Journal of Traditional Chinese Medicine ›› 2025, Vol. 45 ›› Issue (3): 676-684.DOI: 10.19852/j.cnki.jtcm.2025.03.002
Previous Articles Next Articles
A prognostic model of immunoglobulin A nephropathy using artificial neural network: a retrospective study based on integrated Chinese and Western Medicine
CHEN Hongyu, ZHENG Xinyi, WANG Zeng, DING Xiaoyu, XU Luhuan, ZHU Qin( )
)
- Department of Nephrology (Key Laboratory of Zhejiang Province, Management of Kidney Disease), Hangzhou TCM Hopspital Affiliated to Zhejiang Chinese Medical University, Hangzhou 310007, China
-
Received:2024-01-12Accepted:2024-05-15Online:2025-06-15Published:2025-05-21 -
Contact:ZHU Qin, Department of Nephrology (Key Laboratory of Zhejiang Province, Management of Kidney Disease), Hangzhou TCM Hopspital Affiliated to Zhejiang Chinese Medical University, Hangzhou 310007, China. zhuqinfeifei@163.com,Telephone: +86-571-85827631
-
Supported by:Natural Science Foundation-funded Project: Study on the Mechanism of Compound Centella Asiatica Mediate 24‐dehydrocholesterol Reductase/Liver X Receptors (DHCR24/LXR) Signaling Axis to Regulate Macrophage Activation and Alleviate Microinflammation in Diabetic Kidney Disease(82205008);Medical Scientific Research Foundation of Zhejiang Province: Study on the Mechanism of Asiaticoside Mediate DHCR24/LXR Signaling Axis to Regulate Macrophage Innate Immune Response in Diabetic Kidney Disease(2023RC242);Zhejiang Traditional Medicine and Technology Program: Chen Hongyu's Academic Thought and Clinical Experience in the Diagnosis and Treatment of Diabetic Nephropathy by Knowledge Map(2023ZF137);Health Commission of Hangzhou city: Study on Prognosis Model of IgA Nephropathy Combined with Chinese and Western Medicine based on Artificial Neural Network(A20210083);Zhejiang Chinese Medical University Research Foundation: Study on Wang Yongjun's Clinical Decision Model for Diagnosis and Treatment of Diabetic Nephropathy based on Graph Convolutional Neural Network(2022FSYYZZ14)
Cite this article
CHEN Hongyu, ZHENG Xinyi, WANG Zeng, DING Xiaoyu, XU Luhuan, ZHU Qin. A prognostic model of immunoglobulin A nephropathy using artificial neural network: a retrospective study based on integrated Chinese and Western Medicine[J]. Journal of Traditional Chinese Medicine, 2025, 45(3): 676-684.
share this article
| Variable | Study cohorts (n = 735) a | External validation data (n = 105) | |
|---|---|---|---|
| Age (years) | 35 (27-44) | 36 (16-67) | |
| Male [n (%)] | 297 (40.4) | 42 (40) | |
| SBP (mm Hg) | 120 (110-132) | 124 (92-172) | |
| DBP (mm Hg) | 76 (68-84) | 77 (50-106) | |
| BMI (kg/m2) | 23 (20-25) | 22.9 (20-25) | |
| Hb (g/L) | 126 (116-139.5) | 119.8 (74-163) | |
| UA (μmol/L) | 332±97 | 319±87 | |
| TC (mmol/L) | 4.78 (4.16-5.51) | 4.58 (2.35-14.63) | |
| TG (mmol/L) | 1.29 (0.92-1.93) | 1.49 (0.35-10.67) | |
| Alb (g/L) | 40.6 (37.8-43) | 37.62 (23.5-46) | |
| LDL (mmol/L) | 2.7 (2.19-3.33) | 2.75 (0.87-9.86) | |
| Urinary protein (g/24 h) | 0.55 (0.29-1.13) | 1.24 (0.12-7.97) | |
| Microscopic hematuria [n (%)] | - | 378 (51.4) | 37 (35.2) |
| + | 178 (24.2) | 18 (17.1) | |
| ++ | 118 (16.1) | 22 (21) | |
| +++ | 57 (7.8) | 26 (24.8) | |
| ++++ | 4 (0.5) | 2 (1.9) | |
| Scr (μmol/L) | 76 (59-97.5) | 90.91 (39-309) | |
| eGFR (mL/min per 1.73 m2) | 90±34 | 85±33 | |
| IgG (mg/dL) | 1050 (854-1220) | 1053.3 (11.2-2610) | |
| IgA (mg/dL) | 285 (218-358) | 301.9 (3.48-792) | |
| IgM (mg/dL) | 111 (75-148.5) | 111 (0.17-517) | |
| C3 (mg/dL) | 94 (83.5-108.0) | 97.6 (57-168) | |
| C4 (mg/dL) | 22 (18-27) | 23 (6-46) | |
| Renal pathologyb | |||
| M [n (%)] | M0 | 0 (0) | 0 (0) |
| M1 | 735 (100.0) | 105 (100.0) | |
| E [n (%)] | E0 | 616 (83.8) | 79 (75.2) |
| E1 | 119 (16.2) | 26 (24.8) | |
| S [n (%)] | S0 | 145 (19.7) | 16 (15.2) |
| S1 | 590 (80.3) | 89 (84.8) | |
| T [n (%)] | T0 | 475 (64.6) | 72 (68.6) |
| T1 | 215 (29.3) | 25 (23.8) | |
| T2 | 45 (6.1) | 8 (7.6) | |
| C [n (%)] | C0 | 306 (41.6) | 33 (31.4) |
| C1 | 361 (49.1) | 65 (61.9) | |
| C2 | 68 (9.3) | 7 (6.7) | |
| Treatment protocol [n (%)] | RAS blocker | 449 (61.1) | 92 (87.6) |
| Glucocorticoid | 419 (57.0) | 88 (83.8) | |
| Immunosuppressant | 524 (71.3) | 80 (76.2) | |
| New five-type syndrome differentiation [n (%)] | Kidney deficiency | 511 (69.5) | 48 (45.7) |
| Wind-dampness | 209 (28.4) | 50 (47.6) | |
| Stasis | 357 (48.6) | 78 (74.3) | |
| Liver-wind | 132 (18.0) | 14 (13.3) | |
| Uremic | 28 (3.8) | 5 (4.8) | |
Table 1 Baseline data of participants
| Variable | Study cohorts (n = 735) a | External validation data (n = 105) | |
|---|---|---|---|
| Age (years) | 35 (27-44) | 36 (16-67) | |
| Male [n (%)] | 297 (40.4) | 42 (40) | |
| SBP (mm Hg) | 120 (110-132) | 124 (92-172) | |
| DBP (mm Hg) | 76 (68-84) | 77 (50-106) | |
| BMI (kg/m2) | 23 (20-25) | 22.9 (20-25) | |
| Hb (g/L) | 126 (116-139.5) | 119.8 (74-163) | |
| UA (μmol/L) | 332±97 | 319±87 | |
| TC (mmol/L) | 4.78 (4.16-5.51) | 4.58 (2.35-14.63) | |
| TG (mmol/L) | 1.29 (0.92-1.93) | 1.49 (0.35-10.67) | |
| Alb (g/L) | 40.6 (37.8-43) | 37.62 (23.5-46) | |
| LDL (mmol/L) | 2.7 (2.19-3.33) | 2.75 (0.87-9.86) | |
| Urinary protein (g/24 h) | 0.55 (0.29-1.13) | 1.24 (0.12-7.97) | |
| Microscopic hematuria [n (%)] | - | 378 (51.4) | 37 (35.2) |
| + | 178 (24.2) | 18 (17.1) | |
| ++ | 118 (16.1) | 22 (21) | |
| +++ | 57 (7.8) | 26 (24.8) | |
| ++++ | 4 (0.5) | 2 (1.9) | |
| Scr (μmol/L) | 76 (59-97.5) | 90.91 (39-309) | |
| eGFR (mL/min per 1.73 m2) | 90±34 | 85±33 | |
| IgG (mg/dL) | 1050 (854-1220) | 1053.3 (11.2-2610) | |
| IgA (mg/dL) | 285 (218-358) | 301.9 (3.48-792) | |
| IgM (mg/dL) | 111 (75-148.5) | 111 (0.17-517) | |
| C3 (mg/dL) | 94 (83.5-108.0) | 97.6 (57-168) | |
| C4 (mg/dL) | 22 (18-27) | 23 (6-46) | |
| Renal pathologyb | |||
| M [n (%)] | M0 | 0 (0) | 0 (0) |
| M1 | 735 (100.0) | 105 (100.0) | |
| E [n (%)] | E0 | 616 (83.8) | 79 (75.2) |
| E1 | 119 (16.2) | 26 (24.8) | |
| S [n (%)] | S0 | 145 (19.7) | 16 (15.2) |
| S1 | 590 (80.3) | 89 (84.8) | |
| T [n (%)] | T0 | 475 (64.6) | 72 (68.6) |
| T1 | 215 (29.3) | 25 (23.8) | |
| T2 | 45 (6.1) | 8 (7.6) | |
| C [n (%)] | C0 | 306 (41.6) | 33 (31.4) |
| C1 | 361 (49.1) | 65 (61.9) | |
| C2 | 68 (9.3) | 7 (6.7) | |
| Treatment protocol [n (%)] | RAS blocker | 449 (61.1) | 92 (87.6) |
| Glucocorticoid | 419 (57.0) | 88 (83.8) | |
| Immunosuppressant | 524 (71.3) | 80 (76.2) | |
| New five-type syndrome differentiation [n (%)] | Kidney deficiency | 511 (69.5) | 48 (45.7) |
| Wind-dampness | 209 (28.4) | 50 (47.6) | |
| Stasis | 357 (48.6) | 78 (74.3) | |
| Liver-wind | 132 (18.0) | 14 (13.3) | |
| Uremic | 28 (3.8) | 5 (4.8) | |

Figure 2 Results of K-M survival curve of laboratory variables that were statistically different (P < 0.05) A: sex (female = 0, male = 1); B: anemia (without anemia = 0, anemia = 1); C: hyperuricemia (non-hyperuricemia = 0, hyperuricemia = 1); D: hypercholesterolemia (non-hypercholesterolemia = 0, hypercholesterolemia = 1); E: high-LDL (non-high-density lipoprotein = 0, high-density lipoprotein = 1); F: 24-h urinary protein quantitation (24-h urinary protein quantitation ≤ 1 g = 0, 24-h urinary protein quantitation > 1 g = 1); G: Scr (normal range Scr = 0, elevated Scr = 1); H: eGFR (eGFR ≥ 90 mL/min per 1.73 m2 = 0, eGFR < 90 mL/min per 1.73 m2 = 1). K-M: Kaplan-Meier; LDL: low density lipoprotein; eGFR: estimated glomerular filtration rate.
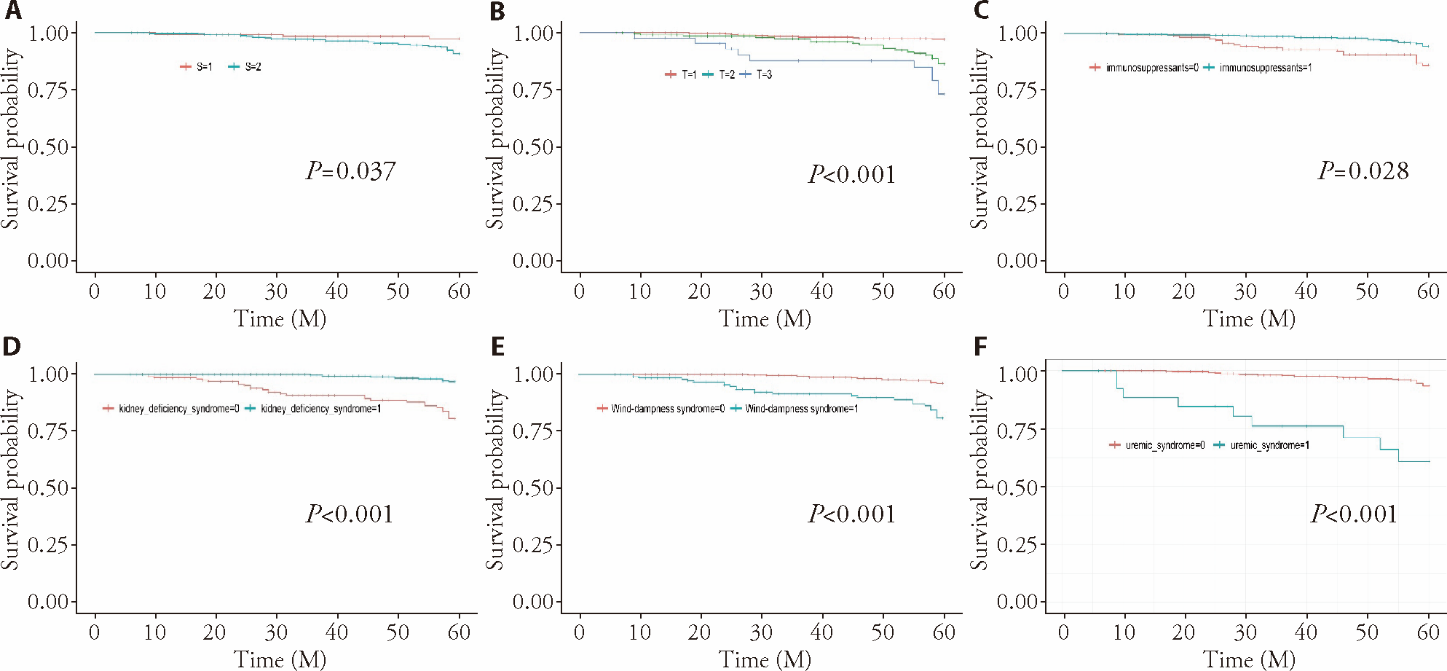
Figure 3 Results of K-M survival curve of renal pathology variables, treatment regimen, and Wang’s new five-type syndrome differentiation that were statistically different (P < 0.05) A: Oxford classification of S (segmental sclerosis) (S = 0 or 1); B: Oxford classification of T (interstitial fibrosis/tubular atrophy) (T = 0 or 1 or 2); C: immunosuppressive agents (without immunosuppressive agents = 0, with immunosuppressant = 1); D: kidney deficiency syndrome (without kidney deficiency syndrome = 0; with kidney deficiency syndrome = 1); E: wind-dampness syndrome (without wind-dampness syndrome = 0; with wind-dampness syndrome = 1); F: uremic syndrome (without uremic syndrome = 0, with uremic syndrome = 1).
| Variable | Comparison | χ 2 value | P value |
|---|---|---|---|
| Sex | Male vs female | 4.827 | 0.028 |
| Obesity | BMI ≥ 25 vs < 25 kg/m2 | 3.341 | 0.068 |
| Hb | Normal vs anemia | 19.270 | 0.000 |
| BP | Normal vs hypertension | 0.391 | 0.532 |
| UA | Normal vs hyperuricemia (F > 360 μmol/L, M > 420 μmol/L) | 24.056 | 0.000 |
| TC | Normal vs hypercholesterolemia | 4.091 | 0.043 |
| LDL | Normal vs high-LDL | 9.534 | 0.002 |
| TG | Normal vs hypertriglyceridemia | 2.298 | 0.130 |
| Alb | Normal vs hypoproteinemia | 3.208 | 0.073 |
| Urinary protein | Urinary protein ≤ 1 vs > 1 g/24 h | 36.415 | 0.000 |
| Microscopic hematuria | -, +, ++, +++, ++++ | 1.641 | 0.801 |
| Scr | Normal vs elevated Scr (F > 84 μmol/L, M > 104 μmol/L) | 61.868 | 0.000 |
| eGFR | eGFR < 90 vs ≥ 90 mL/min/1.73 m2 | 32.097 | 0.000 |
| Oxford classification of renal pathologya,b | E0, E1 | 0.831 | 0.362 |
| S0, S1 | 4.338 | 0.037 | |
| T0, 1, 2 | 36.593 | 0.000 | |
| C0, 1, 2 | 0.679 | 0.712 | |
| Treatment options | Taking vs not taking glucocorticoids | 0.084 | 0.772 |
| Taking vs not taking RAS blockers | 1.135 | 0.287 | |
| Taking vs not taking immunosuppressants | 11.087 | 0.001 | |
| Syndrome differentiation | Presence vs absence of kidney deficiency syndrome | 43.721 | 0.000 |
| Presence vs absence of stasis syndrome | 1.334 | 0.248 | |
| Presence vs absence of wind-dampness syndrome | 36.415 | 0.000 | |
| Presence vs absence of liver- wind syndrome | 0.004 | 0.948 | |
| Presence vs absence of uremic syndrome | 47.383 | 0.000 |
Table 2 Survival curves of each index (Log-rank test)
| Variable | Comparison | χ 2 value | P value |
|---|---|---|---|
| Sex | Male vs female | 4.827 | 0.028 |
| Obesity | BMI ≥ 25 vs < 25 kg/m2 | 3.341 | 0.068 |
| Hb | Normal vs anemia | 19.270 | 0.000 |
| BP | Normal vs hypertension | 0.391 | 0.532 |
| UA | Normal vs hyperuricemia (F > 360 μmol/L, M > 420 μmol/L) | 24.056 | 0.000 |
| TC | Normal vs hypercholesterolemia | 4.091 | 0.043 |
| LDL | Normal vs high-LDL | 9.534 | 0.002 |
| TG | Normal vs hypertriglyceridemia | 2.298 | 0.130 |
| Alb | Normal vs hypoproteinemia | 3.208 | 0.073 |
| Urinary protein | Urinary protein ≤ 1 vs > 1 g/24 h | 36.415 | 0.000 |
| Microscopic hematuria | -, +, ++, +++, ++++ | 1.641 | 0.801 |
| Scr | Normal vs elevated Scr (F > 84 μmol/L, M > 104 μmol/L) | 61.868 | 0.000 |
| eGFR | eGFR < 90 vs ≥ 90 mL/min/1.73 m2 | 32.097 | 0.000 |
| Oxford classification of renal pathologya,b | E0, E1 | 0.831 | 0.362 |
| S0, S1 | 4.338 | 0.037 | |
| T0, 1, 2 | 36.593 | 0.000 | |
| C0, 1, 2 | 0.679 | 0.712 | |
| Treatment options | Taking vs not taking glucocorticoids | 0.084 | 0.772 |
| Taking vs not taking RAS blockers | 1.135 | 0.287 | |
| Taking vs not taking immunosuppressants | 11.087 | 0.001 | |
| Syndrome differentiation | Presence vs absence of kidney deficiency syndrome | 43.721 | 0.000 |
| Presence vs absence of stasis syndrome | 1.334 | 0.248 | |
| Presence vs absence of wind-dampness syndrome | 36.415 | 0.000 | |
| Presence vs absence of liver- wind syndrome | 0.004 | 0.948 | |
| Presence vs absence of uremic syndrome | 47.383 | 0.000 |
| 1. | Rodrigues JC, Haas M, Reich HN. IgA nephropathy. Clin J Am Soc Nephrol 2017; 12: 677-86. |
| 2. |
Kamano C, Shimizu A, Joh K, et al. A cross-sectional study in patients with IgA nephropathy of correlations between clinical data and pathological findings at the time of renal biopsy: a Japanese prospective cohort study. Clin Exp Nephrol 2021; 25: 509-21.
DOI PMID |
| 3. |
Barbour SJ, Espino-Hernandez G, Reich HN, et al. The MEST score provides earlier risk prediction in IgA nephropathy. Kidney Int 2016; 89: 167-75.
DOI PMID |
| 4. |
Barbour SJ, Cattran DC, Kim SJ, et al. Individuals of Pacific Asian origin with IgA nephropathy have an increased risk of progression to end-stage renal disease. Kidney Int 2013; 84: 1017-24.
DOI PMID |
| 5. | Ganzer PD, Loeian MS, Roof SR, et al. Dynamic detection and reversal of myocardial ischemia using an artificially intelligent bioelectronic medicine. Sci Adv 2022; 8: eabj5473. |
| 6. | Wang Y, Chen H, Zhu C, et al. Study on syndrome-manifestation of Traditional Chinese Medicine in 1148 patients with IgA nephropathy. Zhong Xi Yi Jie He Shen Bing Za Zhi 2009; 12: 1054-58. |
| 7. | World Health Organization.ICD-11: International classification of diseases (11th revision) online, 2019-05-25, cited 2021-8-02; 1(1): 3screens. Available from URL: https://www.who.int/news-room/detail/25-05-2019-world-health-assembly-update. |
| 8. | State Bureau of Technology Supervision. National standard for clinical terms of Traditional Chinese Medicine: GB/T 16751.2-1997. Beijing: China Standard Press:1-358. |
| 9. |
Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 1999; 130: 461-70.
DOI PMID |
| 10. |
Kidney Disease: Improving Global Outcomes KDIGO Glomerular Diseases Work Group. KDIGO 2021 clinical practice guideline for the management of glomerular diseases. Kidney Int 2021; 100: S1-S276.
DOI PMID |
| 11. | Trimarchi H, Barratt J, Cattran DC, et al. IgAN Classification Working Group of the International IgA Nephropathy Network and the Renal Pathology Society; Conference Participants. Oxford classification of IgA nephropathy 2016:an update from the IgA Nephropathy Classification Working Group. Kidney Int 2017; 91: 1014-21. |
| 12. |
Deng W, Tan X, Zhou Q, et al. Gender-related differences in clinicopathological characteristics and renal outcomes of Chinese patients with IgA nephropathy. BMC Nephrol 2018; 19: 31.
DOI PMID |
| 13. | Shu D, Xu F, Su Z, et al. Risk factors of progressive IgA nephropathy which progress to end stage renal disease within ten years: a case-control study. BMC Nephrol 2017; 18: 1-6. |
| 14. | Chen T, Xia E, Chen T, et al. Identification and external validation of IgA nephropathy patients benefiting from immunosuppression therapy. EBioMedicine 2020; 52: 102657. |
| 15. | Tan J, Xu Y, Jiang Z, et al. Global glomerulosclerosis and segmental glomerulosclerosis could serve as effective markers for prognosis and treatment of IgA vasculitis with nephritis. Front Med (Lausanne) 2020; 7: 588031. |
| 16. | Shen X. Research on renal histopathological characteristics, clinical manifestations, treatment and prognosis of IgAN. Nanjing: Nanjing University, 2014: 11-39. |
| 17. |
Markowitz G. Updated Oxford classification of IgA nephropathy: a new MEST-C score. Nat Rev Nephrol 2017; 13: 385-86.
DOI PMID |
| 18. | Goto M, Kawamura T, Wakai K, et al. Risk stratification for progression of IgA nephropathy using a decision tree induction algorithm. Nephrol Dial Transplant 2009; 24: 1242-47. |
| 19. | Gu YH. A prognostic model of IgAN based on clinicopathological features and TCM syndromes. Guangzhou: Guangzhou University of Chinese Medicine, 2017: 27-93. |
| 20. | Liu J. Research on prognostic evaluation models of IgA nephropathy. Beijing: Chinese People's Liberation Army (PLA) Medical School, 2018: 35-88. |
| 21. |
Lee HS, Koh HI, Lee HB, Park HC. IgA nephropathy in Korea: a morphological and clinical study. Clin Nephrol 1987; 27: 131-40.
PMID |
| 22. |
Chen T, Li X, Li Y, et al. Prediction and risk stratification of kidney outcomes in IgA nephropathy. Am J Kidney Dis 2019; 74: 300-09.
DOI PMID |
| 23. | Chen XM, Chen Y, Li P, et al. A multicenter epidemiological survey on TCM syndrome in 1016 patients with IgA nephropathy and analysis of its relevant factors. Zhong Xi Yi Jie He Za Zhi 2006; 26: 197-97. |
| 24. | Wang YJ, Chen HY, Zhou LS, et al. Study on the therapy based on tonifying deficiency, resolving blood stasis and dispelling wind-dampness of IgA nephropathy in 123 cases. Zhong Xi Yi Jie He Shen Bing Za Zhi 2008; 9: 879-79. |
| [1] | WANG Bochuan, ZHANG Yong, ZHANG Qiuyun, ZHANG Zhiqiang, LUO Changyong, WANG Zhendong, BAI Chen, WANG Yuhan, GE Xueyi, QIAN Ying, YU He, GU Xiaohong. Reveal the mechanisms of prescriptions for liver cancer' treatment based on two illustrious senior TCM physicians [J]. Journal of Traditional Chinese Medicine, 2023, 43(1): 188-197. |
| [2] | GU Xiaoli, CHEN Menglei, LIU Minghui, ZHANG Zhe, ZHAO Weiwei, CHENG Wenwu. Value of Traditional Chinese Medicine syndrome differentiation in predicting the survival time of patients with advanced cancer [J]. Journal of Traditional Chinese Medicine, 2021, 41(4): 636-641. |
| [3] | Guoqiang Lin, Yingqiu Li, Shengxi Chen, Haihe Jiang. Integrated Chinese-western therapy versus western therapy alone on survival rate in patients with non-small-cell lung cancer at middlelate stage [J]. Journal of Traditional Chinese Medicine, 2013, 33(04): 433-438. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||