renal failure based on the theory of "Same Syndromes in Different Diseases". Biomed Res Int 2017; 2017: 3805806. |
| 28. | Zhong J, He L, Ding X. Study on clinical syndrome classification and related biochemical indexes in 146 cases of chronic renal failure. J Tradit Chin Med 2006; 47: 374-77. |
| 29. |
Nagata M. Podocyte injury and its consequences. Kidney Int 2016; 89: 1221-30.
DOI PMID |
| 30. |
Benzing T. Signaling at the slit diaphragm. J Am Soc Nephrol 2004; 15: 1382-91.
DOI PMID |
| 31. |
Kestilä M, Lenkkeri U, Männikkö M, et al. Positionally cloned gene for a novel glomerular protein--nephrin--is mutated in congenital nephrotic syndrome. Mol Cell 1998; 1: 575-82.
DOI PMID |
| 32. |
Holzman LB, St John PL, Kovari IA, Verma R, Holthofer H, DR A. Nephrin localizes to the slit pore of the glomerular epithelial cell. Kidney Int 1999; 56: 1481-91.
PMID |
| 33. |
Putaala H, Soininen R, Kilpeläinen P, Wartiovaara J, Tryggvason K. The murine nephrin gene is specifically expressed in kidney, brain and pancreas: inactivation of the gene leads to massive proteinuria and neonatal death. Hum Mol Genet 2001; 10: 1-8.
PMID |
| 34. |
Ruotsalainen V, Patrakka J, Tissari P, et al. Role of nephrin in cell junction formation in human nephrogenesis. Am J Pathol 2000; 157: 1905-16.
PMID |
| 35. |
Jalanko H. Pathogenesis of proteinuria: lessons learned from nephrin and podocin. Pediatr Nephrol 2003; 18: 487-91.
PMID |
| 36. |
Roselli S, Gribouval O, Boute N, et al. Podocin localizes in the kidney to the slit diaphragm area. Am J Pathol 2002; 160: 131-9.
DOI PMID |
| 37. |
Huber TB, Simons M, Hartleben B, et al. Molecular basis of the functional podocin-nephrin complex: mutations in the NPHS2 gene disrupt nephrin targeting to lipid raft microdomains. Hum Mol Genet 2003; 12: 3397-405.
PMID |
| 38. |
Kawachi H, Koike H, Kurihara H, Sakai T, Shimizu F. Cloning of rat homologue of podocin: expression in proteinuric states and in developing glomeruli. J Am Soc Nephrol 2003; 14: 46-56.
PMID |
| 39. |
Yu H, Kistler A, Faridi MH, et al. Synaptopodin limits TRPC6 podocyte surface expression and attenuates proteinuria. J Am Soc Nephrol 2016; 27: 3308-19.
PMID |
| 40. |
Ning L, Suleiman HY, Miner JH. Synaptopodin is dispensable for normal podocyte homeostasis but is protective in the context of acute podocyte injury. J Am Soc Nephrol 2020; 31: 2815-32.
DOI PMID |
| 41. | Li H, Wu H, Guo Q, et al. Myeloid-derived suppressor cells promote the progression of primary membranous nephropathy by enhancing Th 17 response. Front Immunol 2020; 11: 1777. |
| 42. |
McGeachy MJ, Cua DJ, Gaffen SL. The IL-17 family of cytokines in health and disease. Immunity 2019; 50: 892-906.
DOI PMID |
| 43. | Chen L, Ruan G, Cheng Y, Yi A, Chen D, Wei Y. The role of Th17 cells in inflammatory bowel disease and the research progress. Front Immunol 2023; 13: 1055914. |
| 44. | GR L. The balance of Th17 versus Treg cells in autoimmunity. Int J Mol Sci 2018; 19: 730. |
| 45. | Bunte K, Beikler T. Th 17 cells and the IL-23/IL-17 axis in the pathogenesis of periodontitis and immune-mediated inflammatory diseases. Int J Mol Sci 2019; 20: 3394. |
| 46. | Yang X, Zhang J, Ding YL. Association of microRNA-155, interleukin 17A, and proteinuria in preeclampsia. Medicine (Baltimore) 2017; 2017: e6509. |
| 47. |
Zhai SB, Sun BC, Zhang Y, Zhao LY, Zhang L. IL-17 aggravates renal injury by promoting podocyte injury in children with primary nephrotic syndrome. Exp Ther Med 2020; 20: 409-17.
DOI PMID |
| 48. | Wang L, Li Q, Wang LJ, et al. The role of Th17/IL-17 in the pathogenesis of primary nephrotic syndrome in children. Kidney Blood Press Res 2013; 337: 332-45. |
| 49. | Liu YB, Su L, Lin QX, Han Y, Peng Y, Fan QF. Induction of C-Mip by IL-17 plays an important role in adriamycin-induced podocyte damage. Cell Physiol Biochem 2015; 36: 1274-90. |
| 50. |
Xu Y, Lin H, Zheng W, et al. Matrine ameliorates adriamycin-induced nephropathy in rats by enhancing renal function and modulating Th17/Treg balance. Eur J Pharmacol 2016; 791: 491-501.
DOI PMID |
| 51. |
Li MO, Wan YY, Flavell RA. T cell-produced transforming growth factor-beta1 controls T cell tolerance and regulates Th1- and Th17-cell differentiation. Immunity 2007; 26: 579-91.
DOI PMID |
| 52. |
Cantarelli C, Jarque M, Angeletti A, et al. A comprehensive phenotypic and functional immune analysis unravels circulating anti-phospholipase A2 receptor antibody secreting cells in membranous nephropathy patients. Kidney Int Rep 2020; 5: 1764-76.
DOI PMID |
| 53. | Le Berre L, Bruneau S, Naulet J, et al. Induction of T regulatory cells attenuates idiopathic nephrotic syndrome. J Am Soc Nephrol 2008; 20: 57-67. |
| 54. | Kim MG, Koo TY, Yan JJ, et al. IL-2/anti-IL-2 complex attenuates renal ischemia-reperfusion injury through expansion of regulatory T cells. J Am Soc Nephrol 2013; 24: 1529-36. |
| [1] | Xiaolan SU, Tao ZHANG, Song GUO, Xin WANG, Lin CHEN, Wei WEI. Efficacy of Wumei Baijiang prescription (乌梅败酱方) on regulatory T cells / helper T cells Immune balance in mice with ulcerative colitiss [J]. Journal of Traditional Chinese Medicine, 2022, 42(1): 30-38. |
| [2] | GONG Hui, KUANG Gaoyan, WU Yongrong, LU Fangguo, DENG Yihui, HE Yanlin, WANG Hengxin. Zhuifeng tougu capsules (追风透骨胶囊) improve rheumatoid arthritis symptoms in rats by regulating the toll-like receptor 2/4-nuclear factor kappa-B signaling pathway [J]. Journal of Traditional Chinese Medicine, 2021, 41(3): 447-454. |
| [3] | Sun Zhigao, Hu Yazhuo, Wang Yuguo, Feng Jian, Dou Yongqi. Bupi Hewei decoction ameliorates 5-fluorouracil-induced intestinal dysbiosis in rats through T helper 17/T regulatory cell signaling pathway [J]. Journal of Traditional Chinese Medicine, 2020, 40(1): 38-48. |
| [4] | Wei Mingyan, Yang Tao, Li Qian, Zhou Dongdong, Du Zongpan, Fan Yongping. Protective effects of catalpol and rhein in murine experimental autoimmune encephalomyelitis via regulation of T helper (Th)1, Th2,Th17, and regulatory T cell responses [J]. Journal of Traditional Chinese Medicine, 2019, 39(06): 809-817. |
| [5] | Qu Tiange, Mei Chencheng, Zeng Yajun, Li Lingling, Duan Xingwu. Correlation analysis of Treg/Th17 cells and related cytokines in patients with psoriasis vulgaris [J]. Journal of Traditional Chinese Medicine, 2019, 39(05): 700-706. |
| [6] | Hou Yi, Wang Tieshan, Guo Xiangyu, Sun Wen, Guo Xuan, Wu Lili, Qin Lingling, Zhang Chengfei, Liu Tonghua. Protective effects of Jiayan Kangtai granules on autoimmune thyroiditis in a rat model by modulating Th17/Treg cell balance [J]. Journal of Traditional Chinese Medicine, 2018, 38(03): 380-390. |
| [7] | Xiaolin Chen, Youwu Lin, Shijun Zhang, Zexiong Chen, Carlini Fan Hardi, Ting Xiang, Baoguo Sun. Correlation between pathogenesis of dampness syndrome and Interleukin-2,Interleukin-8 in rats [J]. Journal of Traditional Chinese Medicine, 2013, 33(01): 114-118. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||

