Journal of Traditional Chinese Medicine ›› 2025, Vol. 45 ›› Issue (4): 896-908.DOI: 10.19852/j.cnki.jtcm.2025.04.020
• Original Articles • Previous Articles Next Articles
Reversal of amino acid metabolism patterns between circulating blood and tumors as a new biomarker for the Zhengxu Xieshi syndrome in patients with esophageal squamous cell carcinoma
WANG Siliang1, MA Yushui1,2, LIU Lei3, WANG Pei4, WU Jia1,2, JIN Xing1,2, JIN Qiang1,2, WANG Congcong1,2, QIN Chentai1,2, ZHENG Miaomiao1,2, YANG Xi5, PAN Jun6, XU Hanchen2,7, DONG Changsheng1,2( ), CHEN Wenlian1,2(
), CHEN Wenlian1,2( )
)
- 1 Cancer Institute, Longhua Hospital Shanghai University of Traditional Chinese Medicine, Shanghai 200032, China
2 Shanghai Frontiers Science Center of Disease and Syndrome Biology of Inflammatory Cancer Transformation, Shanghai 200032, China
3 Department of Thoracic Surgery, the Affiliated Tumor Hospital of Nantong University, Nantong 226300, China
4 Traditional Chinese Medicine department, the Affiliated Tumor Hospital of Nantong University, Nantong 226300, China
5 Department of Oncology, Shanxi Provincial Hospital of Traditional Chinese Medicine, Shanxi 030001, China
6 Department of Medical Oncology, Jinling Hospital, Medical School of Nanjing University, Nanjing 210002, China
7 Institute of Digestive Diseases, Longhua Hospital, Shanghai University of Traditional Chinese Medicine, Shanghai 200032, China
-
Received:2024-04-12Accepted:2024-11-18Online:2025-07-25Published:2025-07-25 -
Contact:DONG Changsheng,CHEN Wenlian -
About author:CHEN Wenlian, Cancer Institute, Longhua Hospital Shanghai University of Traditional Chinese Medicine, Shanghai 200032, China and Shanghai Frontiers Science Center of Disease and Syndrome Biology of Inflammatory Cancer Transformation, Shanghai 200032, China. chenwl8412@shutcm.edu.cn,Telephone: +86-17756177005
DONG Changsheng, Cancer Institute, Longhua Hospital Shanghai University of Traditional Chinese Medicine, Shanghai 200032, China and Shanghai Frontiers Science Center of Disease and Syndrome Biology of Inflammatory Cancer Transformation, Shanghai 200032, China. csdong@shutcm.edu.cn;
-
Supported by:Natural Science Foundation-funded Project: Establishment of Syndrome-Disease Warning System and Investigation of the Clinical Features and Evolution Patterns of Traditional Chinese Medicine Syndromes in Esophageal Cancer under Modern Medical Diagnosis and Treatment(2023YFC3503200);Natural Science Foundation-funded Project: Establishment of Syndrome-Disease Warning System and Investigation of the Clinical Features and Evolution Patterns of Traditional Chinese Medicine Syndromes in Esophageal Cancer under Modern Medical Diagnosis and Treatment(2023YFC3503201);Biological Basis of the Pathogenesis of Cancer Toxin in Traditional Chinese Medicine(2022YFC3500200);Biological Basis of the Pathogenesis of Cancer Toxin in Traditional Chinese Medicine(2022YFC3500202);Natural Science Foundation-funded Project: Mechanistic Study of the Nucleolar Methyltransferase Fibrillarin to Promote Neoplastic Growth of Esophageal Squamous Cell Carcinoma via Activating Nucleoside Synthesis(32170778);Mechanistic Study of Renal Cancer Neoplastic Growth Driven by Glucose Transporter 5-Mediated Fructose Metabolism Reprogramming(31970708);Mechanistic Study of Methionine Cycle Modulating the Growth of Esophageal Squamous Cell Carcinoma through "DNA Methylation-DNA Stability-p53/p21-cell Cycle" Pathway(82002953);Antitumor Mechanism of Oleanolic Acid via Autophagy by Regulating Adenosine Monophosphate-Activated Protein Kinase-Mammalian Target of Rapamycin- Unc51 Like Kinase 1 Pathway and Inhibiting Purine Salvage Pathway(82004177);USP14 Cooperates with Ubiquitin C-Terminal Hydrolase L5 to Regulate Constitutive Photomorphogenic 9 Signalosome-mediated Programmed Death-Ligand 1 Deubiquitination and Promote Immune Evasion in Hepatocellular Carcinoma(81972214);National Scientific and Technological Major Special Project of China: Shuang Huang Sheng Bai Oral Liquid: Efficacy and Safety Evaluation of Traditional Chinese Medicine Compound Based on Systems Biology Approaches(2019ZX09201004-002-013);A special clinical research initiative for the health business sponsored by the Shanghai Municipal Health Commission(202040155);Shanghai Municipal Science and Technology Commissions Special Biomedical Technology Support Plan(20S31904100);Shanghai "Science and Technology Innovation Action Plan" Medical Innovation Research Project--Shanghai Clinical Research Center of Traditional Chinese Medicine Oncology(21MC1930500);Shanghai 13th Five-Year Plan Key Specialty of Traditional Chinese Medicine Oncology(shslczdzk03701);Tracking Programme for Eastern Scholar at Shanghai Institutions of Higher Learning, Shanghai High-level Talent Leadership Programme of Traditional Chinese Medicine(ZY(2021-2023)-0403);Scientific Research Project of Industry Development Center of Shanghai University of Traditional Chinese Medicine(602076D)
Cite this article
WANG Siliang, MA Yushui, LIU Lei, WANG Pei, WU Jia, JIN Xing, JIN Qiang, WANG Congcong, QIN Chentai, ZHENG Miaomiao, YANG Xi, PAN Jun, XU Hanchen, DONG Changsheng, CHEN Wenlian. Reversal of amino acid metabolism patterns between circulating blood and tumors as a new biomarker for the Zhengxu Xieshi syndrome in patients with esophageal squamous cell carcinoma[J]. Journal of Traditional Chinese Medicine, 2025, 45(4): 896-908.
share this article
| Variable | Study 1 | Study 2 | ||||
|---|---|---|---|---|---|---|
| HC (n = 34) | ESCC (n = 34) | P value | ESCC (n = 24) | |||
| Age (years) | Median | 67 | 67 | 0.745 | 68 | |
| Range | 56-78 | 54-77 | 50-76 | |||
| Gender [n (%)] | Male | 21 (61.76) | 26 (76.47) | 0.291 | 15 (62.50) | |
| Female | 13 (38.24) | 8 (23.53) | 9 (37.50) | |||
| BMI | Median | 22.18 | 22.01 | |||
| Range | 18.37-30.37 | 18.37-28.31 | ||||
| Hypertension [n (%)] | Yes | 6 (17.65) | 4 (16.67) | |||
| No | 28 (82.35) | 19 (79.17) | ||||
| Not available | 0 | 1 (4.17) | ||||
| Diabetes [n (%)] | Yes | 0 | 2 (8.33) | |||
| No | 34 (100) | 21 (87.50) | ||||
| Not available | 0 | 1 (4.17) | ||||
| Alcohol use [n (%)] | Never a drinker | 29 (85.29) | 1 (4.17) | |||
| Drinker | 5 (14.71) | 22 (91.67) | ||||
| Not available | 0 | 1 (4.17) | ||||
| Cigarette use [n (%)] | Never a smoker | 20 (58.82) | 4 (16.67) | |||
| Smoker | 14 (41.18) | 19 (79.17) | ||||
| Not available | 0 | 1 (4.17) | ||||
| WBC (109/L) | Median | 5.8 | 5.4 | |||
| Range | 3.4-11.6 | 2.2-8.4 | ||||
| RBC (1012/L) | Median | 4.63 | 4.39 | |||
| Range | 3.59-5.59 | 3.41-5.32 | ||||
| TNM stage [n (%)] | Ⅰ | 9 (26.47) | 1 (4.17) | |||
| Ⅱ | 18 (52.94) | 14 (58.33) | ||||
| III | 6 (17.65) | 7 (29.17) | ||||
| Ⅳ | 1 (2.94) | 2 (8.33) | ||||
| Metastasis [n (%)] | Yes | 12 (35.29) | 8 (33.33) | |||
| No | 22 (64.71) | 16 (66.67) | ||||
Table 1 Basic characteristics of enrolled human subjects
| Variable | Study 1 | Study 2 | ||||
|---|---|---|---|---|---|---|
| HC (n = 34) | ESCC (n = 34) | P value | ESCC (n = 24) | |||
| Age (years) | Median | 67 | 67 | 0.745 | 68 | |
| Range | 56-78 | 54-77 | 50-76 | |||
| Gender [n (%)] | Male | 21 (61.76) | 26 (76.47) | 0.291 | 15 (62.50) | |
| Female | 13 (38.24) | 8 (23.53) | 9 (37.50) | |||
| BMI | Median | 22.18 | 22.01 | |||
| Range | 18.37-30.37 | 18.37-28.31 | ||||
| Hypertension [n (%)] | Yes | 6 (17.65) | 4 (16.67) | |||
| No | 28 (82.35) | 19 (79.17) | ||||
| Not available | 0 | 1 (4.17) | ||||
| Diabetes [n (%)] | Yes | 0 | 2 (8.33) | |||
| No | 34 (100) | 21 (87.50) | ||||
| Not available | 0 | 1 (4.17) | ||||
| Alcohol use [n (%)] | Never a drinker | 29 (85.29) | 1 (4.17) | |||
| Drinker | 5 (14.71) | 22 (91.67) | ||||
| Not available | 0 | 1 (4.17) | ||||
| Cigarette use [n (%)] | Never a smoker | 20 (58.82) | 4 (16.67) | |||
| Smoker | 14 (41.18) | 19 (79.17) | ||||
| Not available | 0 | 1 (4.17) | ||||
| WBC (109/L) | Median | 5.8 | 5.4 | |||
| Range | 3.4-11.6 | 2.2-8.4 | ||||
| RBC (1012/L) | Median | 4.63 | 4.39 | |||
| Range | 3.59-5.59 | 3.41-5.32 | ||||
| TNM stage [n (%)] | Ⅰ | 9 (26.47) | 1 (4.17) | |||
| Ⅱ | 18 (52.94) | 14 (58.33) | ||||
| III | 6 (17.65) | 7 (29.17) | ||||
| Ⅳ | 1 (2.94) | 2 (8.33) | ||||
| Metastasis [n (%)] | Yes | 12 (35.29) | 8 (33.33) | |||
| No | 22 (64.71) | 16 (66.67) | ||||
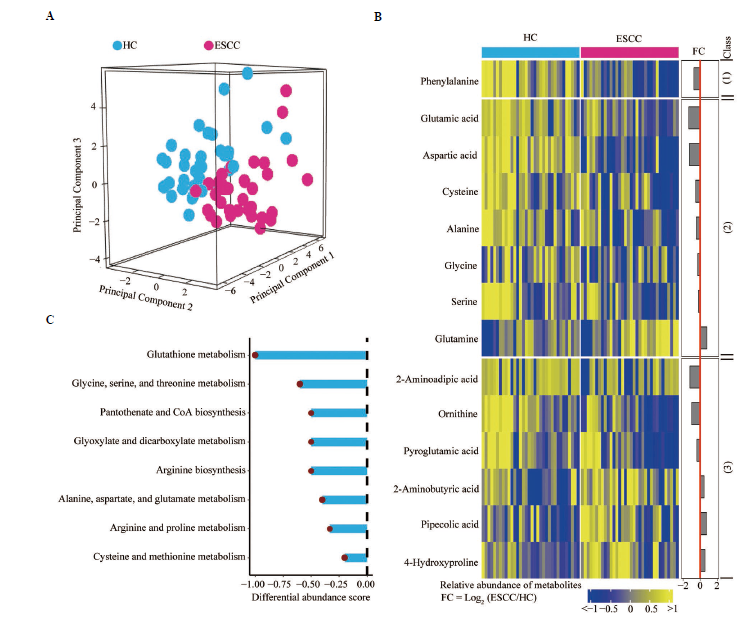
Figure 1 Repressed amino acid metabolism in patient sera A: comparative analysis of amino acid metabolic features in the sera between ESCC and HCs; B: heatmap showing the differentially expressed amino acids in the sera of patients with ESCC compared to those of HCs. (1): essential amino acids; (2): non-essential amino acids; (3): non-canonical amino acids. C: KEGG pathway-based differential abundance analysis revealing differences in amino acid metabolic pathways in the sera of patients relative to those of HCs. HC: healthy control; ESCC: esophageal squamous cell carcinoma; FC: foldchange; KEGG: kyoto encyclopedia of genes and genomes.
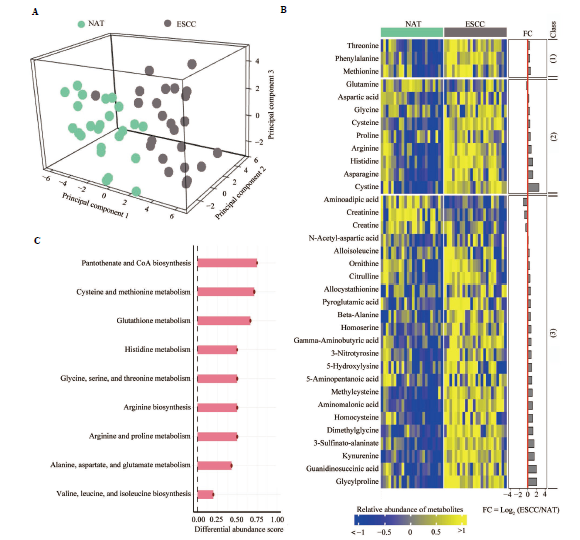
Figure 2 Heightened amino acid metabolism in clinical ESCC tissues A: comparative assessment of amino acid metabolic features of clinical ESCC tissues and matched NATs from patients; B: heatmap presenting differentially expressed amino acids in clinical ESCC tissues and matched NATs from patients. (1): essential amino acids; (2): non-essential amino acids; (3): non-canonical amino acids; C: KEGG pathway-based differential abundance analysis depicting alterations in amino acid metabolic pathways in clinical ESCC tissues compared to those in matched NATs. NATs: paired normal adjacent tissues; ESCC: esophageal squamous cell carcinoma; KEGG: kyoto encyclopedia of genes and genomes dataset.
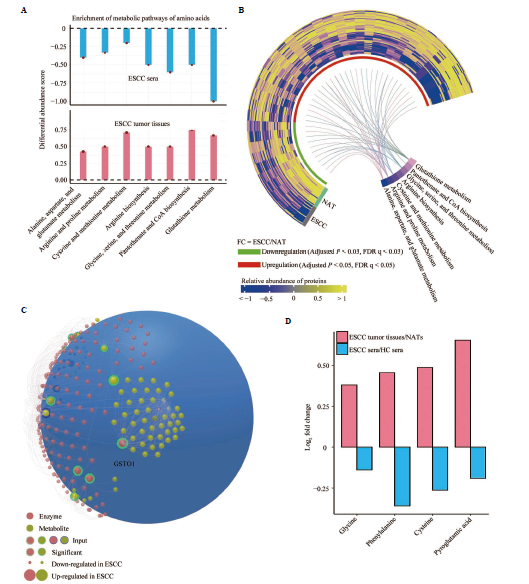
Figure 3 Amino acid metabolic pathways and biomarkers with opposite alterations between patient sera and clinical ESCC tissues A: depiction of seven amino acid metabolic pathways exhibiting opposite changes in patient sera and tumor tissues; B: heatmap illustrating expression shifts in 40 metabolic enzymes involved in the amino acid pathways shown in A, in clinical ESCC tissues and paired NATs in patients; C: comprehensive analysis of metabolic enzyme and amino acid alterations in the top three pathways from A: glutathione metabolism, pantothenate and CoA biosynthesis, and glycine/serine/threonine metabolism; D: identification of four amino acid metabolites with converse alterations in tumor tissues and sera of patients with ESCC. NATs: paired normal adjacent tissues; ESCC: esophageal squamous cell carcinoma; CoA: coenzyme A.
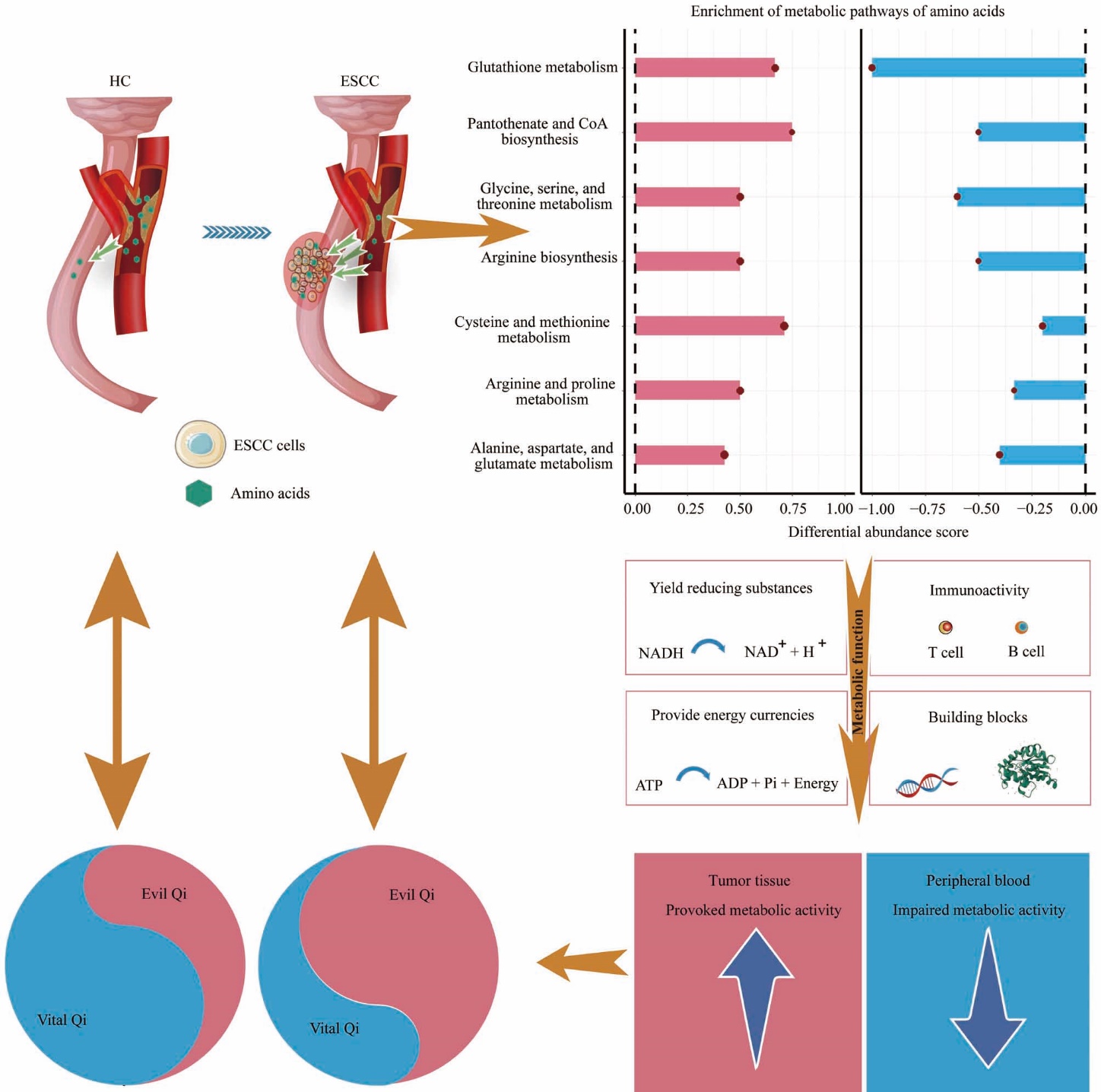
Figure 4 Interpreting ZXXS syndrome via amino acid metabolic reprogramming The interpretation of ZXXS syndrome in patients diagnosed with ESCC is underpinned by the profound perturbations observed across seven amino acid metabolic pathways. These pathways, which are implicated in immune modulation, generation of reducing agents, energy currency supply, and building block synthesis, are substantially dysregulated in both circulating blood and regional neoplastic tissues in patients with ESCC. Within patients, attenuated activity of these pathways in sera signifies compromised vital Qi, whereas enhanced activity in ESCC tissues indicates locally heightened evil Qi. Thus, this study delineates opposing amino acid metabolic changes between the systemic circulation and localized tumor tissues, offering insights into the biological hallmarks of ZXXS syndrome in patients with ESCC. NATs: paired normal adjacent tissues; ESCC: esophageal squamous cell carcinoma; ZXXS: Zhengxu Xieshi.
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Jemal ISA, Bray F. Global Cancer Statistics 2020: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021; 71: 209-49. |
| 2. |
Liang H, Fan JH, Qiao YL. Epidemiology, etiology, and prevention of esophageal squamous cell carcinoma in China. Cancer Biol Med 2017; 14: 33-41.
DOI PMID |
| 3. |
Lagergren J, Smyth E, Cunningham D, Lagergren P. Oesophageal cancer. Lancet 2017; 390: 2383-96.
DOI PMID |
| 4. | Wang ZX, Cui C, Yao J, et al. Toripalimab plus chemotherapy in treatment-naive, advanced esophageal squamous cell carcinoma (JUPITER-06): a multi-center phase 3 trial. Cancer Cell 2022; 40: 277-88. |
| 5. | Wu HX, Pan YQ, He Y, Wang ZX, Guan WL, Chen YX, et al. Clinical benefit of first-line programmed death-1 antibody plus chemotherapy in low programmed cell death ligand 1-expressing esophageal squamous cell carcinoma: a post hoc analysis of jupiter-06 and Meta-analysis. J Clin Oncol 2022: JCO2201490. |
| 6. | Tang JL, Liu BY, Ma KW. Traditional Chinese Medicine. Lancet 2008; 372: 1938-40. |
| 7. | Liu S, Zhu JJ, Li JC. The interpretation of human body in Traditional Chinese Medicine and its influence on the characteristics of TCM theory. Anat Rec (Hoboken) 2021; 304: 2559-65. |
| 8. |
Ji Q, Luo YQ, Wang WH, Liu X, Li Q, Su SB. Research advances in Traditional Chinese Medicine syndromes in cancer patients. J Integr Med 2016; 14: 12-21.
DOI PMID |
| 9. |
Bower H, Bjorkholm M, Dickman PW, Hoglund M, Lambert PC, Andersson TM. Life expectancy of patients with chronic myeloid leukemia approaches the life expectancy of the general population. J Clin Oncol 2016; 34: 2851-7.
DOI PMID |
| 10. |
Hu J, Liu YF, Wu CF, et al. Long-term efficacy and safety of all-trans retinoic acid/arsenic trioxide-based therapy in newly diagnosed acute promyelocytic leukemia. Proc Natl Acad Sci USA 2009; 106: 3342-7.
DOI PMID |
| 11. |
Liu J, Wang S, Zhang Y, Fan HT, Lin HS. Traditional Chinese medicine and cancer: history, present situation, and development. Thorac Cancer 2015; 6: 561-9.
DOI PMID |
| 12. | Cao L, Wang X, Zhu G, et al. Traditional Chinese Medicine therapy for esophageal cancer: a literature review. Integr Cancer Ther 2021; 20: 1-11. |
| 13. | Lopez-Otin C, Kroemer G. Hallmarks of health. Cell 2021; 184: 33-63. |
| 14. | Chen WL, Wang JH, Zhao AH, et al. A distinct glucose metabolism signature of acute myeloid leukemia with prognostic value. Blood 2014; 124: 1645-54. |
| 15. | Chen T, Xie G, Wang X, et al. Serum and urine metabolite profiling reveals potential biomarkers of human hepatocellular carcinoma. Mol Cell Proteomics 2011; 10: M110.004945. |
| 16. | Liu L, Wu J, Shi M, et al. New metabolic alterations and a predictive marker pipecolic acid in sera for esophageal squamous cell carcinoma. Genomics Proteomics Bioinformatics 2022; 20: 670-87. |
| 17. |
Schild T, Low V, Blenis J, Gomes AP. Unique metabolic adaptations dictate distal organ-specific metastatic colonization. Cancer cell 2018; 33: 347-54.
DOI PMID |
| 18. |
Brand A, Singer K, Koehl GE, et al. LDHA-associated lactic acid production blunts tumor immunosurveillance by T and NK cells. Cell Metab 2016; 24: 657-71.
DOI PMID |
| 19. |
Vander Heiden MG, DeBerardinis RJ. Understanding the Intersections between metabolism and cancer biology. Cell 2017; 168: 657-69.
DOI PMID |
| 20. | Chen WL, Jin X, Wang M, et al. GLUT5-mediated fructose utilization drives lung cancer growth by stimulating fatty acid synthesis and AMPK/mTORC 1 signaling. JCI Insight 2020; 5: e131596. |
| 21. |
Jin X, Liang Y, Liu D, et al. An essential role for GLUT5-mediated fructose utilization in exacerbating the malignancy of clear cell renal cell carcinoma. Cell Biol Toxicol 2019; 35: 471-83.
DOI PMID |
| 22. | Jin X, Liu L, Wu J, et al. A multi-omics study delineates new molecular features and therapeutic targets for esophageal squamous cell carcinoma. Clin Transl Med 2021; 11: e538. |
| 23. | Amin MB, Greene FL, Edge SB, et al. The eighth edition AJCC cancer staging manual: continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. CA Cancer J Clin 2017; 67: 93-9. |
| 24. | National Health Commission of The People's Republic of China. Chinese guidelines for diagnosis and treatment of esophageal carcinoma 2018 (English version). Chin J Cancer Res 2019; 31: 223-58. |
| 25. |
Gu Z, Eils R, Schlesner M. Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics 2016; 32: 2847-9.
DOI PMID |
| 26. |
Gu Z, Gu L, Eils R, Schlesner M, Brors B. Circlize implements and enhances circular visualization in R. Bioinformatics 2014; 30: 2811-2.
DOI PMID |
| 27. | Rohart F, Gautier B, Singh A, Lê Cao KA. MixOmics: an R package for 'omics feature selection and multiple data integration. PLoS Comput Biol 2017; 13: e1005752. |
| 28. |
Hakimi AA, Reznik E, Lee CH, et al. An integrated metabolic atlas of clear cell renal cell carcinoma. Cancer Cell 2016; 29: 104-16.
DOI PMID |
| 29. | Zhou G, Pang Z, Lu Y, Ewald J, Xia J. OmicsNet 2.0: a web-based platform for multi-omics integration and network visual analytics. Nucleic Acids Res 2022; 50: 527-33. |
| 30. | Wen Y, Chen H, Zhang L, et al. Glycyrrhetinic acid induces oxidative/nitrative stress and drives ferroptosis through activating NADPH oxidases and iNOS, and depriving glutathione in triple-negative breast cancer cells. Free Radic Biol Med 2021; 173: 41-51. |
| 31. | Qian CJ, Tong YY, Wang YC, Teng XS, Yao J. Circ_ 0001093 promotes glutamine metabolism and cancer progression of esophageal squamous cell carcinoma by targeting miR-579-3p/glutaminase axis. J Bioenerg Biomembr 2022; 54: 119-34. |
| 32. | Liang Z, Zhao B, Hou J, Zheng J, Xin G. CircRNA circ-OGDH (hsa_circ_0003340) acts as a cerna to regulate glutamine metabolism and esophageal squamous cell carcinoma progression by the mir-615-5p/pdx1 axis. Cancer Manag Res 2021; 13: 3041-53. |
| 33. |
Bansal A, Simon MC. Glutathione metabolism in cancer progression and treatment resistance. J Cell Biol 2018; 217: 2291-8.
DOI PMID |
| 34. |
Nunes SC, Ramos C, Lopes-Coelho F, et al. Cysteine allows ovarian cancer cells to adapt to hypoxia and to escape from carboplatin cytotoxicity. Sci Rep 2018; 8: 9513.
DOI PMID |
| 35. | Bonifácio VDB, Pereira SA, Serpa J, Vicente JB. Cysteine metabolic circuitries: druggable targets in cancer. Br J Cancer 2021; 124: 862-79. |
| 36. | Li AM, Ye J. Reprogramming of serine, glycine and one-carbon metabolism in cancer. Biochim Biophys Acta Mol Basis Dis 2020; 1866: 165841. |
| 37. | Dobrzyn P. CoA in Health and Disease. Int J Mol Sci 2022; 23: 4317. |
| 38. |
Lind DS. Arginine and cancer. J Nutr 2004; 134: 2837S-41S.
DOI PMID |
| 39. |
Dolce V, Cappello AR, Capobianco L. Mitochondrial tricarboxylate and dicarboxylate-tricarboxylate carriers: from animals to plants. IUBMB Life 2014; 66: 462-71.
DOI PMID |
| 40. |
Guertin DA, Wellen KE. Acetyl-CoA metabolism in cancer. Nat Rev Cancer 2023; 23: 156-72.
DOI PMID |
| 41. |
Hirschhaeuser F, Sattler UG, Mueller-Klieser W. Lactate: a metabolic key player in cancer. Cancer Res 2011; 71: 6921-5.
DOI PMID |
| 42. | Knott SRV, Wagenblast E, Khan S, et al. Asparagine bioavailability governs metastasis in a model of breast cancer. Nature 2018; 554: 378-81. |
| 43. |
Brosnan ME, Brosnan JT. Histidine metabolism and function. J Nutr 2020; 150: 2570s-5s.
DOI PMID |
| 44. | Peng H, Wang Y, Luo W. Multifaceted role of branched-chain amino acid metabolism in cancer. Oncogene 2020; 39: 6747-56. |
| 45. | Xutian S, Cao D, Wozniak J, Junion J, Boisvert J. Comprehension of the unique characteristics of Traditional Chinese Medicine. Am J Chin Med 2012; 40: 231-44. |
| 46. |
Hu X, Guo F. Amino acid sensing in metabolic homeostasis and health. Endocr Rev 2021; 42: 56-76.
DOI PMID |
| 47. |
Harris IS, Treloar AE, Inoue S, et al. Glutathione and thioredoxin antioxidant pathways synergize to drive cancer initiation and progression. Cancer Cell 2015; 27: 211-22.
DOI PMID |
| 48. |
Wang W, Wu Z, Dai Z, Yang Y, Wang J, Wu G. Glycine metabolism in animals and humans: implications for nutrition and health. Amino Acids 2013; 45: 463-77.
DOI PMID |
| 49. | Naquet P, Kerr EW, Vickers SD, Leonardi R. Regulation of coenzyme a levels by degradation: the 'Ins and Outs'. Prog Lipid Res 2020; 78: 101028. |
| 50. |
Sun C, Wang A, Zhou Y, et al. Spatially resolved multi-omics highlights cell-specific metabolic remodeling and interactions in gastric cancer. Nat Commun 2023; 14: 2692.
DOI PMID |
| 51. |
Liu D, Huang J, Gao S, Jin H, He J. A temporo-spatial pharmacometabolomics method to characterize pharmacokinetics and pharmacodynamics in the brain microregions by using ambient mass spectrometry imaging. Acta Pharm Sin B 2022; 12: 3341-53.
DOI PMID |
| 52. |
Morris SM Jr. Arginine metabolism revisited. J Nutr 2016; 146: 2579S-86S.
PMID |
| 53. |
Eagle H, Barban S, Levy M, Schulze HO. The utilization of carbohydrates by human cell cultures. J Biol Chem 1958; 233: 551-8.
PMID |
| 54. |
Djukic T, Simic T, Pljesa-Ercegovac M, et al. Upregulated glutathione transferase omega-1 correlates with progression of urinary bladder carcinoma. Redox Rep 2017; 22: 486-92.
DOI PMID |
| 55. |
Manupati K, Debnath S, Goswami K, et al. Glutathione S-transferase omega 1 inhibition activates JNK-mediated apoptotic response in breast cancer stem cells. FEBS J 2019; 286: 2167-92.
DOI PMID |
| 56. | Wang K, Zhang FL, Jia W. Glutathione S-transferase omega 1 promotes the proliferation, migration and invasion, and inhibits the apoptosis of non-small cell lung cancer cells, via the JAK/STAT3 signaling pathway. Mol Med Rep 2021; 23:71. |
| 57. |
Locasale JW, Grassian AR, Melman T, et al. Phosphoglycerate dehydrogenase diverts glycolytic flux and contributes to oncogenesis. Nat Genet 2011; 43: 869-74.
DOI PMID |
| 58. | Pataskar A, Champagne J, Nagel R, et al. Tryptophan depletion results in tryptophan-to-phenylalanine substitutants. Nature 2022; 603: 721-7. |
| 59. |
Liu X, Olszewski K, Zhang Y, et al. Cystine transporter regulation of pentose phosphate pathway dependency and disulfide stress exposes a targetable metabolic vulnerability in cancer. Nat Cell Biol 2020; 22: 476-86.
DOI PMID |
| 60. |
Gueta I, Perach Ovadia Y, Markovits N, Schacham YN, Epsztein A, Loebstein R. Is pyroglutamic acid a prognostic factor among patients with suspected infection? A prospective cohort study. Sci Rep 2020; 10: 10128.
DOI PMID |
| 61. |
Sanderson SM, Gao X, Dai Z, Locasale JW. Methionine metabolism in health and cancer: a nexus of diet and precision medicine. Nat Rev Cancer 2019; 19: 625-37.
DOI PMID |
| 62. |
Fernstrom JD, Fernstrom MH. Tyrosine, phenylalanine, and catecholamine synthesis and function in the brain. J Nutr 2007; 137: 1539S-47 S; discussion 48S.
DOI PMID |
| 63. |
Hoy AJ, Nagarajan SR, Butler LM. Tumour fatty acid metabolism in the context of therapy resistance and obesity. Nat Rev Cancer 2021; 21: 753-66.
DOI PMID |
| 64. | Chen WL, Wang YY, Zhao A, et al. Enhanced fructose utilization mediated by SLC2A5 is a unique metabolic feature of acute myeloid leukemia with therapeutic potential. Cancer Cell 2016; 30: 779-91. |
| 65. |
Goncalves MD, Lu C, Tutnauer J, et al. High-fructose corn syrup enhances intestinal tumor growth in mice. Science 2019; 363: 1345-9.
DOI PMID |
| 66. | Hwang JJ, Jiang L, Hamza M, et al. The human brain produces fructose from glucose. JCI Insight 2017; 2: e90508. |
| 67. |
Lane AN, Fan TW. Regulation of mammalian nucleotide metabolism and biosynthesis. Nucleic Acids Res 2015; 43: 2466-85.
DOI PMID |
| No related articles found! |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||

