Journal of Traditional Chinese Medicine ›› 2023, Vol. 43 ›› Issue (4): 686-694.DOI: 10.19852/j.cnki.jtcm.2023.04.005
Previous Articles Next Articles
Analgesic efficacy of median nerve stimulation in mice with chemotherapy-induced peripheral neuropathy via modulation of brain-derived neurotrophic factor expression
Dong-Wook Kang1, Jae-Gyun Choi1, Hee Ju Song1, Jaehyuk Kim1, Miae Lee1, Taehee Kim1, Suk-Yun Kang2, Yeonhee Ryu2, Hwa Seung Yoo3, Jin Sun Lee4, Jin Bong Park5, Sang Do Lee1( ), Hyun-Woo Kim1(
), Hyun-Woo Kim1( )
)
- 1 Department of Physiology and Medical Science, College of Medicine and Brain Research Institute, Chungnam National University, Daejeon 35015, Republic of Korea
2 KM Fundamental Research Division Korea Institute of Oriental Medicine, Daejeon 34054, Republic of Korea
3 East-West Cancer Center, Dunsan Korean Medicine Hospital of Daejeon University, Daejeon 35234, Republic of Korea
4 Department of Surgery, Chungnam National University Hospital, Daejeon 35015, Republic of Korea
5 Laboratory of Veterinary Pharmacology, College of Veterinary Medicine and Research Institute for Veterinary Science, Seoul National University, 1 Gwanak-ro, Gwanak-gu, Seoul 08826, Republic of Korea
-
Received:2021-12-16Accepted:2022-08-03Online:2023-08-15Published:2023-07-03 -
Contact:Hyun-Woo Kim, Department of Physiology and Medical Science, College of Medicine and Brain Research Institute, Chungnam National University, Daejeon 35015, Republic of Korea. kim0827@cnu.ac.kr. Telephone: +82-42-580-8220; +82-42-580-8217
Sang Do Lee, Department of Physiology and Medical Science, College of Medicine, Chungnam National University, Daejeon 35015, Republic of Korea. sdlee@cnu.ac.kr -
Supported by:“Korea Health Technology R & D Project through the Korea Health Industry Development Institute, funded by the Ministry of Health and Welfare, Republic of Korea(HI15C0007);“Chungnam National University, and Basic Science Research Program through the National Research Foundation of Korea(2021R1F1A1062509);Study on the Angiotensin Converting Enzyme Inhibitor (ACEi)-related Pain Mechanism by Mediating Substance P, Bradykinin and Angiotensin II Activities(2021R1A6A3A01086598);Study on the Role and Interaction Mechanisms of BDNF and APE1/Ref-1 in Animal Models of Chronic Pain Accompanied with Depression
Cite this article
Dong-Wook Kang, Jae-Gyun Choi, Hee Ju Song, Jaehyuk Kim, Miae Lee, Taehee Kim, Suk-Yun Kang, Yeonhee Ryu, Hwa Seung Yoo, Jin Sun Lee, Jin Bong Park, Sang Do Lee, Hyun-Woo Kim. Analgesic efficacy of median nerve stimulation in mice with chemotherapy-induced peripheral neuropathy via modulation of brain-derived neurotrophic factor expression[J]. Journal of Traditional Chinese Medicine, 2023, 43(4): 686-694.
share this article
| Group | n | Pre BW | Day 2 BW | Day 4 BW | Day 6 BW | Day 12 BW | Day 20 BW | Day 22 BW |
|---|---|---|---|---|---|---|---|---|
| Vehicle DTX | 5 7 | 30.7±0.5 30.0±1.1 | 31.2±0.5 31.9±0.8 | 32.4±0.5 33.2±0.8 | 33.0±0.6 33.4±0.9 | 35.1±0.8 36.2±1.1 | 36.6±1.0 36.3±1.2 | 36.2±0.9 37.7±1.3 |
Table 1 Changes in body weight after DTX injection (g)
| Group | n | Pre BW | Day 2 BW | Day 4 BW | Day 6 BW | Day 12 BW | Day 20 BW | Day 22 BW |
|---|---|---|---|---|---|---|---|---|
| Vehicle DTX | 5 7 | 30.7±0.5 30.0±1.1 | 31.2±0.5 31.9±0.8 | 32.4±0.5 33.2±0.8 | 33.0±0.6 33.4±0.9 | 35.1±0.8 36.2±1.1 | 36.6±1.0 36.3±1.2 | 36.2±0.9 37.7±1.3 |
| Group | n | Pre PWF | Day 2 PWF | Day 4 PWF | Day 6 PWF | Day 12 PWF | Day 20 PWF | Day 22 PWF |
|---|---|---|---|---|---|---|---|---|
| Vehicle DTX | 5 7 | 9.0±1.9 11.4±2.1 | 9.0±1.9 43.6±8.8a | 10.0±1.6 62.1±6.3a | 9.0±2.9 65.7±3.0a | 11.0±3.7 66.4±4.0a | 10.0±1.6 62.1±5.0a | 11.0±4.0 33.6±5.1b |
Table 2 Changes in pain response after DTX injection (%)
| Group | n | Pre PWF | Day 2 PWF | Day 4 PWF | Day 6 PWF | Day 12 PWF | Day 20 PWF | Day 22 PWF |
|---|---|---|---|---|---|---|---|---|
| Vehicle DTX | 5 7 | 9.0±1.9 11.4±2.1 | 9.0±1.9 43.6±8.8a | 10.0±1.6 62.1±6.3a | 9.0±2.9 65.7±3.0a | 11.0±3.7 66.4±4.0a | 10.0±1.6 62.1±5.0a | 11.0±4.0 33.6±5.1b |
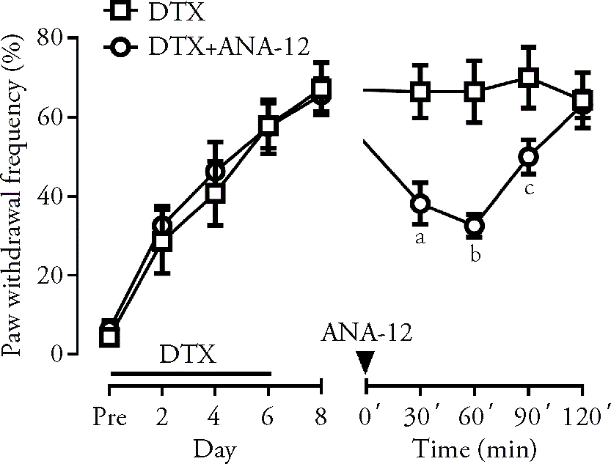
Figure 1 Effect of intraperitoneal administration of ANA-12 on DTX-induced mechanical allodynia Multiple injections of DTX induced mechanical hypersensitivity, and this pain response was significantly attenuated by intraperitoneal administration of ANA-12, the specific TrkB receptor antagonist. DTX group: CIPN model mice without treatment (n = 7); DTX + ANA-12 group: CIPN model mice treated with ANA-12 (1 mg/kg) (n = 8). DTX: docetaxel; ANA-12: N-[2-[(2-oxoazepan-3-yl) carbamoyl] phenyl]-1-benzothiophene-2-carboxamide; CIPN: chemotherapy-induced peripheral neuropathy. aP < 0.01, bP < 0.001 and cP < 0.05 compared with DTX group. Differences among groups analyzed by two-way analysis of variance.
| Group | n | Pre RT | 0 min RT | 20 min RT | 40 min RT | 60 min RT | 80 min RT | 100 min RT |
|---|---|---|---|---|---|---|---|---|
| Vehicle Avertin | 5 5 | 116.6±2.4 120.0±0.0 | 118.6±1.0 19.0±9.9a | 120.0±0.0 0.0±0.0a | 119.6±0.4 0.0±0.0a | 120.0±0.0 15.8±4.9a | 119.4±0.6 56.4±7.4a | 120.0±0.0 117.6±2.4 |
Table 3 Restoration of normal motor function over time after anesthesia (s)
| Group | n | Pre RT | 0 min RT | 20 min RT | 40 min RT | 60 min RT | 80 min RT | 100 min RT |
|---|---|---|---|---|---|---|---|---|
| Vehicle Avertin | 5 5 | 116.6±2.4 120.0±0.0 | 118.6±1.0 19.0±9.9a | 120.0±0.0 0.0±0.0a | 119.6±0.4 0.0±0.0a | 120.0±0.0 15.8±4.9a | 119.4±0.6 56.4±7.4a | 120.0±0.0 117.6±2.4 |
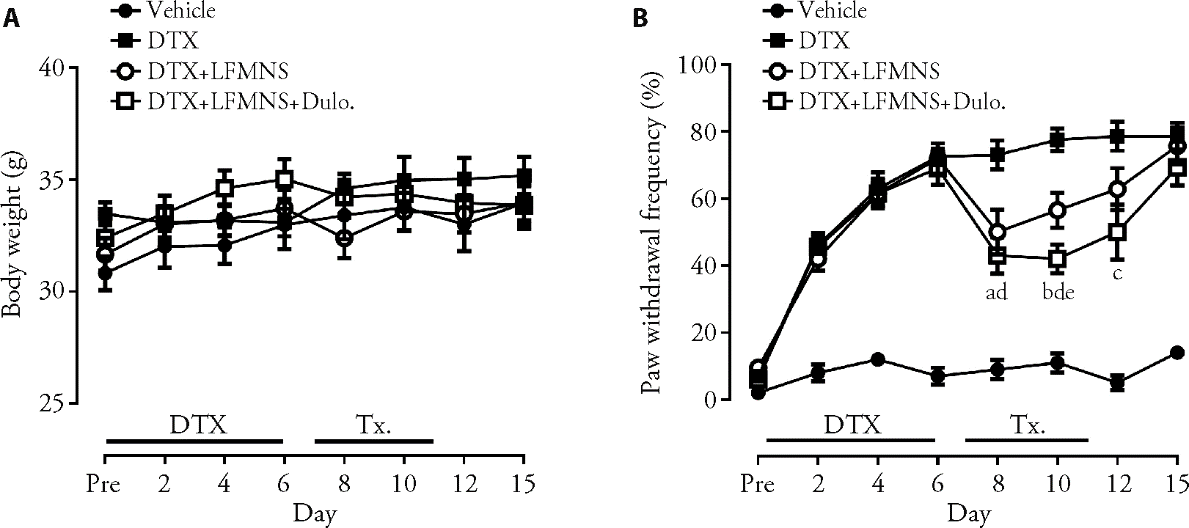
Figure 2 Effect of repeated LFMNS on DTX-induced mechanical allodynia A: changes in body weight during the experiment period. There was no significant weight loss during the experimental period; B: changes in pain response during the experimental period. Multiple injections of DTX induced mechanical hypersensitivity, and this pain response was significantly attenuated by repeated LFMNS at the wrist area. Vehicle group: 5% DMSO injected mice (n = 5); DTX group: CIPN model mice without treatment (n = 10); DTX + LFMNS group: CIPN model mice treated with LFMNS (n = 10); DTX + LFMNS + Dulo. group: CIPN model mice treated with LFMNS combined with Duloxetine (10 mg/kg) (n = 10). DTX: docetaxel; LFMNS: low-frequency median nerve stimulation; Dulo: duloxetine; DMSO: dimethyl sulfate solution; CIPN: chemotherapy-induced peripheral neuropathy. aP < 0.01, bP < 0.001 DTX + LFMNS group vs DTX group; cP < 0.01, dP < 0.001 DTX + LFMNS + Dulo. group vs DTX group; eP < 0.05 DTX + LFMNS group vs DTX + LFMNS + Dulo. group. Differences among groups analyzed by two-way analysis of variance.

Figure 3 Expression change of BDNF protein and mRNA in lumbar 4-5 segment DRG DRG samples were collected 5 d after the first treatment of LFMNS. A: changes in pain response during the experimental period. LFMNS treated groups have shown significant analgesic effects in the pain response test. Vehicle group: 5% DMSO injected mice (n = 5); DTX group: CIPN model mice without treatment (n = 7); DTX + LFMNS group: CIPN model mice treated with LFMNS (n = 8); DTX + LFMNS + Dulo. group: CIPN model mice treated with LFMNS combined with Duloxetine (10 mg/kg) (n = 8). aP < 0.01 DTX + LFMNS group vs DTX group, bP < 0.01 DTX + LFMNS + Dulo. group vs DTX group. Differences among groups analyzed by two-way analysis of variance. B-C: changes in the molecular expression of DRG neurons. DTX injected mice showed a significantly enhanced BDNF protein expression in DRG neurons and repeated LFMNS were suppressed this increment (B1/B2/B3: G1; B4/B5/B6: G2; B7/B8/B9: G3; B10/B11/B12: G4, × 100). G1: Vehicle group; G2: DTX group; G3: DTX + LFMNS group; G4: DTX + LFMNS + Dulo. group. Vehicle group: 5% DMSO injected mice (n = 5); DTX group: CIPN model mice without treatment (n = 6); DTX + LFMNS group: CIPN model mice treated with LFMNS (n = 6); DTX + LFMNS + Dulo. group: CIPN model mice treated with LFMNS combined with Duloxetine (10 mg/kg) (n = 6). D: changes in the BDNF mRNA expression of DRG. DTX-injected mice showed significantly enhanced BDNF mRNA expression in DRG, and repeated LFMNS suppressed this increment. G1: Vehicle group; G2: DTX group; G3: DTX + LFMNS group; G4: DTX + LFMNS + Dulo. group. Vehicle group: 5% DMSO injected mice (n = 5); DTX group: CIPN model mice without treatment (n = 7); DTX + LFMNS group: CIPN model mice treated with LFMNS (n = 7); DTX + LFMNS + Dulo. group: CIPN model mice treated with LFMNS combined with Duloxetine (10 mg/kg) (n = 7). B1/B4/B7/B10: expressed NeuN in the DRG; B2/B5/B8/B11: expressed BDNF in the DRG; B3/B6/B9/B12: merged images of NeuN and BDNF. DTX: docetaxel; LFMNS: low-frequency median nerve stimulation; Dulo.: duloxetine; DMSO: dimethyl sulfate solution; CIPN: chemotherapy-induced peripheral neuropathy; DRG: dorsal root ganglion; BDNF: brain-derived neurotrophic factor. cP < 0.01 and dP < 0.001 compared with DTX group. eP > 0.05, no significant difference DTX + LFMNS group vs DTX + LFMNS + Dulo. group. Differences among groups analyzed by one-way analysis of variance.

Figure 4 Expression change of BDNF protein and mRNA in lumbar 4-5 segment spinal cord dorsal area Lumbar spinal cord samples were collected 5 d after the first treatment of LFMNS. A-B: changes in the molecular expression of spinal cord after treatment. DTX injected mice showed a significantly enhanced BDNF protein expression in the Lumbar spinal cord dorsal area, and repeated LFMNS have suppressed this increment. G1: Vehicle group; G2: DTX group; G3: DTX + LFMNS group; G4: DTX + LFMNS + Dulo. group. Vehicle group: 5% DMSO injected mice (n = 3); DTX group: CIPN model mice without treatment (n = 3); DTX + LFMNS group: CIPN model mice treated with LFMNS (n = 3); DTX + LFMNS + Dulo. group: CIPN model mice treated with LFMNS combined with Duloxetine (10 mg/kg) (n = 3). C: changes in the BDNF mRNA expression of spinal cord. There are no changes observed in spinal cord expressions of BDNF mRNA. G1: Vehicle group; G2: DTX group; G3: DTX + LFMNS group; G4: DTX + LFMNS + Dulo. group. Vehicle group: 5% DMSO injected mice (n = 5); DTX group: CIPN model mice without treatment (n = 7); DTX + LFMNS group: CIPN model mice treated with LFMNS (n = 7); DTX + LFMNS + Dulo. group: CIPN model mice treated with LFMNS combined with Duloxetine (10 mg/kg) (n = 7). DTX: docetaxel; LFMNS: low-frequency median nerve stimulation; Dulo.: duloxetine; DMSO: dimethyl sulfate solution; CIPN: chemotherapy-induced peripheral neuropathy; BDNF: brain-derived neurotrophic factor. aP < 0.05, bP < 0.01, compared with DTX group. Differences among groups analyzed by one-way analysis of variance.
| 1. |
Cavaletti G, Marmiroli P. Chemotherapy-induced peripheral neurotoxicity. Curr Opin Neurol 2015; 28: 500-7.
DOI PMID |
| 2. | Fehrenbacher JC. Chemotherapy-induced peripheral neuropathy. Prog Mol Biol Transl Sci 2015; 131: 471-508. |
| 3. |
Matsuo M, Ito H, Takemura Y, et al. Increased risk of paclitaxel-induced peripheral neuropathy in patients using clopidogrel: a retrospective pilot study. J Anesth 2017; 31: 631-5.
DOI PMID |
| 4. |
Lyseng-Williamson KA, Fenton C. Docetaxel: a review of its use in metastatic breast cancer. Drugs 2005; 65: 2513-31.
PMID |
| 5. |
Pazdur R, Kudelka AP, Kavanagh JJ, Cohen PR, Raber MN. The taxoids: paclitaxel (Taxol) and docetaxel (Taxotere). Cancer Treat Rev 1993; 19: 351-86.
PMID |
| 6. |
Yvon AM, Wadsworth P, Jordan MA. Taxol suppresses dynamics of individual microtubules in living human tumor cells. Mol Biol Cell 1999; 10: 947-59.
DOI PMID |
| 7. | Park SB, Goldstein D, Krishnan AV, et al. Chemotherapy-induced peripheral neurotoxicity: a critical analysis. CA Cancer J Clin 2013; 63: 419-37. |
| 8. |
Cervellini I, Bello E, Frapolli R, et al. The neuroprotective effect of erythropoietin in docetaxel-induced peripheral neuropathy causes no reduction of antitumor activity in 13762 adenocarcinoma-bearing rats. Neurotox Res 2010; 18: 151-60.
DOI PMID |
| 9. | Ventzel L, Jensen AB, Jensen AR, Jensen TS, Finnerup NB. Chemotherapy-induced pain and neuropathy: a prospective study in patients treated with adjuvant oxaliplatin or docetaxel. Pain 2016; 157: 560-8. |
| 10. |
Celik EC, Erhan B, Gunduz B, Lakse E. The effect of low-frequency TENS in the treatment of neuropathic pain in patients with spinal cord injury. Spinal Cord 2013; 51: 334-7.
DOI PMID |
| 11. |
Guo S, Falkenberg K, Schytz HW, Caparso A, Jensen RH, Ashina M. Low frequency activation of the sphenopalatine ganglion does not induce migraine-like attacks in migraine patients. Cephalalgia 2020; 40: 966-77.
DOI PMID |
| 12. | Walsh DM, Liggett C, Baxter D, Allen JM. A double-blind investigation of the hypoalgesic effects of transcutaneous electrical nerve stimulation upon experimentally induced ischaemic pain. Pain 1995; 61: 39-45. |
| 13. | Ainsworth L, Budelier K, Clinesmith M, et al. Transcutaneous electrical nerve stimulation (TENS) reduces chronic hyperalgesia induced by muscle inflammation. Pain 2006; 120: 182-7. |
| 14. | Chen YH, Lee HJ, Lee MT, et al. Median nerve stimulation induces analgesia via orexin-initiated endocannabinoid disinhibition in the periaqueductal gray. Proc Natl Acad Sci USA 2018; 115: E10720-9. |
| 15. | Jung K, Larsen LE, Rottmann S, Ellrich J. Heterotopic low-frequency stimulation induces nociceptive LTD within the same central receptive field in man. Exp Brain Res 2011; 212: 189-98. |
| 16. | Jung K, Lelic D, Rottmann S, Drewes AM, Petrini L, Ellrich J. Electrical low-frequency stimulation induces central neuroplastic changes of pain processing in man. Eur J Pain 2012; 16: 509-21. |
| 17. |
Lindelof K, Jung K, Ellrich J, Jensen R, Bendtsen L. Low-frequency electrical stimulation induces long-term depression in patients with chronic tension-type headache. Cephalalgia 2010; 30: 860-7.
DOI PMID |
| 18. | Rottmann S, Jung K, Ellrich J. Electrical low-frequency stimulation induces homotopic long-term depression of nociception and pain from hand in man. Clin Neurophysiol 2008; 119: 1895-904. |
| 19. | Shetter AG, Racz GB, Lewis R, Heavner JE. Peripheral Nerve Stimulation. North RB, Levy RM, editor. Neurosurgical management of pain. New York: Springer, 1997: 261-70. |
| 20. | Wall PD, Sweet WH. Temporary abolition of pain in man. Science 1967; 155: 108-9. |
| 21. |
Jeon IC, Kim MS, Kim SH. Median nerve stimulation in a patient with complex regional pain syndrome type Ⅱ. J Korean Neurosurg Soc 2009; 46: 273-6.
DOI URL |
| 22. |
Lee M, Kiernan MC, Macefield VG, Lee BB, Lin CS. Short-term peripheral nerve stimulation ameliorates axonal dysfunction after spinal cord injury. J Neurophysiol 2015; 113: 3209-18.
DOI PMID |
| 23. |
Kang SY, Bang SK, Kwon OS, et al. Treatment of electrical wrist stimulation reduces chemotherapy-induced neuropathy and ultrasound vocalization via modulation of spinal NR2B phosphorylation. Brain Res Bull 2020; 162: 237-44.
DOI URL |
| 24. | Groth R, Aanonsen L. Spinal brain-derived neurotrophic factor (BDNF) produces hyperalgesia in normal mice while antisense directed against either BDNF or trkB, prevent inflammation-induced hyperalgesia. Pain 2002; 100: 171-81. |
| 25. |
Obata K, Noguchi K. BDNF in sensory neurons and chronic pain. Neurosci Res 2006; 55: 1-10.
PMID |
| 26. |
Duan B, Liu DS, Huang Y, et al. PI3-kinase/Akt pathway-regulated membrane insertion of acid-sensing ion channel 1a underlies BDNF-induced pain hypersensitivity. J Neurosci 2012; 32: 6351-63.
DOI PMID |
| 27. |
Khan N, Smith MT. Neurotrophins and neuropathic pain: role in pathobiology. Molecules 2015; 20: 10657-88.
DOI PMID |
| 28. |
Narita M, Yajima Y, Aoki T, et al. Up-regulation of the TrkB receptor in mice injured by the partial ligation of the sciatic nerve. Eur J Pharmacol 2000; 401: 187-90.
PMID |
| 29. |
Yajima Y, Narita M, Usui A, et al. Direct evidence for the involvement of brain-derived neurotrophic factor in the development of a neuropathic pain-like state in mice. J Neurochem 2005; 93: 584-94.
PMID |
| 30. |
Geng SJ, Liao FF, Dang WH, et al. Contribution of the spinal cord BDNF to the development of neuropathic pain by activation of the NR2B-containing NMDA receptors in rats with spinal nerve ligation. Exp Neurol 2010; 222: 256-66.
DOI URL |
| 31. | Marcos JL, Galleguillos D, Pelissier T, et al. Role of the spinal TrkB-NMDA receptor link in the BDNF-induced long-lasting mechanical hyperalgesia in the rat: a behavioural study. Eur J Pain 2017; 21: 1688-96. |
| 32. |
Choi JW, Kang SY, Choi JG, et al. Analgesic effect of electroacupuncture on paclitaxel-induced neuropathic pain via spinal opioidergic and adrenergic mechanisms in mice. Am J Chin Med 2015; 43: 57-70.
DOI URL |
| 33. |
Kim HW, Roh DH, Yoon SY, et al. The anti-inflammatory effects of low- and high-frequency electroacupuncture are mediated by peripheral opioids in a mouse air pouch inflammation model. J Altern Complement Med 2006; 12: 39-44.
DOI URL |
| 34. |
Kang DW, Choi JG, Kim J, Park JB, Lee JH, Kim HW. Bee venom reduces burn-induced pain via the suppression of peripheral and central substance P expression in mice. J Vet Sci 2021; 22: e9.
DOI URL |
| 35. |
Altar CA, DiStefano PS. Neurotrophin trafficking by anterograde transport. Trends Neurosci 1998; 21: 433-7.
PMID |
| 36. | Moy JK, Khoutorsky A, Asiedu MN, Dussor G, Price TJ. eIF4E phosphorylation influences BDNF mRNA translation in mouse dorsal root ganglion neurons. Front Cell Neurosci 2018; 12: 29. |
| 37. |
Argyriou AA, Koltzenburg M, Polychronopoulos P, Papapetropoulos S, Kalofonos HP. Peripheral nerve damage associated with administration of taxanes in patients with cancer. Crit Rev Oncol Hematol 2008; 66: 218-28.
DOI URL |
| 38. | Rowinsky EK, Chaudhry V, Cornblath DR, Donehower RC. Neurotoxicity of Taxol. J Natl Cancer Inst Monogr 1993; 15: 107-15. |
| 39. |
Persohn E, Canta A, Schoepfer S, et al. Morphological and morphometric analysis of paclitaxel and docetaxel-induced peripheral neuropathy in rats. Eur J Cancer 2005; 41: 1460-6.
DOI PMID |
| 40. |
Ji RR, Kohno T, Moore KA, Woolf CJ. Central sensitization and LTP: do pain and memory share similar mechanisms? Trends Neurosci 2003; 26: 696-705.
DOI URL |
| 41. |
Sandkuhler J. Models and mechanisms of hyperalgesia and allodynia. Physiol Rev 2009; 89: 707-58.
DOI PMID |
| 42. |
Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Science 2000; 288: 1765-9.
DOI PMID |
| 43. |
Merighi A, Salio C, Ghirri A, et al. BDNF as a pain modulator. Prog Neurobiol 2008; 85: 297-317.
DOI URL |
| 44. |
Garraway SM, Petruska JC, Mendell LM. BDNF sensitizes the response of lamina Ⅱ neurons to high threshold primary afferent inputs. Eur J Neurosci 2003; 18: 2467-76.
PMID |
| 45. |
Pezet S, Malcangio M, Lever IJ, et al. Noxious stimulation induces Trk receptor and downstream ERK phosphorylation in spinal dorsal horn. Mol Cell Neurosci 2002; 21: 684-95.
PMID |
| 46. |
Slack SE, Pezet S, McMahon SB, Thompson SW, Malcangio M. Brain-derived neurotrophic factor induces NMDA receptor subunit one phosphorylation via ERK and PKC in the rat spinal cord. Eur J Neurosci 2004; 20: 1769-78.
DOI URL |
| 47. |
Coutaux A. Non-pharmacological treatments for pain relief: TENS and acupuncture. Joint Bone Spine 2017; 84: 657-61.
DOI PMID |
| 48. | Walker JB, Katz RL. Non-opioid pathways suppress pain in humans. Pain 1981; 11: 347-54. |
| 49. | hung JM, Lee KH, Hori Y, Endo K, Willis WD. Factors influencing peripheral nerve stimulation produced inhibition of primate spinothalamic tract cells. Pain 1984; 19: 277-93. |
| [1] | HE Jiakai, ZHANG Jinling, WANG Yu, LI Shaoyuan, FANG Jiliang, ZHANG Shuai, ZHAO Yanan, ZHAI Weihang, GAO Deqiang, LI Ran, JIANG Yuhang, CHEN Zehao, JIA Baohui, RONG Peijing. Transcutaneous auricular vagus nerve stimulation would be an alternative to implantable cervical vagus nerve stimulation in some situation [J]. Journal of Traditional Chinese Medicine, 2023, 43(3): 627-630. |
| [2] | CHEN Limei, SUN Jifei, GUO Chunlei, LI Xiaojiao, WANG Zhi, Hong Yang, FANG Jiliang. Preliminary single-arm study of brain effects during transcutaneous auricular vagus nerve stimulation treatment of recurrent depression by resting-state functional magnetic resonance imaging [J]. Journal of Traditional Chinese Medicine, 2022, 42(5): 818-824. |
| [3] | WANG Yifei, YANG Yi, WANG Yu, ZHANG Jinling, ZHAI Weihang, LI Shaoyuan, WU Mozheng, HE Jianghong, RONG Peijing. Transcutaneous auricular vague nerve stimulation improved brain connection activity on patients of disorders of consciousness: a pilot study [J]. Journal of Traditional Chinese Medicine, 2022, 42(3): 463-471. |
| [4] | Wang Wei, Zhang Yixin, Yu Wentao, Gao Weijuan, Shen Ning, Jin Bing, Wang Xiangting, Fang Chaoyi, Wang Yanjun. Bushenhuoxue improves cognitive function and activates brain-derived neurotrophic factor-mediated signaling in a rat model of vascular dementia [J]. Journal of Traditional Chinese Medicine, 2020, 40(1): 49-58. |
| [5] | Li Yuan, Yang Mingxiao, Wu Fan, Cheng Ke, Chen Haiyong, Shen Xueyong, Lao Lixing. Mechanism of electroacupuncture on inflammatory pain: neural-immune-endocrine interactions [J]. Journal of Traditional Chinese Medicine, 2019, 39(05): 740-749. |
| [6] | Huang Wei, Yu Tingyu, Long Wenfei, Xiao Jianbin, Zhao Gaofeng. Effect of electrically stimulating acupoint, Zusanli(ST 36), on patient's recovery after laparoscopic colorectal cancer resection: a randomized controlled trial [J]. Journal of Traditional Chinese Medicine, 2019, 39(03): 433-439. |
| [7] | Ai Nogami-Hara, Kaori Kubota, Kotaro Takasaki, Takuya Watanabe, Nobuaki Egahira, Hikari Iba, Risako Fujikawa, Shutaro Katsurabayashi, Funda Bolukbasi Hatip, Izzettin Hatip-Al-Khatib, Katsunori Iwasaki. Extract of Yokukansan improves anxiety-like behavior and increases serum brain-derived neurotrophic factor in rats with cerebral ischemia combined with amyloid-β42 peptide [J]. Journal of Traditional Chinese Medicine, 2019, 39(01): 50-58. |
| [8] | Zhang Shiqiang, Wu Tingting, Zhang Haisheng, Yang Yun, Jiang Haiyan, Cao Shengcheng, Xie Fang, Xia Xiaoting, Lü Junqiang, Zhong Yi. Effect of electroacupuncture on chemotherapy-induced peripheral neuropathy in patients with malignant tumor:a single-blinded,randomized controlled trial [J]. Journal of Traditional Chinese Medicine, 2017, 37(02): 179-184. |
| [9] | Yeh Tsui-Yun, Lin Jen-Chien, Liu Chi-Feng. Effect of transcutaneous electrical nerve stimulation through acupoints of Pucan(BL 61) and Shenmai(BL 62) on intraocular pressure in patients with glaucoma: a randomized controlled trial [J]. Journal of Traditional Chinese Medicine, 2016, 36(01): 51-56. |
| [10] | Jiang Huili, Yu Xue, Ren Xiujun, Tu Ya. Electroacupuncture alters pain-related behaviors and expression of spinal prostaglandin E_2 in a rat model of neuropathic pain [J]. Journal of Traditional Chinese Medicine, 2016, 36(01): 85-91. |
| [11] | Liu Ye, Xu Mingjun, Che Xiangming, He Junqin, Guo Dandan, Zhao Guosheng, Zhang Guogang, Zhang Shuo, Kang Kai, Zhang Chunlei, Wang Yinan, Li Shan, Zhang Qinglin, Xu Li, Zhang Ming, Han Bin, Jing Yumiao, Zhang Ning. Effect of direct current pulse stimulating acupoints of JiaJi (T10-L3) and Ciliao (BL 32) with Han's Acupoint Nerve Stimulator on labour pain in women: a randomized controlled clinical study [J]. Journal of Traditional Chinese Medicine, 2015, 35(06): 620-625. |
| [12] | Wu Zhiyuan, Yang Ming, Jia Jie, Wu Yi, Huang Tiansheng, Li Mingfen, He Zhijie, Guo Zhenzhen, Leung Mason Chin Pang. Effect of transcutaneous electrical nerve stimulation at acupoints on patients with type 2 diabetes mellitus:a randomized controlled trial [J]. Journal of Traditional Chinese Medicine, 2015, 35(02): 134-140. |
| Viewed | ||||||||||||||||||||||||||||||||||||||||||||||||||
|
Full text 224
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||
|
Abstract 189
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||

