Journal of Traditional Chinese Medicine ›› 2025, Vol. 45 ›› Issue (3): 473-484.DOI: 10.19852/j.cnki.jtcm.2025.03.006
Matrine alleviates coronary microvascular dysfunction in ischemia with non-obstructive coronary artery disease mice induced by advanced glycation end products via inhibition of the reactive oxygen species-mediated endoplasmic reticulum stress in cardiac microvascular endothelial cells
DU Haixia1, QIU Chuan2, MA Yanpeng3,4,5,6, PAN Shuo3,4,5,6, WANG Xiqiang3,4,5,6, WANG Junkui3,4,5,6( ), LIU Zhongwei3,4,5,6(
), LIU Zhongwei3,4,5,6( )
)
- 1 Department of Cardiology, Shaanxi Provincial People’s Hospital, Xi’an 710068, China; Department 403, PLA Rocket Force University of Engineering, Xi’an 710025, China
2 Division of Biomedical Informatics and Genomics, Deming Department of Medicine, Tulane Center of Biomedical Informatics and Genomics, Tulane University, New Orleans 70112, USA
3 Department of Cardiology, Shaanxi Provincial People’s Hospital, Xi’an 710068, China; Scientific and Technology Transfer Office, Shaanxi Provincial People’s Hospital, Xi’an 710068, China
4 Atherosclerosis Integrated Chinese and Western Medicine Key Research Laboratory, Research Office of Shaanxi Administration of Traditional Chinese Medicine, Xi’an 710003, China
5 Traditional Chinese Medicine Inheritance and Innovation Platform, Shaanxi Provincial People’s Hospital, Xi’an 710068, China
6 Shaanxi Belt and Road Joint Laboratory of Precision Medicine for Cardiovascular and Cerebrovascular Diseases; Xi’an 710068, China
-
Received:2024-03-22Accepted:2024-09-08Online:2025-06-15Published:2025-05-21 -
Contact:Prof. LIU Zhongwei, Department of Cardiology, Shaanxi Provincial People’s Hospital, Xi’an 710068, China. medicalman@163.com;Prof. WANG Junkui, Department of Cardiology, Shaanxi Provincial People’s Hospital, Xi’an 710068, China. junkuiwang@yeah.net, Telephone: +86-29-85251331-3378 -
Supported by:National Natural Scientific Foundation of China: Mechanisms of Macrophage-Mediated Vascular Smooth Muscle Cells Phenotypic Conversion in Advanced Glycation End Products-induced Atherosclerosis and Therapeutic Effects of Targeted Gene Silencing(82070858);Youth Scientific Research and Innovation Team Program of Shaanxi Province: Diabetes-Related Atherosclerosis Basic Research and Application Research Team(2022-SLRH-LJ-014)
Cite this article
DU Haixia, QIU Chuan, MA Yanpeng, PAN Shuo, WANG Xiqiang, WANG Junkui, LIU Zhongwei. Matrine alleviates coronary microvascular dysfunction in ischemia with non-obstructive coronary artery disease mice induced by advanced glycation end products via inhibition of the reactive oxygen species-mediated endoplasmic reticulum stress in cardiac microvascular endothelial cells[J]. Journal of Traditional Chinese Medicine, 2025, 45(3): 473-484.
share this article
| Group | n | 0 d | 10 d | 20 d |
|---|---|---|---|---|
| control | 6 | 19.3±2.8 | 20.6±4.2 | 20.6±4.2 |
| matrine | 6 | 19.4±1.9a | 22.7±5.6a | 22.7±5.6a |
| INOCA | 6 | 17.5±4.0b | 17.8±6.1b | 17.8±6.1b |
| INOCA+AGEs | 6 | 18.8±2.9c | 20.7±4.1c | 20.7±4.1c |
| INOCA+AGEs+matrine | 6 | 19.9±2.3d | 20.6±2.1d | 20.6±2.1d |
Table 1 Serum cTNT levels in animals at 0, 10 and 20 d after injections of AGEs-BSA or control BSA ($\bar{x}±s$, μg/L)
| Group | n | 0 d | 10 d | 20 d |
|---|---|---|---|---|
| control | 6 | 19.3±2.8 | 20.6±4.2 | 20.6±4.2 |
| matrine | 6 | 19.4±1.9a | 22.7±5.6a | 22.7±5.6a |
| INOCA | 6 | 17.5±4.0b | 17.8±6.1b | 17.8±6.1b |
| INOCA+AGEs | 6 | 18.8±2.9c | 20.7±4.1c | 20.7±4.1c |
| INOCA+AGEs+matrine | 6 | 19.9±2.3d | 20.6±2.1d | 20.6±2.1d |
| Group | n | CFVrest (cm/s) | CFVhypereamia (cm/s) | CFVR |
|---|---|---|---|---|
| Control | 6 | 210.88±13.02 | 701.44±10.33 | 3.34±0.23 |
| Matrine | 6 | 208.42±18.54a | 695.39±15.19a | 3.37±0.33a |
| INOCA | 6 | 212.64±12.76b | 664.36±12.21e | 3.14±0.19e |
| INOCA+AGEs | 6 | 209.44±7.84c | 584.45±15.61f | 2.79±0.13f |
| INOCA+AGEs+matrine | 6 | 214.35±7.74d | 648.94±11.86g | 3.03±0.12g |
Table 2 CFV at rest, during hypereamia and their ratio ($\bar{x}±s$)
| Group | n | CFVrest (cm/s) | CFVhypereamia (cm/s) | CFVR |
|---|---|---|---|---|
| Control | 6 | 210.88±13.02 | 701.44±10.33 | 3.34±0.23 |
| Matrine | 6 | 208.42±18.54a | 695.39±15.19a | 3.37±0.33a |
| INOCA | 6 | 212.64±12.76b | 664.36±12.21e | 3.14±0.19e |
| INOCA+AGEs | 6 | 209.44±7.84c | 584.45±15.61f | 2.79±0.13f |
| INOCA+AGEs+matrine | 6 | 214.35±7.74d | 648.94±11.86g | 3.03±0.12g |
| Biomarker | Control (n = 6) | Martine treatment (n = 6) |
|---|---|---|
| ALT (U/L) | 31.10±3.20 | 30.46±3.39a |
| AST (U/L) | 99.10±5.27 | 101.08±6.31a |
| CRE (mmol/L) | 26.70±1.76 | 25.26±4.45a |
| CK (U/L) | 527.12±16.20 | 519.93±23.39a |
| CK-MB (U/L) | 196.02±14.51 | 200.32±14.82a |
| Na+ (mmol/L) | 130.71±3.07 | 129.91±1.51a |
| K+ (mmol/L) | 4.81±0.45 | 5.14±0.16a |
| Cl- (mmol/L) | 100.47±3.59 | 102.55±4.19a |
Table 3 Blood biochemical markers determination in matrine-treated animals ($\bar{x}±s$)
| Biomarker | Control (n = 6) | Martine treatment (n = 6) |
|---|---|---|
| ALT (U/L) | 31.10±3.20 | 30.46±3.39a |
| AST (U/L) | 99.10±5.27 | 101.08±6.31a |
| CRE (mmol/L) | 26.70±1.76 | 25.26±4.45a |
| CK (U/L) | 527.12±16.20 | 519.93±23.39a |
| CK-MB (U/L) | 196.02±14.51 | 200.32±14.82a |
| Na+ (mmol/L) | 130.71±3.07 | 129.91±1.51a |
| K+ (mmol/L) | 4.81±0.45 | 5.14±0.16a |
| Cl- (mmol/L) | 100.47±3.59 | 102.55±4.19a |
| Group | n | FITC-Dextran leakage (fold of control) | TER (Ω·cm2) |
|---|---|---|---|
| Control | 6 | 1.02±0.08 | 642.14±29.75 |
| Matrine | 6 | 0.97±0.06a | 666.73±14.65a |
| AGEs | 6 | 2.17±0.14b | 325.75±9.47b |
| AGEs+matrine | 6 | 1.43±0.04c | 613.74±13.67c |
Table 4 FITC-dextran leakage and TER of CEMCs ($\bar{x}±s$)
| Group | n | FITC-Dextran leakage (fold of control) | TER (Ω·cm2) |
|---|---|---|---|
| Control | 6 | 1.02±0.08 | 642.14±29.75 |
| Matrine | 6 | 0.97±0.06a | 666.73±14.65a |
| AGEs | 6 | 2.17±0.14b | 325.75±9.47b |
| AGEs+matrine | 6 | 1.43±0.04c | 613.74±13.67c |

Figure 1 Effect of matrine on AGEs-induced coronary microcirculatory apoptosis, inflammation, and microthrombosis A: images of isolated CMECs (×400), with TUNEL-positive cells indicated by red fluorescence in Control (A1), Matrine (A2), INOCA (A3), INOCA + AGEs (A4), INOCA + AGEs + Matrine (A5). Cell nuclei were stained with DAPI; D: apoptotic rate of CMECs calculated from TUNEL staining (n = 6); B: cardiac tissue sections stained for CD45 by immunofluorescence (× 400) in Control (B1), Matrine (B2), INOCA (B3), INOCA + AGEs (B4), INOCA + AGEs + Matrine (B5). Cell nuclei were stained with DAPI; E: mean fluorescence intensities of CD45 staining (n = 6); C: cardiac tissue sections stained for CD42b by immunofluorescence (× 400) in Control (C1), Matrine (C2), INOCA (C3), INOCA + AGEs (C4), INOCA + AGEs + Matrine (C5). Cell nuclei were stained with DAPI; F: mean fluorescence intensities of CD42b staining (n = 6). Model groups: Control: treated with control BSA; matrine: treated with matrine at dosage of 200 mg/kg bodyweight; INOCA: INOCA model (ob/ob-/- mice); INOCA + AGEs: INOCA model treated with AGEs at 1 mg/d; INOCA + AGEs + matrine: INOCA model co-treated with matrine and AGEs. AGEs: advanced glycation end products; TUNEL: terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling; DAPI: 4,6-diamidino-2-phenylindole; CMECs: cardiac microvascular endothelial cells; IL6: interleukin 6; TNFα: tumor necrosis factor alpha; TXB2: thromboxane B2. Differences between two groups were compared using t-test. Data are presented as mean±standard deviation. Compared with control, aP > 0.05; compared with INOCA, bP < 0.001; compared with INOCA + AGEs, cP < 0.001.

Figure 2 Effect of matrine on AGEs-induced ROS-mediated ER signaling in CMECs A: images of isolated CMECs (× 400) with DCFH-DA fluorescent staining in Control (A1), Matrine (A2), INOCA (A3), INOCA + AGEs (A4), INOCA + AGEs + Matrine (A5). Cell nuclei were stained with DAPI ; B: mean fluorescence intensities of DCFH-DA in CMECs (n = 6); C: immunoblots of phosphorylated PERK (p-PERK), PERK, phosphorylated IRE1 (p-IRE1), and IRE1 in CMECs isolated from animals; Relative phosphorylation levels of PERK (D) and IRE1 (E) were indicated by the columns (n = 6); F: immunoblots of GRP78, ATF4, XBP1s, CHOP, and GAPDH in CMECs; Columns indicate the relative expression levels of GRP78 (G), ATF4 (H), XBP1s (I), and CHOP (J) in isolated CMECs (n = 6); K: enzymatic activity of calcineurin in isolated CMECs (n = 6). Model groups: control: treated with control BSA; matrine: treated with matrine at dosage of 200 mg/kg bodyweight; INOCA: INOCA model (ob/ob-/- mice); INOCA+AGEs: INOCA model treated with AGEs at 1 mg/d; INOCA + AGEs + matrine: INOCA model co-treated with matrine and AGEs. AGEs: advanced glycation end products; ROS: reactive oxygen species; DAPI: 4,6-diamidino-2-phenylindole; CMECs: cardiac microvascular endothelial cells; DCFH-DA: 2,7-dichlorofluorescein; PERK: protein kinase R-like endoplasmic reticulum kinase; IRE1: inositol-requiring enzyme 1; GRP78: glucose regulated protein 78; ATF4: activating transcription factor 4; XBP1s: X-box binding protein 1s; CHOP: C/EBP-homologous protein. Differences between two groups were compared using t-test. Data are presented as mean ± standard deviation. Compared with control, aP > 0.05; compared with INOCA, bP < 0.001; compared with INOCA + AGEs, cP < 0.001.

Figure 3 Effect of matrine on NFAT-mediated pathways in CMECs A: immunofluorescent staining of NFATc4 in isolated CMECs in Control (A1), Matrine (A2), INOCA (A3), INOCA + AGEs (A4), INOCA + AGEs + Matrine (A5). Cell nuclei were stained with DAPI; B: NFATc4 nuclear translocation rate (n = 6); C: immunoblots of NFATc4 and histone H3 in nuclear protein extracted from CMECs; D: relative nuclear translocation of NFATc4 (n = 6); E: immunoblots of COX2, IL6, TNFα, Fas, FasL, and GAPDH in isolated CMECs; F-J: relative expression levels of COX2 (F), FAS (G), FASL (H), IL6 (I), and TNFα (J) (n = 6). Model groups: Control: treated with control BSA; matrine: treated with matrine at dosage of 200 mg/kg bodyweight; INOCA: INOCA model (ob/ob-/- mice); INOCA + AGEs: INOCA model treated with AGEs at 1 mg/d; INOCA + AGEs + matrine: INOCA model co-treated with matrine and AGEs. AGEs: advanced glycation end products; DAPI: 4,6-diamidino-2-phenylindole; CMECs: cardiac microvascular endothelial cells; NFAT: nuclear factor of activated T-cells; COX2: cyclooxygenase 2; IL6: interleukin 6; TNFα: tumor necrosis factor alpha; FASL: FAS ligand. Differences between two groups were compared using t-test. Data are presented as mean ± standard deviation. Compared with control, aP > 0.05; compared with INOCA, bP < 0.001; compared with INOCA + AGEs, cP < 0.001; compared with INOCA + AGEs, dP < 0.01.
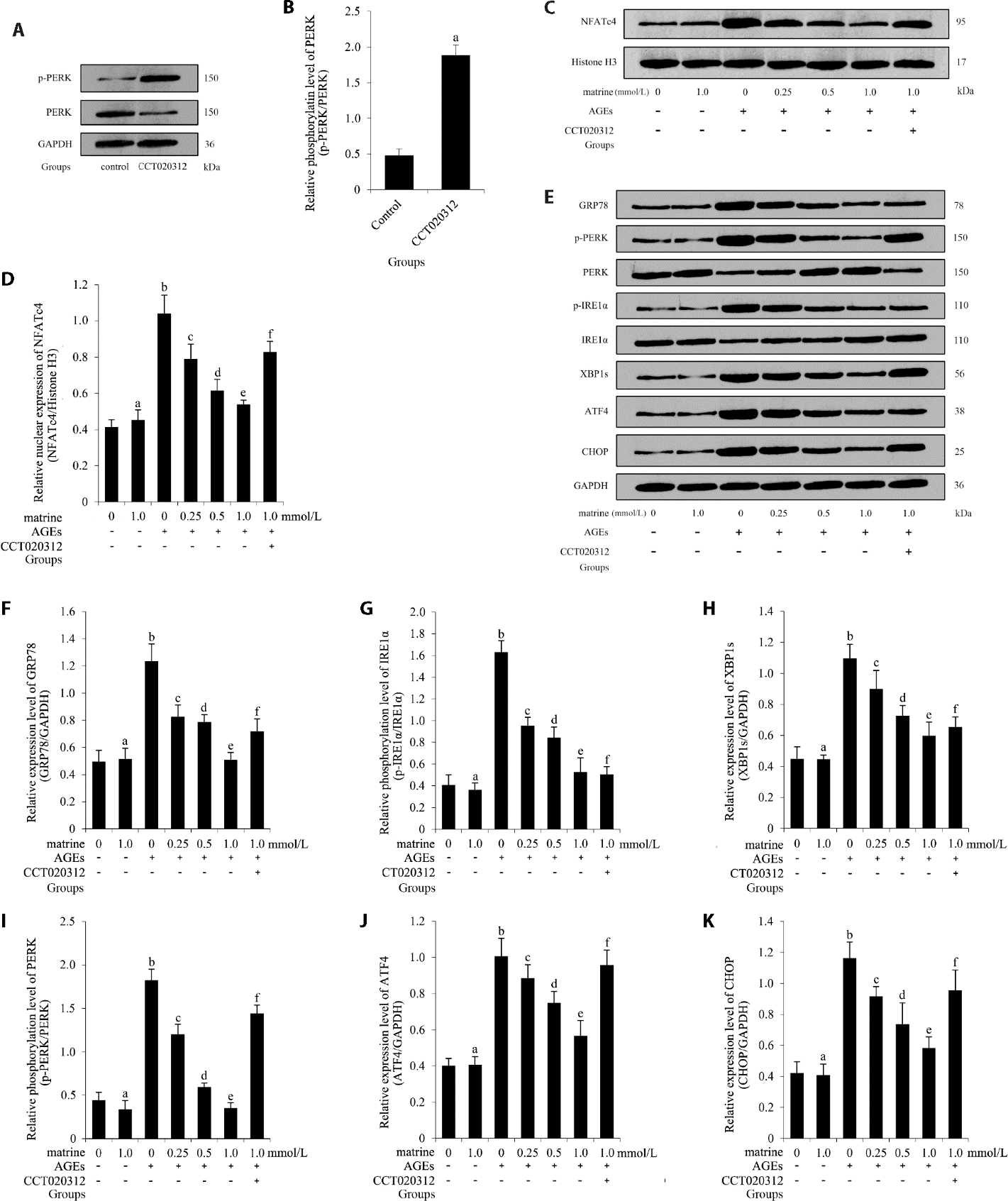
Figure 4 Effect of matrine on AGEs-induced PERK/NFAT signaling in primary CMECs A: immunoblots of p-PERK, PERK, and GAPDH in primary CMECs treated with CCT020312; B: relative phosphorylation levels of PERK (n = 3). Compared with control, aP < 0.05; C: immunoblots of NFATc4 and histone H3 in AGEs-exposed primary CMECs treated with matrine and/or CCT020312; D: relative nuclear levels of NFATc4 (n = 3); E: immunoblots of GRP78, p-PERK, PERK, p-IRE1α, IRE1α, XBP1s, ATF4, CHOP, and GAPDH in AGEs-exposed primary CMECs treated with matrine and/or CCT020312; F: relative expression level of GRP78 (n = 3); G: relative phosphorylation level of IRE1α (n = 3); H: relative expression level of XBP1s (n = 3); I: relative phosphorylation level of PERK (n = 3); J: relative expression level of ATF4 (n = 3); K: relative expression level of CHOP (n = 3). Model groups: control: untreated primary CMECs; primary CMECs treated with CCT020312 at 10 μmol/L. AGEs: advanced glycation end products; CMECs: cardiac microvascular endothelial cells; NFAT: nuclear factor of activated T-cells; PERK: protein kinase R-like endoplasmic reticulum kinase; IRE1: inositol-requiring enzyme 1; GRP78: glucose regulated protein 78; ATF4: activating transcription factor 4; XBP1s: X-box binding protein 1s; CHOP: C/EBP-homologous protein. Differences between two groups were compared using t-test. Data are presented as mean ± standard deviation. Compared with control CMECs, aP > 0.05; compared with CMECs treated with matrine at 1.0 mmol/L, bP < 0.05; compared with CMECs treated with AGEs, cP < 0.05; compared with CMECs co-treated with matrine at 0.25 mmol/L and AGEs, dP < 0.05; compared with CMECs co-treated with matrine at 0.5 mmol/L and AGEs, eP < 0.05; CMECs co-treated with matrine at 1.0 mmol/L and AGEs, fP < 0.05.
| 1. | Bertil L, Tomasz B, Mario A, Francesco P. Myocardial infarction with non-obstructive coronary artery disease. Euro Intervention 2021; 17: e875-87. |
| 2. | Bairey Merz CN, Pepine CJ, Walsh MN, Fleg JL. Ischemia and no obstructive coronary artery disease (INOCA): developing evidence-based therapies and research agenda for the next decade. Circulation 2017; 135: 1075-92. |
| 3. |
Kunadian V, Chieffo A, Camici PG, et al. An EAPCI expert consensus document on ischaemia with non-obstructive coronary arteries in collaboration with European Society of Cardiology working group on coronary pathophysiology & microcirculation endorsed by coronary vasomotor disorders international study group. Eur Heart J 2020; 41: 3504-20.
DOI PMID |
| 4. |
Jespersen L, Hvelplund A, Abildstrøm SZ, et al. Stable angina pectoris with no obstructive coronary artery disease is associated with increased risks of major adverse cardiovascular events. Eur Heart J 2012; 33: 734-44.
DOI PMID |
| 5. | Vancheri F, Longo G, Vancheri S, et al. Coronary microvascular dysfunction. J Clin Med 2020; 9: 2880. |
| 6. |
Konijnenberg LSF, Damman P, Duncker DJ, et al. Pathophysiology and diagnosis of coronary microvascular dysfunction in ST-elevation myocardial infarction. Cardiovasc Res 2020; 116: 787-805.
DOI PMID |
| 7. |
Jansen TPJ, Konst RE, Elias-Smale SE, et al. Assessing microvascular dysfunction in angina with unobstructed coronary arteries: JACC review topic of the week. J Am Coll Cardiol 2021; 78: 1471-9.
DOI PMID |
| 8. | Kloner RA, King KS, Harrington MG. No-reflow phenomenon in the heart and brain. Am J Physiol Heart Circ Physiol 2018; 315: H550-62. |
| 9. | Liu ZW, Zhu HT, Ma YP, et al. AGEs exacerbates coronary microvascular dysfunction in NoCAD by activating endoplasmic reticulum stress-mediated PERK signaling pathway. Metabolism 2021; 117: 154710. |
| 10. |
Gyldenkerne C, Olesen KKW, Madsen M, et al. Extent of coronary artery disease is associated with myocardial infarction and mortality in patients with diabetes mellitus. Clin Epidemiol 2019; 11: 419-28.
DOI PMID |
| 11. |
Henning RJ. Type-2 diabetes mellitus and cardiovascular disease. Future cardiology. Future Cardiol 2018; 14: 491-509.
DOI PMID |
| 12. |
Zhang H, Chen LL, Sun XP, et al. Matrine: a promising natural product with various pharmacological activities. Front Pharmacol 2020; 11: 588.
DOI PMID |
| 13. | Zhang X, Hu C, Zhang N, et al. Matrine attenuates pathological cardiac fibrosis via RPS5/p38 in mice. Acta Pharmacol Sin 2021; 42: 573-84. |
| 14. | Hu C, Zhang X, Wei WY, et al. Matrine attenuates oxidative stress and cardiomyocyte apoptosis in doxorubicin-induced cardiotoxicity via maintaining AMPKα/UCP2 pathway. Acta Pharm Sin B 2019; 9: 690-701. |
| 15. |
Gao XB, Guo S, Zhang S, et al. Matrine attenuates endoplasmic reticulum stress and mitochondrion dysfunction in nonalcoholic fatty liver disease by regulating SERCA pathway. J Transl Med 2018; 16: 319.
DOI PMID |
| 16. |
Guo S, Chen YF, Pang CL, et al. Matrine is a novel inhibitor of the TMEM16A chloride channel with antilung adenocarcinoma effects. J Cell Physiol 2019; 234: 8698-708.
DOI PMID |
| 17. | Jin BJ, Jin H. Oxymatrine attenuates lipopolysaccharide-induced acute lung injury by activating the epithelial sodium channel and suppressing the JNK signaling pathway. Exp Anim 2018; 67: 337-47. |
| 18. |
Zhang YF, Wang SZ, Li YY, et al. Sophocarpine and matrine inhibit the production of TNF-alpha and IL-6 in murine macrophages and prevent cachexia-related symptoms induced by colon26 adenocarcinoma in mice. Int Immunopharmacol 2008; 8: 1767-72.
DOI PMID |
| 19. |
Yang FJ, Liu Y, Ren HZ, et al. ER-stress regulates macrophage polarization through pancreatic EIF-2alpha kinase. Cell Immunol 2019; 336: 40-7.
DOI PMID |
| 20. |
Liu ZW, Zhang Y, Tang ZG, et al. Matrine attenuates cardiac fibrosis by affecting ATF6 signaling pathway in diabetic cardiomyopathy. Eur J Pharmacol 2017; 804: 21-30.
DOI PMID |
| 21. |
Liu ZW, Wang Y, Zhu HT, et al. Matrine blocks AGEs- induced HCSMCs phenotypic conversion via suppressing Dll4-Notch pathway. Eur J Pharmacol 2018; 835: 126-31.
DOI PMID |
| 22. |
Liu ZW, Zhang Y, Pan S, et al. Activation of RAGE-dependent endoplasmic reticulum stress associates with exacerbated postmyocardial infarction ventricular arrhythmias in diabetes. Am J Physiol Endocrinol Metab 2021; 320: E539-50.
DOI PMID |
| 23. | Adingupu DD, Göpel SO, Grönros J, et al. SGLT2 inhibition with empagliflozin improves coronary microvascular function and cardiac contractility in prediabetic ob/ob(-/-) mice. Cardiovasc Diabetol 2019; 18: 16. |
| 24. | Liu ZW, Lyu Y, Zhang Y, et al. Matrine-type alkaloids inhibit advanced glycation end products induced reactive oxygen species-mediated apoptosis of aortic endothelial cells in vivo and in vitro by targeting MKK3 and p38MAPK signaling. J Am Heart Assoc 2017; 6: e007441. |
| 25. |
Xu YX, Lin HZ, Zheng WJ, et al. Matrine ameliorates adriamycin-induced nephropathy in rats by enhancing renal function and modulating Th17/Treg balance. Eur J Pharmacol 2016; 791: 491-501.
DOI PMID |
| 26. | Liou CJ, Lai YR, Chen YL, et al. Matrine attenuates COX-2 and ICAM-1 expressions in human lung epithelial cells and prevents acute lung injury in LPS-induced mice. Mediators Inflamm 2016; 2016: 3630485. |
| 27. |
Zhou T, Xiang DK, Li SN, et al. MicroRNA-495 ameliorates cardiac microvascular endothelial cell injury and inflammatory reaction by suppressing the NLRP3 inflammasome signaling pathway. Cell Physiol Biochem 2018; 49: 798-815.
DOI PMID |
| 28. | Qiao L, Yan SQ, Dou XN, et al. Biogenic selenium nanoparticles alleviate intestinal epithelial barrier damage through regulating endoplasmic reticulum stress-mediated mitophagy. Oxid Med Cell Longev 2022; 2022: 3982613. |
| 29. | Zhang X, Zhu JX, Ma ZG, et al. Rosmarinic acid alleviates cardiomyocyte apoptosis via cardiac fibroblast in doxorubicin-induced cardiotoxicity. Int J Bio Sci 2019; 15: 556-67. |
| 30. |
Zhang X, Hu C, Kong CY, et al. FNDC5 alleviates oxidative stress and cardiomyocyte apoptosis in doxorubicin-induced cardiotoxicity via activating AKT. Cell Death Differ 2020; 27: 540-55.
DOI PMID |
| 31. | Hu C, Zhang X, Zhang N, et al. Osteocrin attenuates inflammation, oxidative stress, apoptosis, and cardiac dysfunction in doxorubicin-induced cardiotoxicity. Clin Transl Med 2020; 10: e124. |
| 32. | Zhao Y, Sedighi R, Wang P, et al. Carnosic acid as a major bioactive component in rosemary extract ameliorates high-fat-diet-induced obesity and metabolic syndrome in mice. J Agric Food Chem 2015; 63: 4843-52. |
| 33. | Nakayama H, Mitsuhashi T, Kuwajima S, et al. Immunochemical detection of advanced glycation end products in lens crystallins from streptozocin-induced diabetic rat. Diabetes 1993; 42: 345-50. |
| 34. | Lee J, Yun JS, Ko SH. Advanced glycation end products and their effect on vascular complications in type 2 diabetes mellitus. Nutrients, 2022; 14: 3086. |
| 35. | Liu ZW, Ma YP, Cui QW, et al. Toll-like receptor 4 plays a key role in advanced glycation end products-induced M1 macrophage polarization. Biochem Biophys Res Commun 2020; 531: 602-8. |
| 36. |
Liu ZW, Cai H, Zhu HT, et al. Protein kinase RNA-like endoplasmic reticulum kinase (PERK)/calcineurin signaling is a novel pathway regulating intracellular calcium accumulation which might be involved in ventricular arrhythmias in diabetic cardiomyopathy. Cell Signal 2014; 26: 2591-600.
DOI PMID |
| 37. |
Li M, Fang XZ, Liu XT, et al. Inhibition of calcineurin/NFATc4 signaling attenuates ventilator‑induced lung injury. Mol Med Rep 2020; 21: 607-14.
DOI PMID |
| 38. |
Hernández M, Wicz S, Corral RS. Cardioprotective actions of curcumin on the pathogenic NFAT/COX-2/prostaglandin E(2) pathway induced during trypanosoma cruzi infection. Phytomedicine 2016; 23: 1392-400.
DOI PMID |
| 39. | Jayanthi S, Deng X, Ladenheim B, et al. Calcineurin/NFAT-induced up-regulation of the Fas ligand/Fas death pathway is involved in methamphetamine-induced neuronal apoptosis. Proc Natl Acad Sci U S A 2005; 102: 868-73. |
| 40. | Sun HN, Yang YM, Gu MY, et al. The role of Fas-FasL-FADD signaling pathway in arsenic-mediated neuronal apoptosis in vivo and in vitro. Toxicol Lett 2022; 356: 143-50. |
| 41. | Yu BX, Yuan JN, Zhang FR, et al. Inhibition of Orai1-mediated Ca2+ entry limits endothelial cell inflammation by suppressing calcineurin-NFATc4 signaling pathway. Biochem Biophys Res Commun 2018; 495: 1864-70. |
| 42. | Hu C, Zhang X, Song P, et al. Meteorin-like protein attenuates doxorubicin-induced cardiotoxicity via activating cAMP/PKA/SIRT1 pathway. Redox Biol 2020; 37: 101747. |
| 43. | Hu C, Zhang X, Hu M, et al. Fibronectin type Ⅲ domain-containing 5 improves aging-related cardiac dysfunction in mice. Aging Cell 2022; 21: e13556. |
| 44. |
Caughey GE, Cleland LG, Gamble JR, et al. Up-regulation of endothelial cyclooxygenase-2 and prostanoid synthesis by platelets. Role of thromboxane A2. J Biol Chem 2001; 276: 37839-45.
DOI PMID |
| 45. |
Wang M, Murdoch CE, Brewer AC, et al. Endothelial NADPH oxidase 4 protects against angiotensin II-induced cardiac fibrosis and inflammation. ESC Heart Fail 2021; 8: 1427-37.
DOI PMID |
| 46. |
Matheny HE, Deem TL, Cook-Mills JM. Lymphocyte migration through monolayers of endothelial cell lines involves VCAM-1 signaling via endothelial cell NADPH oxidase. J Immunol 2000; 164: 6550-9.
DOI PMID |
| 47. | Cui QW, Du HX, Ma YP, et al. Matrine inhibits advanced glycation end products-induced macrophage M1 polarization by reducing DNMT3a/b-mediated DNA methylation of GPX1 promoter. Eur J Pharmacol 2022; 926: 175039. |
| 48. | Zhao L, Cai H, Tang ZG, et al. Matrine suppresses advanced glycation end products-induced human coronary smooth muscle cells phenotype conversion by regulating endoplasmic reticulum stress-dependent Notch signaling. Eur J Pharmacol 2020; 882: 173257. |
| 49. |
Soma P, Swanepoel AC, du Plooy JN, et al. Flow cytometric analysis of platelets type 2 diabetes mellitus reveals 'angry' platelets. Cardiovasc Diabetol 2016 ; 15: 52.
DOI PMID |
| 50. | Kroschinsky F, Stölzel F, von Bonin S, et al. New drugs, new toxicities: severe side effects of modern targeted and immunotherapy of cancer and their management. Crit Care 2017; 21: 89. |
| 51. | Zhang QY, Wang FX, Jia KK, et al. Natural product interventions for chemotherapy and radiotherapy-induced side effects. Front Pharmacol 2018; 9: 1253. |
| [1] | CHEN Youlan, DING Mingming, HUANG Chaoyuan, ZHENG Yiyuan, LIU Fengbin. Chang'an decoction (肠安方) alleviates endoplasmic reticulum stress by regulating mitofusin 2 to improve colitis [J]. Journal of Traditional Chinese Medicine, 2024, 44(3): 427-436. |
| [2] | JIANG Wen, ZHANG Wei, ZHANG Yuxiang, YANG Hao, PAN Xiaomei, CHEN Qiang, CHEN Junhui. Tilianin extracted from Xiangqinglan (Herba Dracocephali Moldovicae) inhibits apoptosis induced by mitochondrial pathway and endoplasmic reticulum stress in H9c2 cells after oxygen-glucose deprivation/reoxygenation [J]. Journal of Traditional Chinese Medicine, 2023, 43(1): 42-50. |
| [3] | CHEN Xiaoqing, ZHANG Yong, HUANG Chunlai, FU Tingting, TAO Qinghua, MA Liqiang, WANG Liping. Efficacy of Huanglian root decoction(黄连煎剂) on kidney injury in rat's model of metabolic syndrome [J]. Journal of Traditional Chinese Medicine, 2021, 41(1): 117-124. |
| [4] | Xu Zhiwei, Wang Tong, Chen Liang, Lou Ying, Sun Xiaoyan, Jiang Jinqi. Treatment of dilated cardiomyopathy caused by coronary microvascular dysfunction with anisodamine: a report of 5 cases [J]. Journal of Traditional Chinese Medicine, 2020, 40(2): 340-342. |
| [5] | Zhang Yi, Mo Fangfang, Zhang Dongwei, Gao Sihua, Zhao Dandan, Yu Na, Mu Qianqian, Zuo Jiacheng, Ma Yue. Jiangtang Xiaoke granule attenuates glucose metabolism disorder via regulating endoplasmic reticulum stress in the liver of type 2 diabetes mellitus mice [J]. Journal of Traditional Chinese Medicine, 2018, 38(04): 570-578. |
| [6] | Wang Xiu, Li Jianchun, Hu Yuzhen, Chen Wenyang, Jin Yiguang. Effect of Sophora flavescens alkaloid on aerobic vaginitis in gel form for local treatment [J]. Journal of Traditional Chinese Medicine, 2017, 37(03): 314-320. |
| [7] | He Ling, Fang Meixia, Chen Liguo, Zhou Jianhua, Yuan Jing, Xu Jing, Shan Yan, Xu Qingyun, Xiong Tingting. Transcriptome analysis of blood stasis syndrome in subjects with hypertension [J]. Journal of Traditional Chinese Medicine, 2016, 36(02): 173-180. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||

